Aplastic Anemia as a Roadmap for Bone Marrow Failure: An Overview and a Clinical Workflow
Abstract
1. Introduction
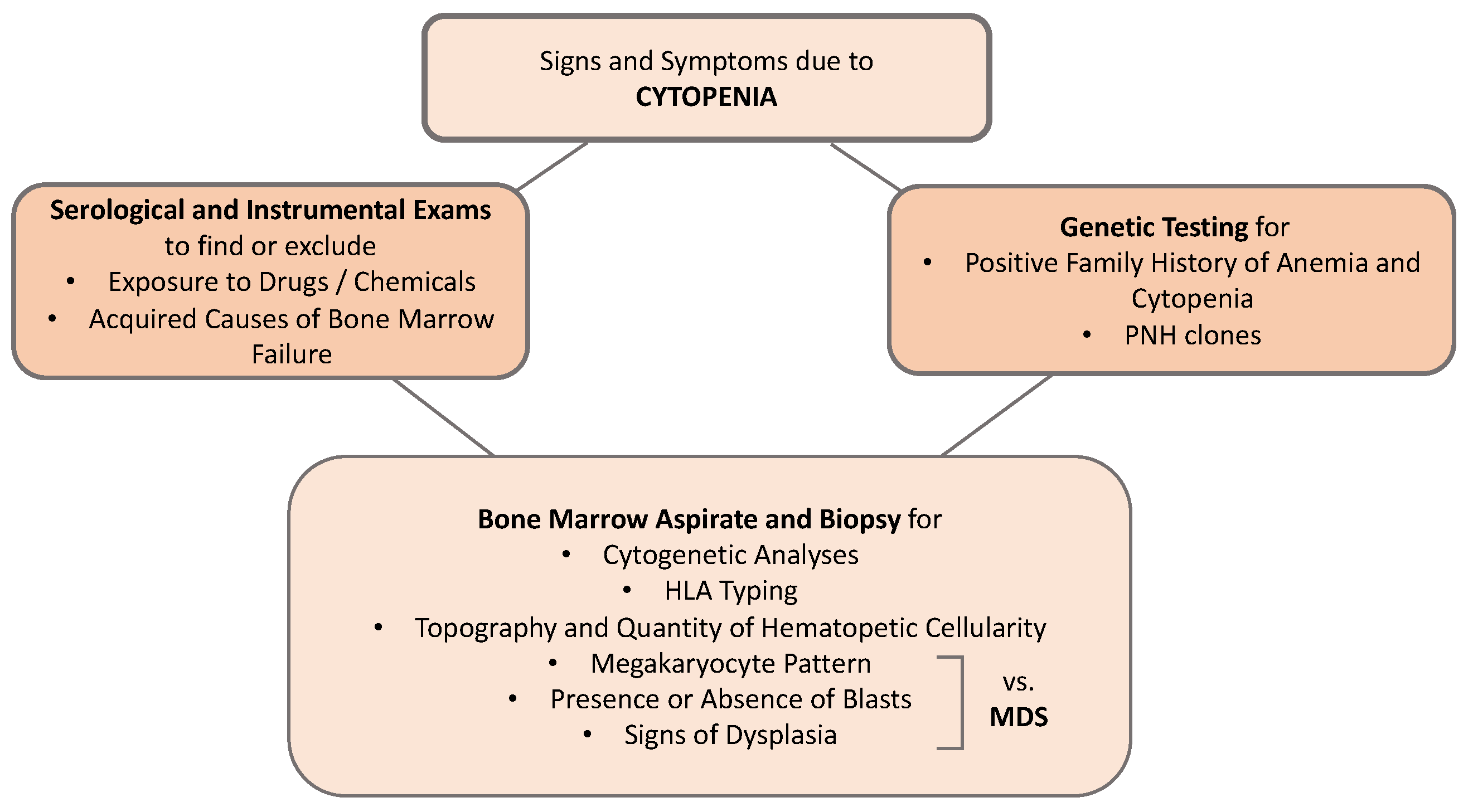
2. Approach to the Patient with Hypocellular Bone Marrow Failure
2.1. Focused Medical History and Physical Examination
2.2. Clonal Evolution
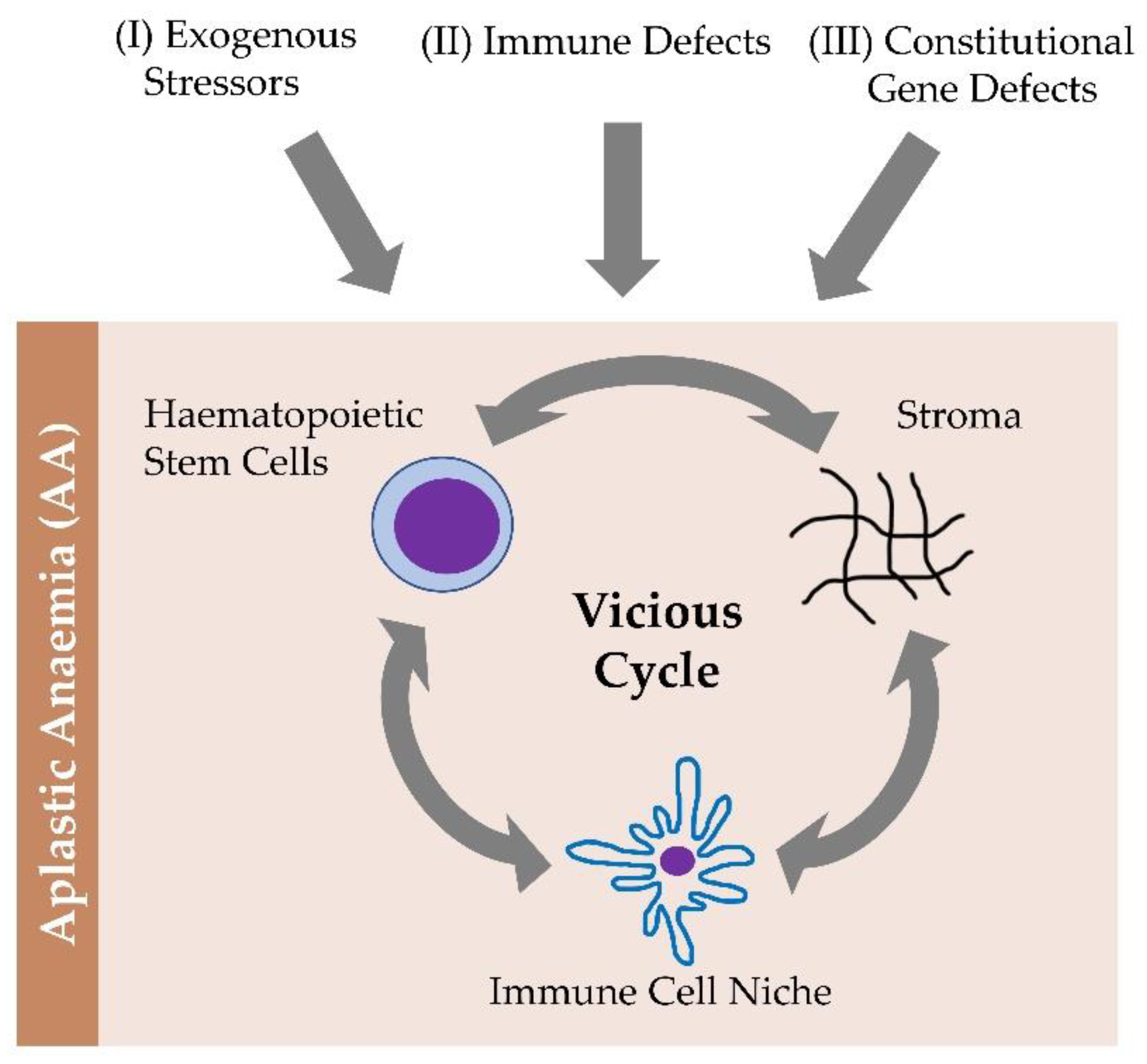
3. Pathobiology
Defects in the Innate Immune Stress Response
4. Treatment
4.1. Supportive Therapies
4.2. Specific Therapies
4.2.1. Hematopoietic Stem Cell Transplantation: Identical HLA Family Donor Transplant
4.2.2. Hematopoietic Stem Cell Transplantation: HSCT from a Non-Familial Donor Identical HLA (MUD)
4.2.3. HSCT from an Alternative Donor
4.3. Immunosuppressive Therapy (IST)
4.4. Forms Refractory to First- and Second-Line Therapies
4.5. Moderate Aplasia
4.6. Treatment Selection and Follow-up Summary
5. Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria: Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaht, K.; Göransson, M.; Carlson, K.; Isaksson, C.; Lenhoff, S.; Sandstedt, A.; Uggla, B.; Winiarski, J.; Ljungman, P.; Brune, M.; et al. Incidence and Outcome of Acquired Aplastic Anemia: Real-World Data from Patients Diagnosed in Sweden from 2000–2011. Haematologica 2017, 102, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.B.; Scott, A.; Sanchez-Bonilla, M.; Ho, P.A.; Gulsuner, S.; Pritchard, C.C.; Abkowitz, J.L.; King, M.-C.; Walsh, T.; Shimamura, A. Genetic Features of Myelodysplastic Syndrome and Aplastic Anemia in Pediatric and Young Adult Patients. Haematologica 2016, 101, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Feurstein, S.; Drazer, M.W.; Godley, L.A. Genetic Predisposition to Leukemia and Other Hematologic Malignancies. Semin. Oncol. 2016, 43, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Iwafuchi, H. The Histopathology of Bone Marrow Failure in Children. J. Clin. Exp. Hematop. 2018, 58, 68–86. [Google Scholar] [CrossRef]
- Antonio, G.; Oronzo, B.; Vito, L.; Angela, C.; Antonel-la, A.; Roberto, C.; Giovanni, S.A.; Antonella, L. Immune System and Bone Microenvironment: Rationale for Targeted Cancer Therapies. Oncotarget 2020, 11, 480–487. [Google Scholar] [CrossRef][Green Version]
- Keel, S.; Geddis, A. The Clinical and Laboratory Evaluation of Patients with Suspected Hypocellular Marrow Failure. Hematology 2021, 2021, 134–142. [Google Scholar] [CrossRef]
- Spinner, M.A.; Sanchez, L.A.; Hsu, A.P.; Shaw, P.A.; Zerbe, C.S.; Calvo, K.R.; Arthur, D.C.; Gu, W.; Gould, C.M.; Brewer, C.C.; et al. GATA2 Deficiency: A Protean Disorder of Hematopoiesis, Lymphatics, and Immunity. Blood 2014, 123, 809–821. [Google Scholar] [CrossRef]
- Weinberg, O.K.; Kuo, F.; Calvo, K.R. Germline Predisposition to Hematolymphoid Neoplasia. Am. J. Clin. Pathol. 2019, 152, 258–276. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Denton, C.; Keel, S.; Geddis, A.E.; Xu, M.; Appel, B.E.; Cantor, A.B.; Fleming, M.D.; Shimamura, A. Bone Marrow Morphology Associated With Germline RUNX1 Mutations in Patients with Familial Platelet Disorder with Associated Myeloid Malignancy. Pediatr. Dev. Pathol. 2019, 22, 315–328. [Google Scholar] [CrossRef]
- Matsui, W.H.; Brodsky, R.A.; Smith, B.D.; Borowitz, M.J.; Jones, R.J. Quantitative Analysis of Bone Marrow CD34 Cells in Aplastic Anemia and Hypoplastic Myelodysplastic Syndromes. Leukemia 2006, 20, 458–462. [Google Scholar] [CrossRef]
- Solimando, A.G.; Vacca, A.; Ribatti, D. Inborn Error of Immunity: A Journey Through Novel Genes and Clinical Presentation. In Encyclopedia of Infection and Immunity; Elsevier: Amsterdam, The Netherlands, 2022; pp. 798–818. ISBN 978-0-323-90303-5. [Google Scholar]
- Hosokawa, K.; Sugimori, N.; Katagiri, T.; Sasaki, Y.; Saito, C.; Seiki, Y.; Mochizuki, K.; Yamazaki, H.; Takami, A.; Nakao, S. Increased Glycosylphosphatidylinositol-Anchored Protein-Deficient Granulocytes Define a Benign Subset of Bone Marrow Failures in Patients with Trisomy 8. Eur. J. Haematol. 2015, 95, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Chuhjo, T.; Wang, H.; Yachie, A.; Omine, M.; Nakao, S. Polyclonal Hematopoiesis Maintained in Patients with Bone Marrow Failure Harboring a Minor Population of Paroxysmal Nocturnal Hemoglobinuria-Type Cells. Blood 2003, 102, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, M.W.; Hirabayashi, S.; Pastor, V.; Starý, J.; Hasle, H.; Masetti, R.; Dworzak, M.; Schmugge, M.; van den Heuvel-Eibrink, M.; Ussowicz, M.; et al. Prevalence, Clinical Characteristics, and Prognosis of GATA2-Related Myelodysplastic Syndromes in Children and Adolescents. Blood 2016, 127, 1387–1397, quiz 1518. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Ma, J.; Lamprecht, T.; Walsh, M.; Wang, S.; Bryant, V.; Song, G.; Wu, G.; Easton, J.; Kesserwan, C.; et al. The Genomic Landscape of Pediatric Myelodysplastic Syndromes. Nat. Commun. 2017, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.W.; Kelly, J.P.; Jurgelon, J.M.; Anderson, T.; Issaragrisil, S.; Wiholm, B.E.; Young, N.S.; Leaverton, P.; Levy, M.; Shapiro, S. Drugs in the Aetiology of Agranulocytosis and Aplastic Anaemia. Eur. J. Haematol. Suppl. 1996, 60, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Makoni, S.N.; Laber, D.A. Clinical Spectrum of Myelophthisis in Cancer Patients. Am. J. Hematol. 2004, 76, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers 2019, 11, 1270. [Google Scholar] [CrossRef]
- Solimando, A.G.; Melaccio, A.; Vacca, A.; Ria, R. The Bone Marrow Niche Landscape: A Journey through Aging, Extrinsic and Intrinsic Stressors in the Haemopoietic Milieu. JCMT 2022, 8, 9. [Google Scholar] [CrossRef]
- Young, N.S. Aplastic Anemia. N. Engl. J. Med. 2018, 379, 1643–1656. [Google Scholar] [CrossRef]
- Solimando, A.G.; Susca, N.; Borrelli, P.; Prete, M.; Lauletta, G.; Pappagallo, F.; Buono, R.; Inglese, G.; Forina, B.M.; Bochicchio, D.; et al. Short-Term Variations in Neutrophil-to-Lymphocyte and Urea-to-Creatinine Ratios Anticipate Intensive Care Unit Admission of COVID-19 Patients in the Emergency Department. Front. Med. 2020, 7, 625176. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Stragapede, A.; Solimando, A.G.; Albanese, F.; Capobianco, M.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Vacca, A.; et al. COVID-19 and the Endocrine System: A Comprehensive Review on the Theme. J. Clin. Med. 2021, 10, 2920. [Google Scholar] [CrossRef] [PubMed]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Narla, A. When to Worry about Inherited Bone Marrow Failure and Myeloid Malignancy Predisposition Syndromes in the Setting of a Hypocellular Marrow. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 153–156. [Google Scholar] [CrossRef]
- Sugimori, C.; Yamazaki, H.; Feng, X.; Mochizuki, K.; Kondo, Y.; Takami, A.; Chuhjo, T.; Kimura, A.; Teramura, M.; Mizoguchi, H.; et al. Roles of DRB1 *1501 and DRB1 *1502 in the Pathogenesis of Aplastic Anemia. Exp. Hematol. 2007, 35, 13–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miano, M.; Dufour, C. The Diagnosis and Treatment of Aplastic Anemia: A Review. Int. J. Hematol. 2015, 101, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yoshizato, T.; Dumitriu, B.; Hosokawa, K.; Makishima, H.; Yoshida, K.; Townsley, D.; Sato-Otsubo, A.; Sato, Y.; Liu, D.; Suzuki, H.; et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N. Engl. J. Med. 2015, 373, 35–47. [Google Scholar] [CrossRef]
- Malcovati, L.; Gallì, A.; Travaglino, E.; Ambaglio, I.; Rizzo, E.; Molteni, E.; Elena, C.; Ferretti, V.V.; Catricalà, S.; Bono, E.; et al. Clinical Significance of Somatic Mutation in Unexplained Blood Cytopenia. Blood 2017, 129, 3371–3378. [Google Scholar] [CrossRef]
- Babushok, D.V. When Does a PNH Clone Have Clinical Significance? Hematology 2021, 2021, 143–152. [Google Scholar] [CrossRef]
- Füreder, W.; Krauth, M.-T.; Sperr, W.R.; Sonneck, K.; Simonitsch-Klupp, I.; Müllauer, L.; Willmann, M.; Horny, H.-P.; Valent, P. Evaluation of Angiogenesis and Vascular Endothelial Growth Factor Expression in the Bone Marrow of Patients with Aplastic Anemia. Am. J. Pathol. 2006, 168, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Solimando, A.G.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Calado, R.T.; Ly, H.; Kajigaya, S.; Baerlocher, G.M.; Chanock, S.J.; Lansdorp, P.M.; Young, N.S. Mutations in TERT, the Gene for Telomerase Reverse Transcriptase, in Aplastic Anemia. N. Engl. J. Med. 2005, 352, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Bär, C.; Povedano, J.M.; Serrano, R.; Benitez-Buelga, C.; Popkes, M.; Formentini, I.; Bobadilla, M.; Bosch, F.; Blasco, M.A. Telomerase Gene Therapy Rescues Telomere Length, Bone Marrow Aplasia, and Survival in Mice with Aplastic Anemia. Blood 2016, 127, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, P.; Gu, J.; Bishop, A.J.R.; Scott, L.M.; Hasty, P.; Rebel, V.I. Potential Relationship between Inadequate Response to DNA Damage and Development of Myelodysplastic Syndrome. Int. J. Mol. Sci. 2015, 16, 966–989. [Google Scholar] [CrossRef]
- Tiwari, V.; Wilson, D.M. DNA Damage and Associated DNA Repair Defects in Disease and Premature Aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef]
- Green, A.M.; Kupfer, G.M. Fanconi Anemia. Hematol. Oncol. Clin. N. Am. 2009, 23, 193–214. [Google Scholar] [CrossRef]
- Nalepa, G.; Clapp, D.W. Fanconi Anaemia and Cancer: An Intricate Relationship. Nat. Rev. Cancer 2018, 18, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Zimmer, J.; Tarsounas, M. Interplay between Fanconi Anemia and Homologous Recombination Pathways in Genome Integrity. EMBO J. 2016, 35, 909–923. [Google Scholar] [CrossRef]
- Liang, J.; Yagasaki, H.; Kamachi, Y.; Hama, A.; Matsumoto, K.; Kato, K.; Kudo, K.; Kojima, S. Mutations in Telomerase Catalytic Protein in Japanese Children with Aplastic Anemia. Haematologica 2006, 91, 656–658. [Google Scholar] [PubMed]
- Kamata, M.; Okitsu, Y.; Fujiwara, T.; Kanehira, M.; Nakajima, S.; Takahashi, T.; Inoue, A.; Fukuhara, N.; Onishi, Y.; Ishizawa, K.; et al. GATA2 Regulates Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Haematologica 2014, 99, 1686–1696. [Google Scholar] [CrossRef]
- Tolkachov, A.; Fischer, C.; Ambrosi, T.H.; Bothe, M.; Han, C.-T.; Muenzner, M.; Mathia, S.; Salminen, M.; Seifert, G.; Thiele, M.; et al. Loss of the Hematopoietic Stem Cell Factor GATA2 in the Osteogenic Lineage Impairs Trabecularization and Mechanical Strength of Bone. Mol. Cell. Biol. 2018, 38, e00599-17. [Google Scholar] [CrossRef]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Vacca, A.; Ribatti, D. A Comprehensive Biological and Clinical Perspective Can Drive a Patient-Tailored Approach to Multiple Myeloma: Bridging the Gaps between the Plasma Cell and the Neoplastic Niche. J. Oncol. 2020, 2020, 6820241. [Google Scholar] [CrossRef] [PubMed]
- Desantis, V.; Saltarella, I.; Lamanuzzi, A.; Melaccio, A.; Solimando, A.G.; Mariggiò, M.A.; Racanelli, V.; Paradiso, A.; Vacca, A.; Frassanito, M.A. MicroRNAs-Based Nano-Strategies as New Therapeutic Approach in Multiple Myeloma to Overcome Disease Progression and Drug Resistance. Int. J. Mol. Sci. 2020, 21, 3084. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Maciejewski, J.P.; Green, S.; Plasilova, M.; Zeng, W.; Young, N.S. In-Vivo Dominant Immune Responses in Aplastic Anaemia: Molecular Tracking of Putatively Pathogenetic T-Cell Clones by TCR Beta-CDR3 Sequencing. Lancet 2004, 364, 355–364. [Google Scholar] [CrossRef]
- Derakhshani, A.; Hashemzadeh, S.; Asadzadeh, Z.; Shadbad, M.A.; Rasibonab, F.; Safarpour, H.; Jafarlou, V.; Solimando, A.G.; Racanelli, V.; Singh, P.K.; et al. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers 2021, 13, 2414. [Google Scholar] [CrossRef]
- Li, B.; Guo, L.; Zhang, Y.; Xiao, Y.; Wu, M.; Zhou, L.; Chen, S.; Yang, L.; Lu, X.; Li, Y. Molecular Alterations in the TCR Signaling Pathway in Patients with Aplastic Anemia. J. Hematol. Oncol. 2016, 9, 32. [Google Scholar] [CrossRef]
- Risitano, A.M.; Kook, H.; Zeng, W.; Chen, G.; Young, N.S.; Maciejewski, J.P. Oligoclonal and Polyclonal CD4 and CD8 Lymphocytes in Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria Measured by V Beta CDR3 Spectratyping and Flow Cytometry. Blood 2002, 100, 178–183. [Google Scholar] [CrossRef]
- Giudice, V.; Feng, X.; Lin, Z.; Hu, W.; Zhang, F.; Qiao, W.; Ibanez, M.D.P.F.; Rios, O.; Young, N.S. Deep Sequencing and Flow Cytometric Characterization of Expanded Effector Memory CD8+CD57+ T Cells Frequently Reveals T-Cell Receptor Vβ Oligoclonality and CDR3 Homology in Acquired Aplastic Anemia. Haematologica 2018, 103, 759–769. [Google Scholar] [CrossRef]
- Dufour, C.; Ferretti, E.; Bagnasco, F.; Burlando, O.; Lanciotti, M.; Ramenghi, U.; Saracco, P.; Van Lint, M.T.; Longoni, D.; Torelli, G.F.; et al. Changes in Cytokine Profile Pre- and Post-Immunosuppression in Acquired Aplastic Anemia. Haematologica 2009, 94, 1743–1747. [Google Scholar] [CrossRef]
- Dubey, S.; Shukla, P.; Nityanand, S. Expression of Interferon-Gamma and Tumor Necrosis Factor-Alpha in Bone Marrow T Cells and Their Levels in Bone Marrow Plasma in Patients with Aplastic Anemia. Ann. Hematol. 2005, 84, 572–577. [Google Scholar] [CrossRef]
- Solomou, E.E.; Rezvani, K.; Mielke, S.; Malide, D.; Keyvanfar, K.; Visconte, V.; Kajigaya, S.; Barrett, A.J.; Young, N.S. Deficient CD4+ CD25+ FOXP3+ T Regulatory Cells in Acquired Aplastic Anemia. Blood 2007, 110, 1603–1606. [Google Scholar] [CrossRef]
- Mohr, A.; Atif, M.; Balderas, R.; Gorochov, G.; Miyara, M. The Role of FOXP3+ Regulatory T Cells in Human Autoimmune and Inflammatory Diseases. Clin. Exp. Immunol. 2019, 197, 24–35. [Google Scholar] [CrossRef]
- Kordasti, S.; Costantini, B.; Seidl, T.; Perez Abellan, P.; Martinez Llordella, M.; McLornan, D.; Diggins, K.E.; Kulasekararaj, A.; Benfatto, C.; Feng, X.; et al. Deep Phenotyping of Tregs Identifies an Immune Signature for Idiopathic Aplastic Anemia and Predicts Response to Treatment. Blood 2016, 128, 1193–1205. [Google Scholar] [CrossRef]
- Dulmovits, B.M.; Olson, T.S. Does Immune Destruction Drive All Forms of Bone Marrow Failure? J. Clin. Investig. 2022, 132, e161288. [Google Scholar] [CrossRef]
- Dufour, C.; Capasso, M.; Svahn, J.; Marrone, A.; Haupt, R.; Bacigalupo, A.; Giordani, L.; Longoni, D.; Pillon, M.; Pistorio, A.; et al. Homozygosis for (12) CA Repeats in the First Intron of the Human IFN-Gamma Gene Is Significantly Associated with the Risk of Aplastic Anaemia in Caucasian Population. Br. J. Haematol. 2004, 126, 682–685. [Google Scholar] [CrossRef]
- Zeng, Y.; Katsanis, E. The Complex Pathophysiology of Acquired Aplastic Anaemia. Clin. Exp. Immunol. 2015, 180, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Elbadry, M.I.; Chonabayashi, K.; Yoshida, Y.; Katagiri, T.; Harada, K.; Nakagawa, N.; Zaimoku, Y.; Imi, T.; Takamatsu, H.; et al. Hematopoiesis by IPSC-Derived Hematopoietic Stem Cells of Aplastic Anemia That Escape Cytotoxic T-Cell Attack. Blood Adv. 2018, 2, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Imi, T.; Katagiri, T.; Hosomichi, K.; Zaimoku, Y.; Hoang Nguyen, V.; Nakagawa, N.; Tajima, A.; Yoshizato, T.; Ogawa, S.; Nakao, S. Sustained Clonal Hematopoiesis by HLA-Lacking Hematopoietic Stem Cells without Driver Mutations in Aplastic Anemia. Blood Adv. 2018, 2, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S. Clonal Hematopoiesis in Acquired Aplastic Anemia. Blood 2016, 128, 337–347. [Google Scholar] [CrossRef]
- Katagiri, T.; Sato-Otsubo, A.; Kashiwase, K.; Morishima, S.; Sato, Y.; Mori, Y.; Kato, M.; Sanada, M.; Morishima, Y.; Hosokawa, K.; et al. Frequent Loss of HLA Alleles Associated with Copy Number-Neutral 6pLOH in Acquired Aplastic Anemia. Blood 2011, 118, 6601–6609. [Google Scholar] [CrossRef]
- Zaimoku, Y.; Takamatsu, H.; Hosomichi, K.; Ozawa, T.; Nakagawa, N.; Imi, T.; Maruyama, H.; Katagiri, T.; Kishi, H.; Tajima, A.; et al. Identification of an HLA Class I Allele Closely Involved in the Autoantigen Presentation in Acquired Aplastic Anemia. Blood 2017, 129, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Valle, M.; Podestà, M.; Pitto, A.; Zocchi, E.; De Flora, A.; Pozzi, S.; Luchetti, S.; Frassoni, F.; Van Lint, M.T.; et al. T-Cell Suppression Mediated by Mesenchymal Stem Cells Is Deficient in Patients with Severe Aplastic Anemia. Exp. Hematol. 2005, 33, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Da Vià, M.C.; Solimando, A.G.; Garitano-Trojaola, A.; Barrio, S.; Munawar, U.; Strifler, S.; Haertle, L.; Rhodes, N.; Teufel, E.; Vogt, C.; et al. CIC Mutation as a Molecular Mechanism of Acquired Resistance to Combined BRAF-MEK Inhibition in Extramedullary Multiple Myeloma with Central Nervous System Involvement. Oncologist 2020, 19, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Deb, D.K.; Sassano, A.; Uddin, S.; Varga, J.; Wickrema, A.; Platanias, L.C. Activation of the P38 Mitogen-Activated Protein Kinase Mediates the Suppressive Effects of Type I Interferons and Transforming Growth Factor-Beta on Normal Hematopoiesis. J. Biol. Chem. 2002, 277, 7726–7735. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Deb, D.K.; Sassano, A.; Kambhampati, S.; Wickrema, A.; Uddin, S.; Mohindru, M.; Van Besien, K.; Platanias, L.C. Cutting Edge: Activation of the P38 Mitogen-Activated Protein Kinase Signaling Pathway Mediates Cytokine-Induced Hemopoietic Suppression in Aplastic Anemia. J. Immunol. 2002, 168, 5984–5988. [Google Scholar] [CrossRef]
- Gargiulo, L.; Zaimoku, Y.; Scappini, B.; Maruyama, H.; Ohumi, R.; Luzzatto, L.; Nakao, S.; Notaro, R. Glycosylphosphatidylinositol-Specific T Cells, IFN-γ-Producing T Cells, and Pathogenesis of Idiopathic Aplastic Anemia. Blood 2017, 129, 388–392. [Google Scholar] [CrossRef]
- Giudice, V.; Cardamone, C.; Triggiani, M.; Selleri, C. Bone Marrow Failure Syndromes, Overlapping Diseases with a Common Cytokine Signature. Int. J. Mol. Sci. 2021, 22, 705. [Google Scholar] [CrossRef]
- Shi, J.; Ge, M.; Lu, S.; Li, X.; Shao, Y.; Huang, J.; Huang, Z.; Zhang, J.; Nie, N.; Zheng, Y. Intrinsic Impairment of CD4+CD25+ Regulatory T Cells in Acquired Aplastic Anemia. Blood 2012, 120, 1624–1632. [Google Scholar] [CrossRef]
- Young, N.S.; Calado, R.T.; Scheinberg, P. Current Concepts in the Pathophysiology and Treatment of Aplastic Anemia. Blood 2006, 108, 2509–2519. [Google Scholar] [CrossRef]
- Young, N.S. Current Concepts in the Pathophysiology and Treatment of Aplastic Anemia. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 76–81. [Google Scholar] [CrossRef]
- Park, M.; Park, C.-J.; Cho, Y.W.; Jang, S.; Lee, J.-H.; Lee, J.-H.; Lee, K.-H.; Lee, Y.H. Alterations in the Bone Marrow Microenvironment May Elicit Defective Hematopoiesis: A Comparison of Aplastic Anemia, Chronic Myeloid Leukemia, and Normal Bone Marrow. Exp. Hematol. 2017, 45, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Z.; Sheng, W.; Fu, R.; Li, L.; Zhang, T.; Wu, Y.; Xing, L.; Song, J.; Wang, H.; et al. Abnormalities of Quantities and Functions of Natural Killer Cells in Severe Aplastic Anemia. Immunol. Investig. 2014, 43, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Solomou, E.E.; Gibellini, F.; Stewart, B.; Malide, D.; Berg, M.; Visconte, V.; Green, S.; Childs, R.; Chanock, S.J.; Young, N.S. Perforin Gene Mutations in Patients with Acquired Aplastic Anemia. Blood 2007, 109, 5234–5237. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Q.; Ge, M.; Shi, X. Cluster Analysis of Lymphocyte Subset from Peripheral Blood in Newly Diagnosed Idiopathic Aplastic Anaemia Patients. Ann. Med. 2022, 54, 2431–2439. [Google Scholar] [CrossRef]
- Hanaoka, N.; Nakakuma, H.; Horikawa, K.; Nagakura, S.; Tsuzuki, Y.; Shimanuki, M.; Kojima, K.; Yonemura, Y.; Kawaguchi, T. NKG2D-Mediated Immunity Underlying Paroxysmal Nocturnal Haemoglobinuria and Related Bone Marrow Failure Syndromes. Br. J. Haematol. 2009, 146, 538–545. [Google Scholar] [CrossRef]
- Murata, S.; Mushino, T.; Hosoi, H.; Kuriyama, K.; Nishikawa, A.; Nagakura, S.; Horikawa, K.; Yonemura, Y.; Nakakuma, H.; Sonoki, T.; et al. Soluble NKG2D Ligands Are Potential Biomarkers and Sentinels of Immune-Mediated Bone Marrow Injury in Bone Marrow Failure Syndromes. Acta Haematol. 2020, 143, 33–39. [Google Scholar] [CrossRef]
- Hanaoka, N.; Kawaguchi, T.; Horikawa, K.; Nagakura, S.; Mitsuya, H.; Nakakuma, H. Immunoselection by Natural Killer Cells of PIGA Mutant Cells Missing Stress-Inducible ULBP. Blood 2006, 107, 1184–1191. [Google Scholar] [CrossRef]
- Bessho, M.; Hirashima, K.; Asano, S.; Ikeda, Y.; Ogawa, N.; Tomonaga, M.; Toyama, K.; Nakahata, T.; Nomura, T.; Mizoguchi, H.; et al. Treatment of the Anemia of Aplastic Anemia Patients with Recombinant Human Erythropoietin in Combination with Granulocyte Colony-Stimulating Factor: A Multicenter Randomized Controlled Study. Multicenter Study Group. Eur. J. Haematol. 1997, 58, 265–272. [Google Scholar] [CrossRef]
- Desantis, V.; Frassanito, M.A.; Tamma, R.; Saltarella, I.; Di Marzo, L.; Lamanuzzi, A.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; et al. Rhu-Epo down-Regulates pro-Tumorigenic Activity of Cancer-Associated Fibroblasts in Multiple Myeloma. Ann. Hematol. 2018, 97, 1251–1258. [Google Scholar] [CrossRef]
- Fattizzo, B.; Kulasekararaj, A.G.; Hill, A.; Benson-Quarm, N.; Griffin, M.; Munir, T.; Arnold, L.; Riley, K.; Ireland, R.; De Lavallade, H.; et al. Clinical and Morphological Predictors of Outcome in Older Aplastic Anemia Patients Treated with Eltrombopag. Haematologica 2019, 104, e494–e496. [Google Scholar] [CrossRef]
- Williams, D.A.; Bennett, C.; Bertuch, A.; Bessler, M.; Coates, T.; Corey, S.; Dror, Y.; Huang, J.; Lipton, J.; Olson, T.S.; et al. Diagnosis and Treatment of Pediatric Acquired Aplastic Anemia (AAA): An Initial Survey of the North American Pediatric Aplastic Anemia Consortium (NAPAAC). Pediatr. Blood Cancer 2014, 61, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Quillen, K.; Wong, E.; Scheinberg, P.; Young, N.S.; Walsh, T.J.; Wu, C.O.; Leitman, S.F. Granulocyte Transfusions in Severe Aplastic Anemia: An Eleven-Year Experience. Haematologica 2009, 94, 1661–1668. [Google Scholar] [CrossRef]
- Locasciulli, A.; Oneto, R.; Bacigalupo, A.; Socié, G.; Korthof, E.; Bekassy, A.; Schrezenmeier, H.; Passweg, J.; Führer, M. Severe Aplastic Anemia Working Party of the European Blood and Marrow Transplant Group Outcome of Patients with Acquired Aplastic Anemia given First Line Bone Marrow Transplantation or Immunosuppressive Treatment in the Last Decade: A Report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2007, 92, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kobayashi, R.; Yabe, H.; Kosaka, Y.; Yagasaki, H.; Watanabe, K.-I.; Kudo, K.; Morimoto, A.; Ohga, S.; Muramatsu, H.; et al. First-Line Treatment for Severe Aplastic Anemia in Children: Bone Marrow Transplantation from a Matched Family Donor versus Immunosuppressive Therapy. Haematologica 2014, 99, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Doney, K.; Leisenring, W.; Storb, R.; Appelbaum, F.R. Primary Treatment of Acquired Aplastic Anemia: Outcomes with Bone Marrow Transplantation and Immunosuppressive Therapy. Seattle Bone Marrow Transplant Team. Ann. Intern. Med. 1997, 126, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A. How I Treat Acquired Aplastic Anemia. Blood 2017, 129, 1428–1436. [Google Scholar] [CrossRef]
- Pulsipher, M.A.; Lehmann, L.E.; Bertuch, A.A.; Sasa, G.; Olson, T.; Nakano, T.; Gilio, A.; Burroughs, L.M.; Lipton, J.M.; Huang, J.N.; et al. A Study Assessing the Feasibility of Randomization of Pediatric and Young Adult Patients between Matched Unrelated Donor Bone Marrow Transplantation and Immune-Suppressive Therapy for Newly Diagnosed Severe Aplastic Anemia: A Joint Pilot Trial of the North American Pediatric Aplastic Anemia Consortium and the Pediatric Transplantation and Cellular Therapy Consortium. Pediatr. Blood Cancer 2020, 67, e28444. [Google Scholar] [CrossRef]
- Dufour, C.; Pillon, M.; Sociè, G.; Rovò, A.; Carraro, E.; Bacigalupo, A.; Oneto, R.; Passweg, J.; Risitano, A.; Tichelli, A.; et al. Outcome of Aplastic Anaemia in Children. A Study by the Severe Aplastic Anaemia and Paediatric Disease Working Parties of the European Group Blood and Bone Marrow Transplant. Br. J. Haematol. 2015, 169, 565–573. [Google Scholar] [CrossRef]
- Dufour, C.; Veys, P.; Carraro, E.; Bhatnagar, N.; Pillon, M.; Wynn, R.; Gibson, B.; Vora, A.J.; Steward, C.G.; Ewins, A.M.; et al. Similar Outcome of Upfront-Unrelated and Matched Sibling Stem Cell Transplantation in Idiopathic Paediatric Aplastic Anaemia. A Study on Behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br. J. Haematol. 2015, 171, 585–594. [Google Scholar] [CrossRef]
- Peinemann, F.; Bartel, C.; Grouven, U. First-Line Allogeneic Hematopoietic Stem Cell Transplantation of HLA-Matched Sibling Donors Compared with First-Line Ciclosporin and/or Antithymocyte or Antilymphocyte Globulin for Acquired Severe Aplastic Anemia. Cochrane Database Syst. Rev. 2013, 7, CD006407. [Google Scholar] [CrossRef]
- Dufour, C.; Pillon, M.; Passweg, J.; Socié, G.; Bacigalupo, A.; Franceschetto, G.; Carraro, E.; Oneto, R.; Risitano, A.M.; Peffault de Latour, R.; et al. Outcome of Aplastic Anemia in Adolescence: A Survey of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica 2014, 99, 1574–1581. [Google Scholar] [CrossRef]
- Marsh, J.C.; Gupta, V.; Lim, Z.; Ho, A.Y.; Ireland, R.M.; Hayden, J.; Potter, V.; Koh, M.B.; Islam, M.S.; Russell, N.; et al. Alemtuzumab with Fludarabine and Cyclophosphamide Reduces Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation for Acquired Aplastic Anemia. Blood 2011, 118, 2351–2357. [Google Scholar] [CrossRef]
- Kekre, N.; Zhang, Y.; Zhang, M.-J.; Carreras, J.; Ahmed, P.; Anderlini, P.; Atta, E.H.; Ayas, M.; Boelens, J.J.; Bonfim, C.; et al. Effect of Antithymocyte Globulin Source on Outcomes of Bone Marrow Transplantation for Severe Aplastic Anemia. Haematologica 2017, 102, 1291–1298. [Google Scholar] [CrossRef]
- Mortensen, B.K.; Jacobsen, N.; Heilmann, C.; Sengeløv, H. Allogeneic Hematopoietic Cell Transplantation for Severe Aplastic Anemia: Similar Long-Term Overall Survival after Transplantation with Related Donors Compared to Unrelated Donors. Bone Marrow Transplant. 2016, 51, 288–290. [Google Scholar] [CrossRef]
- Choi, Y.B.; Yi, E.S.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Immunosuppressive Therapy versus Alternative Donor Hematopoietic Stem Cell Transplantation for Children with Severe Aplastic Anemia Who Lack an HLA-Matched Familial Donor. Bone Marrow Transplant. 2017, 52, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Kondo, T.; Yamazaki, H.; Takenaka, K.; Sugita, J.; Kobayashi, T.; Ozawa, Y.; Uchida, N.; Iwato, K.; Kobayashi, N.; et al. Allogeneic Unrelated Bone Marrow Transplantation from Older Donors Results in Worse Prognosis in Recipients with Aplastic Anemia. Haematologica 2016, 101, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Wang, T.; Haagenson, M.; Spellman, S.R.; Lee, S.J.; Williams, K.M.; Wong, J.Y.; De Vivo, I.; Savage, S.A. Association between Donor Leukocyte Telomere Length and Survival after Unrelated Allogeneic Hematopoietic Cell Transplantation for Severe Aplastic Anemia. JAMA 2015, 313, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Socié, G.; Schrezenmeier, H.; Tichelli, A.; Locasciulli, A.; Fuehrer, M.; Risitano, A.M.; Dufour, C.; Passweg, J.R.; Oneto, R.; et al. Bone Marrow versus Peripheral Blood as the Stem Cell Source for Sibling Transplants in Acquired Aplastic Anemia: Survival Advantage for Bone Marrow in All Age Groups. Haematologica 2012, 97, 1142–1148. [Google Scholar] [CrossRef]
- Devillier, R.; Dalle, J.-H.; Kulasekararaj, A.; D’aveni, M.; Clément, L.; Chybicka, A.; Vigouroux, S.; Chevallier, P.; Koh, M.; Bertrand, Y.; et al. Unrelated Alternative Donor Transplantation for Severe Acquired Aplastic Anemia: A Study from the French Society of Bone Marrow Transplantation and Cell Therapies and the EBMT Severe Aplastic Anemia Working Party. Haematologica 2016, 101, 884–890. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Socie, G.; Hamladji, R.M.; Aljurf, M.; Maschan, A.; Kyrcz-Krzemien, S.; Cybicka, A.; Sengelov, H.; Unal, A.; Beelen, D.; et al. Current Outcome of HLA Identical Sibling versus Unrelated Donor Transplants in Severe Aplastic Anemia: An EBMT Analysis. Haematologica 2015, 100, 696–702. [Google Scholar] [CrossRef]
- DeZern, A.E.; Zahurak, M.; Symons, H.; Cooke, K.; Jones, R.J.; Brodsky, R.A. Alternative Donor Transplantation with High-Dose Post-Transplantation Cyclophosphamide for Refractory Severe Aplastic Anemia. Biol. Blood Marrow Transplant. 2017, 23, 498–504. [Google Scholar] [CrossRef]
- Townsley, D.M.; Winkler, T. Nontransplant Therapy for Bone Marrow Failure. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, P.; Nunez, O.; Weinstein, B.; Scheinberg, P.; Biancotto, A.; Wu, C.O.; Young, N.S. Horse versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N. Engl. J. Med. 2011, 365, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.C.; Bacigalupo, A.; Schrezenmeier, H.; Tichelli, A.; Risitano, A.M.; Passweg, J.R.; Killick, S.B.; Warren, A.J.; Foukaneli, T.; Aljurf, M.; et al. Prospective Study of Rabbit Antithymocyte Globulin and Cyclosporine for Aplastic Anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood 2012, 119, 5391–5396. [Google Scholar] [CrossRef] [PubMed]
- Peffault de Latour, R.; Tabrizi, R.; Marcais, A.; Leblanc, T.; Lamy, T.; Mohty, M.; Tavitian, S.; Jubert, C.; Pasquet, M.; Galambrun, C.; et al. Nationwide Survey on the Use of Horse Antithymocyte Globulins (ATGAM) in Patients with Acquired Aplastic Anemia: A Report on Behalf of the French Reference Center for Aplastic Anemia. Am. J. Hematol. 2018, 93, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Oneto, R.; Schrezenmeier, H.; Hochsmann, B.; Dufour, C.; Kojima, S.; Zhu, X.; Chen, X.; Issaragrisil, S.; Chuncharunee, S.; et al. First Line Treatment of Aplastic Anemia with Thymoglobuline in Europe and Asia: Outcome of 955 Patients Treated 2001–2012. Am. J. Hematol. 2018, 93, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Saracco, P.; Quarello, P.; Iori, A.P.; Zecca, M.; Longoni, D.; Svahn, J.; Varotto, S.; Del Vecchio, G.C.; Dufour, C.; Ramenghi, U.; et al. Cyclosporin A Response and Dependence in Children with Acquired Aplastic Anaemia: A Multicentre Retrospective Study with Long-Term Observation Follow-up. Br. J. Haematol. 2008, 140, 197–205. [Google Scholar] [CrossRef]
- Tichelli, A.; Schrezenmeier, H.; Socié, G.; Marsh, J.; Bacigalupo, A.; Dührsen, U.; Franzke, A.; Hallek, M.; Thiel, E.; Wilhelm, M.; et al. A Randomized Controlled Study in Patients with Newly Diagnosed Severe Aplastic Anemia Receiving Antithymocyte Globulin (ATG), Cyclosporine, with or without G-CSF: A Study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood 2011, 117, 4434–4441. [Google Scholar] [CrossRef]
- Socie, G.; Mary, J.-Y.; Schrezenmeier, H.; Marsh, J.; Bacigalupo, A.; Locasciulli, A.; Fuehrer, M.; Bekassy, A.; Tichelli, A.; Passweg, J. Granulocyte-Stimulating Factor and Severe Aplastic Anemia: A Survey by the European Group for Blood and Marrow Transplantation (EBMT). Blood 2007, 109, 2794–2796. [Google Scholar] [CrossRef]
- Gurion, R.; Gafter-Gvili, A.; Paul, M.; Vidal, L.; Ben-Bassat, I.; Yeshurun, M.; Shpilberg, O.; Raanani, P. Hematopoietic Growth Factors in Aplastic Anemia Patients Treated with Immunosuppressive Therapy-Systematic Review and Meta-Analysis. Haematologica 2009, 94, 712–719. [Google Scholar] [CrossRef][Green Version]
- Peffault de Latour, R.; Kulasekararaj, A.; Iacobelli, S.; Terwel, S.R.; Cook, R.; Griffin, M.; Halkes, C.J.M.; Recher, C.; Barraco, F.; Forcade, E.; et al. Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. N. Engl. J. Med. 2022, 386, 11–23. [Google Scholar] [CrossRef]
- Scheinberg, P.; Wu, C.O.; Nunez, O.; Young, N.S. Long-Term Outcome of Pediatric Patients with Severe Aplastic Anemia Treated with Antithymocyte Globulin and Cyclosporine. J. Pediatr. 2008, 153, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Henry-Amar, M.; Bacigalupo, A.; Hows, J.; Tichelli, A.; Ljungman, P.; McCann, S.R.; Frickhofen, N.; Van’t Veer-Korthof, E.; Gluckman, E. Malignant Tumors Occurring after Treatment of Aplastic Anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N. Engl. J. Med. 1993, 329, 1152–1157. [Google Scholar] [CrossRef]
- van der Hem, J.G.K.; de Wreede, L.C.; Brand, A.; Veelken, H.; Falkenburg, J.H.F.; Halkes, C.J.M. Long-Term Risk of Cancer Development in Adult Patients with Idiopathic Aplastic Anemia after Treatment with Anti-Thymocyte Globulin. Haematologica 2017, 102, e382–e383. [Google Scholar] [CrossRef][Green Version]
- Lengline, E.; Drenou, B.; Peterlin, P.; Tournilhac, O.; Abraham, J.; Berceanu, A.; Dupriez, B.; Guillerm, G.; Raffoux, E.; de Fontbrune, F.S.; et al. Nationwide Survey on the Use of Eltrombopag in Patients with Severe Aplastic Anemia: A Report on Behalf of the French Reference Center for Aplastic Anemia. Haematologica 2018, 103, 212–220. [Google Scholar] [CrossRef]
- Howard, S.C.; Naidu, P.E.; Hu, X.J.; Jeng, M.R.; Rodriguez-Galindo, C.; Rieman, M.D.; Wang, W.C. Natural History of Moderate Aplastic Anemia in Children. Pediatr. Blood Cancer 2004, 43, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Muus, P.; Dührsen, U.; Risitano, A.M.; Schubert, J.; Luzzatto, L.; Schrezenmeier, H.; Szer, J.; Brodsky, R.A.; Hill, A.; et al. Effect of the Complement Inhibitor Eculizumab on Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria. Blood 2007, 110, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Hill, A.; Arnold, L.M.; Brooksbank, G.L.; Richards, S.J.; Cullen, M.; Mitchell, L.D.; Cohen, D.R.; Gregory, W.M.; Hillmen, P. Long-Term Treatment with Eculizumab in Paroxysmal Nocturnal Hemoglobinuria: Sustained Efficacy and Improved Survival. Blood 2011, 117, 6786–6792. [Google Scholar] [CrossRef]
- Pagliuca, S.; Risitano, A.M.; De Fontbrune, F.S.; Robin, M.; Iori, A.P.; Marotta, S.; Michonneau, D.; Villate, A.; Desmier, D.; Socié, G.; et al. Combined Intensive Immunosuppression and Eculizumab for Aplastic Anemia in the Context of Hemolytic Paroxysmal Nocturnal Hemoglobinuria: A Retrospective Analysis. Bone Marrow Transplant. 2018, 53, 105–107. [Google Scholar] [CrossRef]

| Agents Implicated in AA |
Drugs
|
Chemicials
|
| Acquired | Inherited |
| Acquired aplastic anemia * | Inherited bone marrow failure and myeloid malignancies predisposition syndromes (IBMF/MMPS) -Fanconi anemia -Short telomere syndromes -Shwachman-Diamond Syndrome -GATA2 deficiency -SAMD9/SAMD9L disorders |
| Hypocellular MDS | |
| Medications/toxins | |
| Anorexia nervosa | Inborn errors of immunity (i.e., X-linked lymphoproliferative disorder) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solimando, A.G.; Palumbo, C.; Pragnell, M.V.; Bittrich, M.; Argentiero, A.; Krebs, M. Aplastic Anemia as a Roadmap for Bone Marrow Failure: An Overview and a Clinical Workflow. Int. J. Mol. Sci. 2022, 23, 11765. https://doi.org/10.3390/ijms231911765
Solimando AG, Palumbo C, Pragnell MV, Bittrich M, Argentiero A, Krebs M. Aplastic Anemia as a Roadmap for Bone Marrow Failure: An Overview and a Clinical Workflow. International Journal of Molecular Sciences. 2022; 23(19):11765. https://doi.org/10.3390/ijms231911765
Chicago/Turabian StyleSolimando, Antonio G., Carmen Palumbo, Mary Victoria Pragnell, Max Bittrich, Antonella Argentiero, and Markus Krebs. 2022. "Aplastic Anemia as a Roadmap for Bone Marrow Failure: An Overview and a Clinical Workflow" International Journal of Molecular Sciences 23, no. 19: 11765. https://doi.org/10.3390/ijms231911765
APA StyleSolimando, A. G., Palumbo, C., Pragnell, M. V., Bittrich, M., Argentiero, A., & Krebs, M. (2022). Aplastic Anemia as a Roadmap for Bone Marrow Failure: An Overview and a Clinical Workflow. International Journal of Molecular Sciences, 23(19), 11765. https://doi.org/10.3390/ijms231911765








