Soluble Epoxide Hydrolase Is Associated with Postprandial Anxiety Decrease in Healthy Adult Women
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Participants
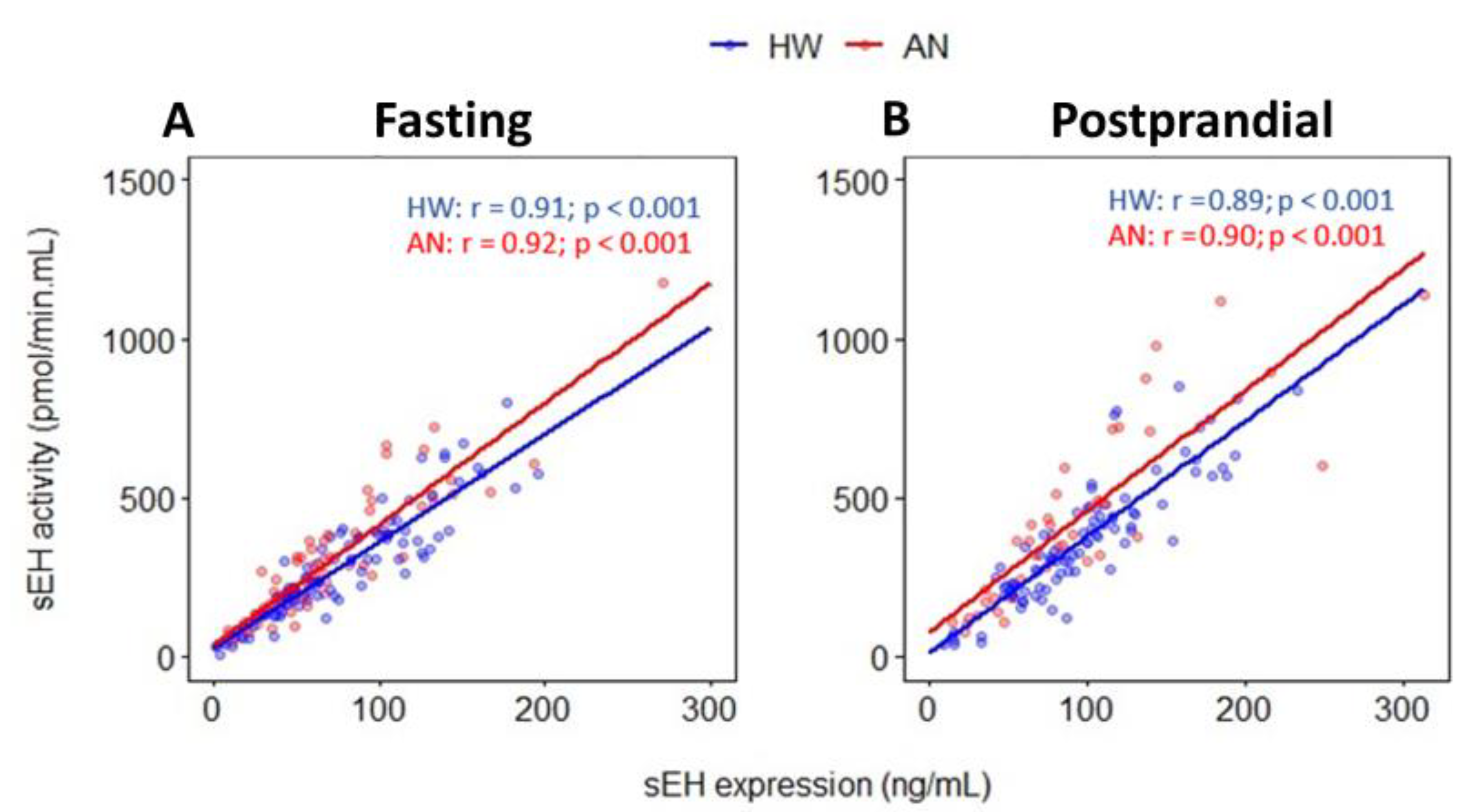
2.2. Relationship between sEH Expression and sEH Activity
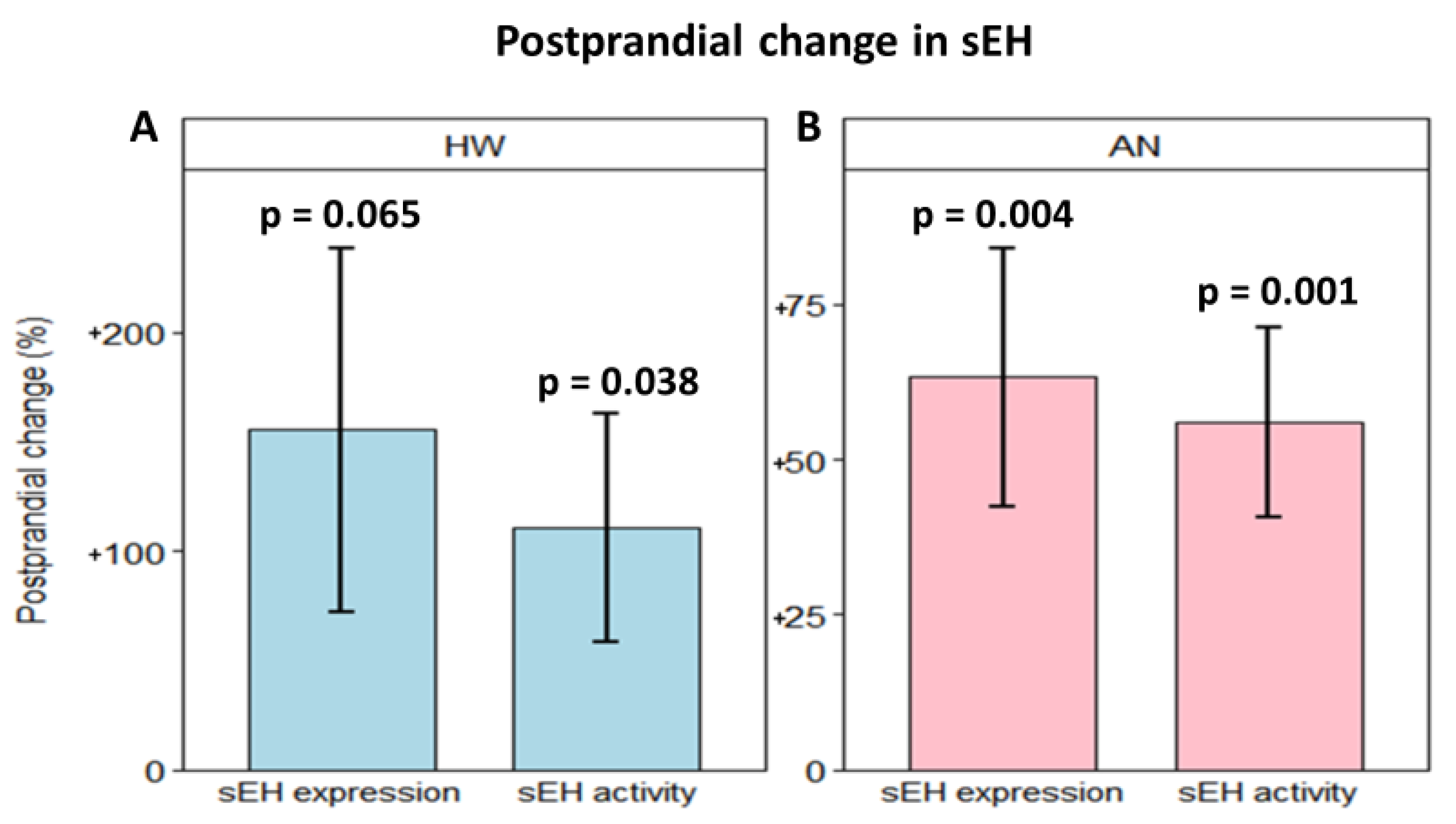
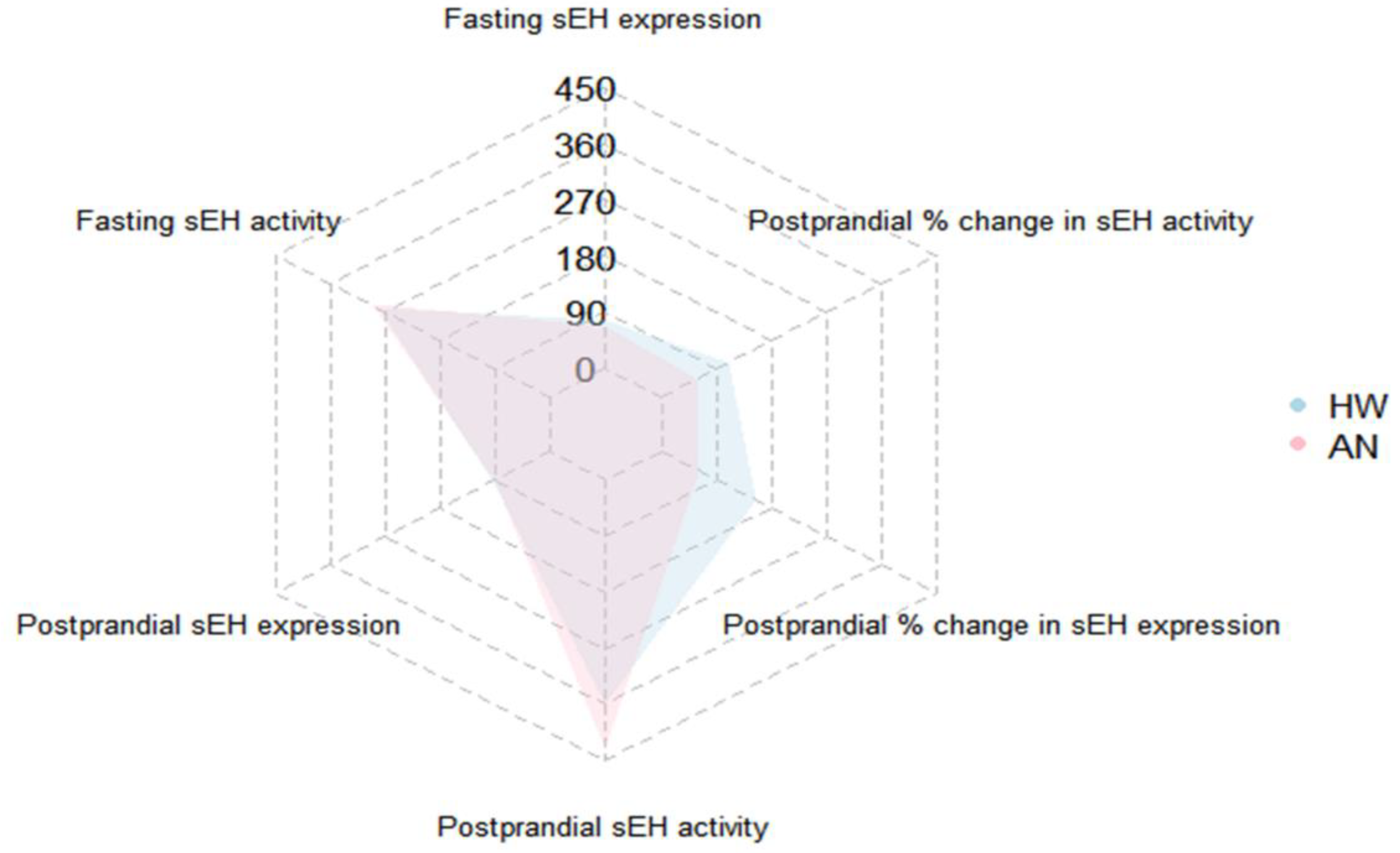
2.3. Effect of a High-Fat Meal on sEH Levels
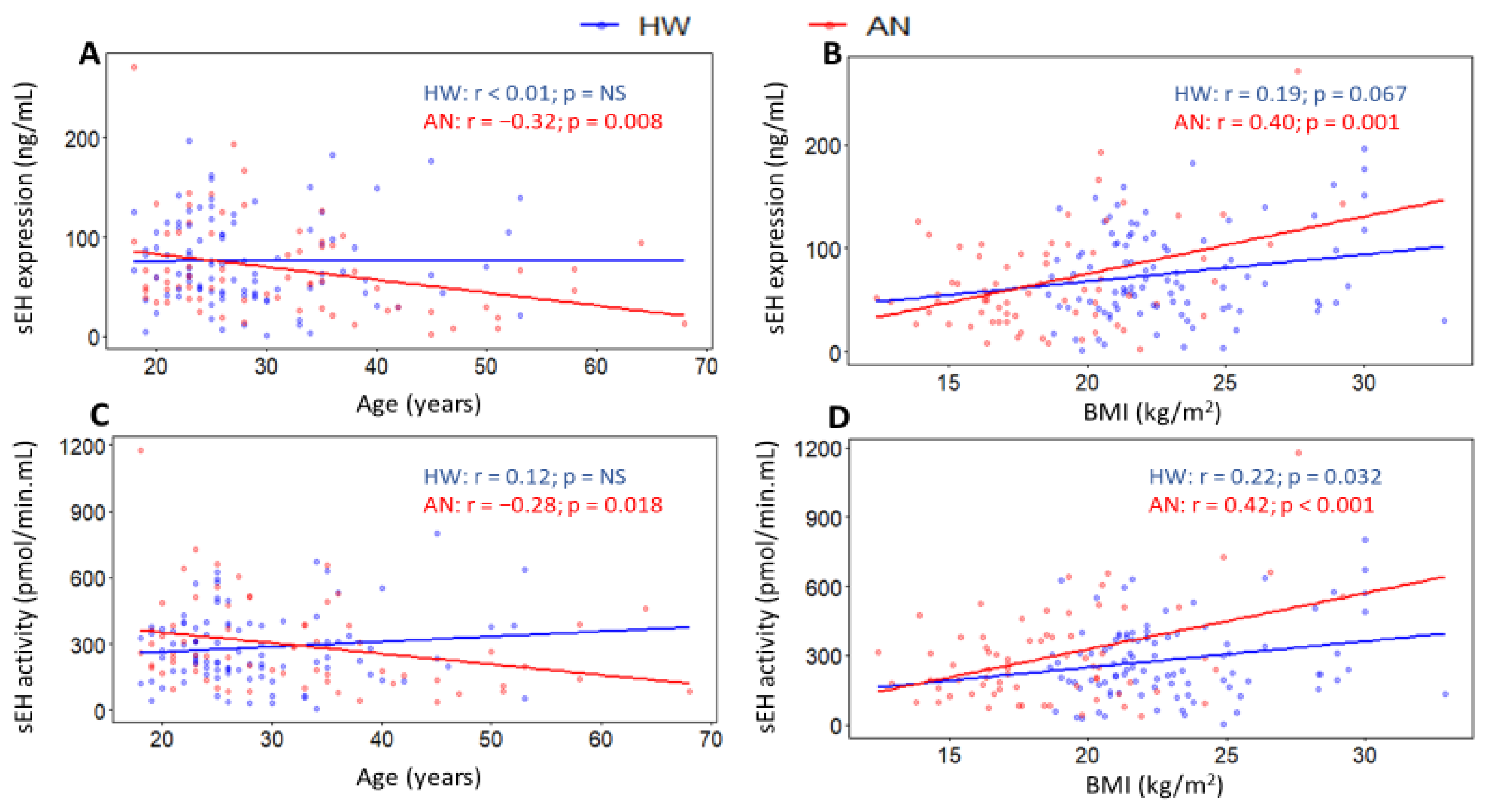
2.4. Associations of sEH with Age and Body Mass Index (BMI)
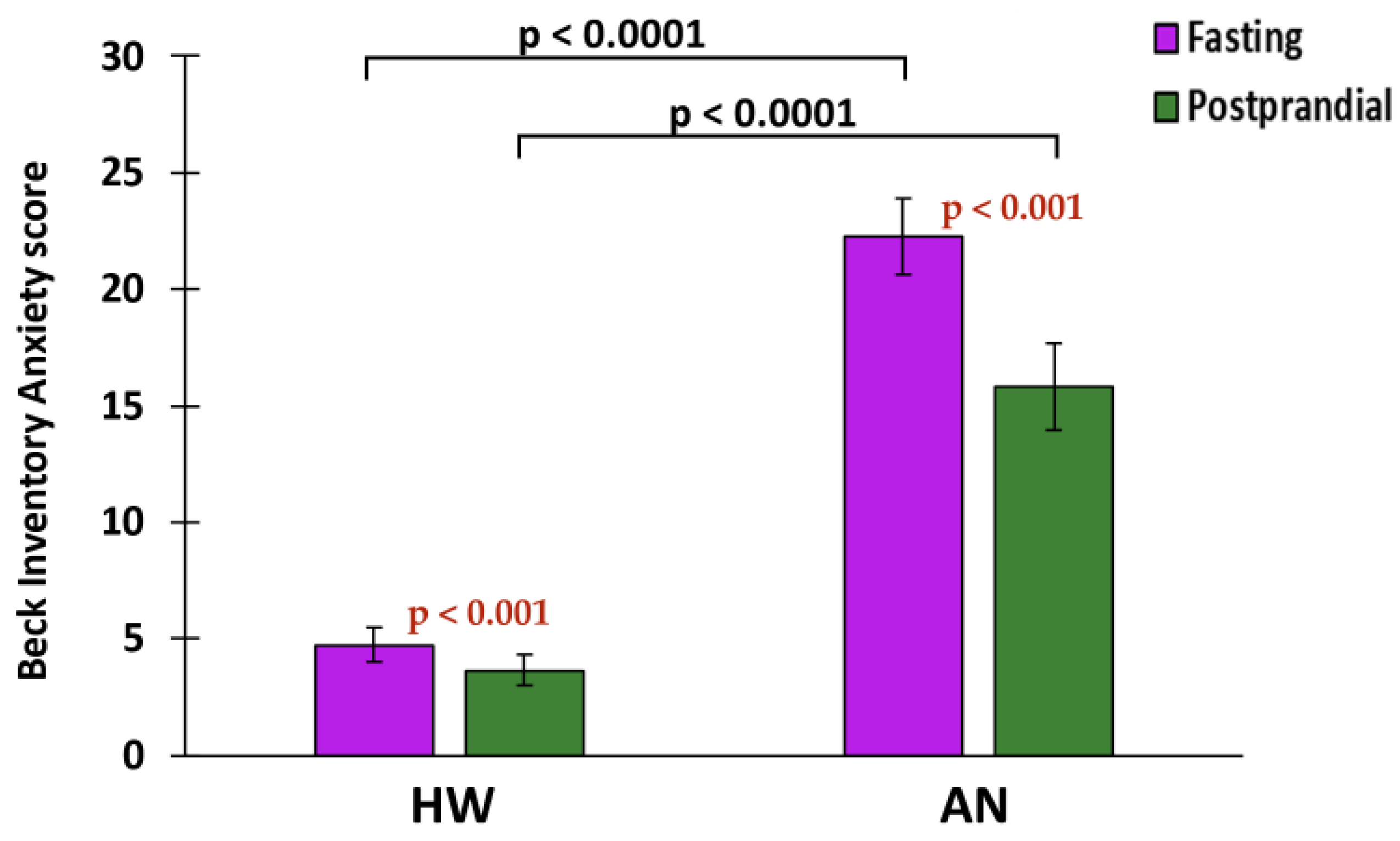
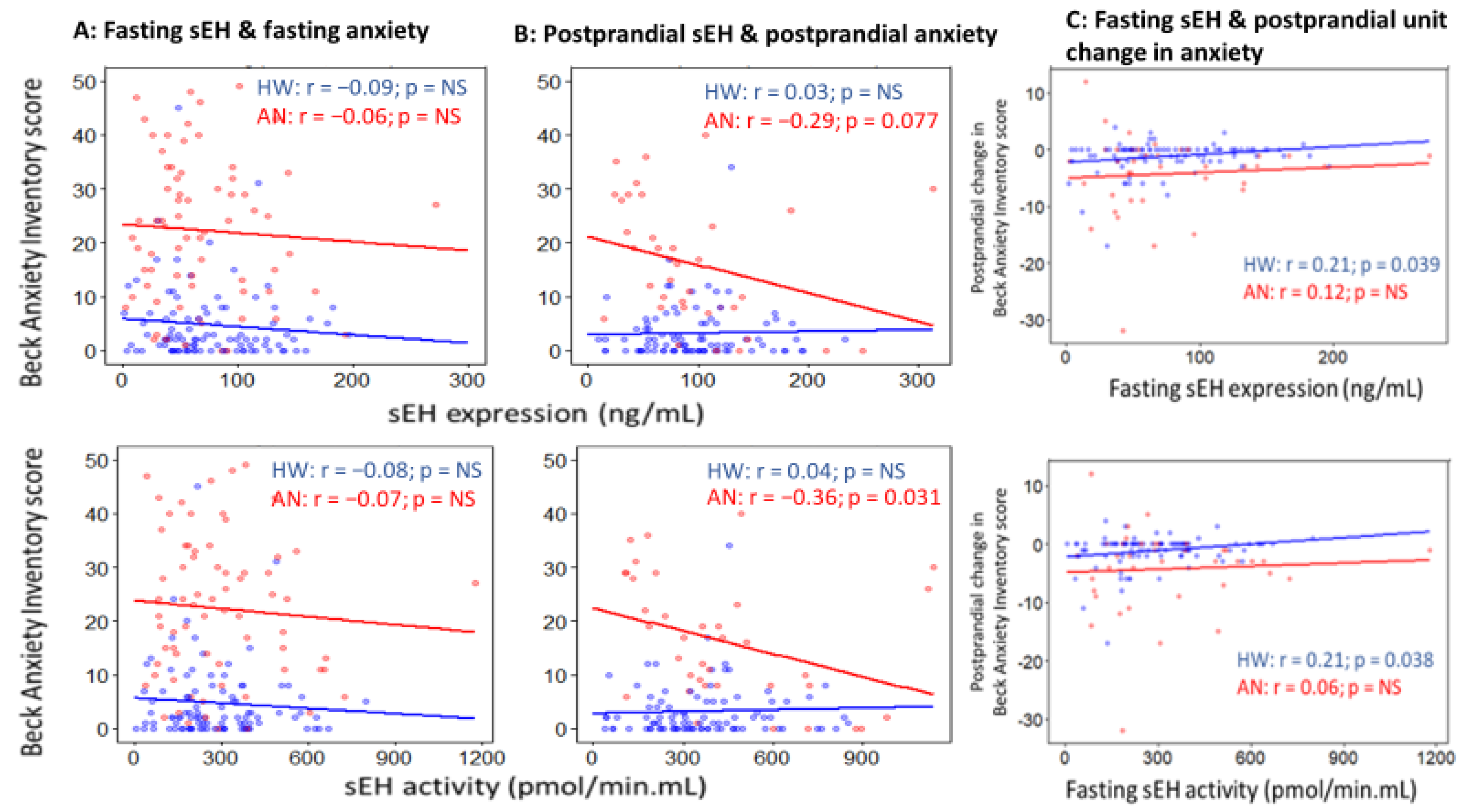
2.5. Associations of sEH with Anxiety and Depression
2.6. Race and sEH
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. sEH Protein Level Quantification
4.3. sEH Cellular Activity
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Spector, A.A.; Kim, H.Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kodani, S.; Hammock, B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014, 53, 108–123. [Google Scholar] [CrossRef]
- Zeldin, D.C.; Kobayashi, J.; Falck, J.R.; Winder, B.S.; Hammock, B.D.; Snapper, J.R.; Capdevila, J.H. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J. Biol. Chem. 1993, 268, 6402–6407. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.W.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D.; et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Front. Pharmacol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.W.; Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 2005, 44, 1–51. [Google Scholar] [CrossRef]
- Enayetallah, A.E.; French, R.A.; Barber, M.; Grant, D.F. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J. Histochem. Cytochem. 2006, 54, 329–335. [Google Scholar] [CrossRef]
- De Taeye, B.M.; Morisseau, C.; Coyle, J.; Covington, J.W.; Luria, A.; Yang, J.; Murphy, S.B.; Friedman, D.B.; Hammock, B.B.; Vaughan, D.E. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity 2010, 18, 489–498. [Google Scholar] [CrossRef]
- Yu, Z.; Davis, B.B.; Morisseau, C.; Hammock, B.D.; Olson, J.L.; Kroetz, D.L.; Weiss, R.H. Vascular localization of soluble epoxide hydrolase in the human kidney. Am. J. Physiol. Renal Physiol. 2004, 286, F720–F726. [Google Scholar] [CrossRef]
- Pacifici, G.M.; Temellini, A.; Giuliani, L.; Rane, A.; Thomas, H.; Oesch, F. Cytosolic epoxide hydrolase in humans: Development and tissue distribution. Arch. Toxicol. 1988, 62, 254–257. [Google Scholar] [CrossRef]
- Gill, S.S.; Hammock, B.D. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem. Pharmacol. 1980, 29, 389–395. [Google Scholar] [CrossRef]
- Enayetallah, A.E.; French, R.A.; Thibodeau, M.S.; Grant, D.F. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J. Histochem. Cytochem. 2004, 52, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130. [Google Scholar] [CrossRef] [PubMed]
- EPHX2 epoxide hydrolase 2 [Homo sapiens (human)]. Gene ID: 2053; 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/2053 (accessed on 25 April 2022).
- Yang, J.; Oh, Y.T.; Wan, D.; Watanabe, R.M.; Hammock, B.D.; Youn, J.H. Postprandial effect to decrease soluble epoxide hydrolase activity: Roles of insulin and gut microbiota. J. Nutr. Biochem. 2017, 49, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Beals, E.; Kamita, S.G.; Sacchi, R.; Demmer, E.; Rivera, N.; Rogers-Soeder, T.S.; Gertz, E.R.; Van Loan, M.D.; German, J.B.; Hammock, B.D.; et al. Addition of milk fat globule membrane-enriched supplement to a high-fat meal attenuates insulin secretion and induction of soluble epoxide hydrolase gene expression in the postprandial state in overweight and obese subjects. J. Nutr. Sci. 2019, 8, e16. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Hsu, M.F.; Bettaieb, A.; Chu, B.; Matsumoto, N.; Morisseau, C.; Havel, P.J.; Huising, M.O.; Hammock, B.D.; Haj, F.G. Genetic deficiency or pharmacological inhibition of soluble epoxide hydrolase ameliorates high fat diet-induced pancreatic β-cell dysfunction and loss. Free Radic. Biol. Med. 2021, 172, 48–57. [Google Scholar] [CrossRef]
- Wagner, K.M.; Yang, J.; Morisseau, C.; Hammock, B.D. Soluble Epoxide Hydrolase Deletion Limits High-Fat Diet-Induced Inflammation. Front. Pharmacol. 2021, 12, 778470. [Google Scholar] [CrossRef]
- Bettaieb, A.; Nagata, N.; AbouBechara, D.; Chahed, S.; Morisseau, C.; Hammock, B.D.; Haj, F.G. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J. Biol. Chem. 2013, 288, 14189–14199. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 267. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, M.; Yang, J.; Nonaka, R.; Yamaguchi, A.; Ishikawa, K.I.; Kobayashi, K.; Murayama, S.; Hwang, S.H.; Saiki, S.; et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E5815–E5823. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.M.; McReynolds, C.B.; Schmidt, W.K.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 2017, 180, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Park, J.A.; Joiakim, A.; Putt, D.A.; Taylor, R.N.; Kim, H. The role of soluble epoxide hydrolase in preeclampsia. Med. Hypotheses 2017, 108, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bode, E.D.; Mathias, K.C.; Stewart, D.F.; Moffatt, S.M.; Jack, K.; Smith, D.L. Cardiovascular Disease Risk Factors by BMI and Age in United States Firefighters. Obesity 2021, 29, 1186–1194. [Google Scholar] [CrossRef]
- Chatterjee, A.; Harris, S.B.; Leiter, L.A.; Fitchett, D.H.; Teoh, H.; Bhattacharyya, O.K. Managing cardiometabolic risk in primary care: Summary of the 2011 consensus statement. Can. Fam. Physician 2012, 58, 389–393. [Google Scholar]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Abell, J.E.; Egan, B.M.; Wilson, P.W.; Lipsitz, S.; Woolson, R.F.; Lackland, D.T. Age and race impact the association between BMI and CVD mortality in women. Public Health Rep. 2007, 122, 507–512. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Age and Cancer Risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age#:~:text=Age%20and%20Cancer%20Risk&text=The%20incidence%20rates%20for%20cancer,groups%2060%20years%20and%20older (accessed on 1 May 2022).
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Mayrink, J.; Souza, R.T.; Feitosa, F.E.; Rocha Filho, E.A.; Leite, D.F.; Vettorazzi, J.; Calderon, I.M.; Sousa, M.H.; Costa, M.L.; Baker, P.N.; et al. Incidence and risk factors for Preeclampsia in a cohort of healthy nulliparous pregnant women: A nested case-control study. Sci. Rep. 2019, 9, 9517. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.M.; Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad. Med. 2017, 63, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Dragioti, E.; Solmi, M.; Cortese, S.; Domschke, K.; Murray, R.M.; Jones, P.B.; Uher, R.; Carvalho, A.F.; Reichenberg, A.; et al. Risk and protective factors for mental disorders beyond genetics: An evidence-based atlas. World Psychiatry 2021, 20, 417–436. [Google Scholar] [CrossRef]
- Reynolds, K.; Pietrzak, R.H.; El-Gabalawy, R.; Mackenzie, C.S.; Sareen, J. Prevalence of psychiatric disorders in U.S. older adults: Findings from a nationally representative survey. World Psychiatry 2015, 14, 74–81. [Google Scholar] [CrossRef]
- Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [CrossRef]
- Bray, G.A. Obesity increases risk for diabetes. Int. J. Obes. Relat. Metab. Disord. 1992, 16 (Suppl. 4), S13–S17. [Google Scholar]
- Hancock, A.M.; Witonsky, D.B.; Gordon, A.S.; Eshel, G.; Pritchard, J.K.; Coop, G.; Di Rienzo, A. Adaptations to climate in candidate genes for common metabolic disorders. PLoS Genet. 2008, 4, e32. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Zhang, J.; Wang, Y.; Hwang, S.H.; Qi, W.; Wan, D.; Kim, D.; Sun, J.; Sanidad, K.Z.; et al. Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, 5283–5288. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Madhu, D.; Cherian, P.; Al-Mulla, F.; Abubaker, J.; Tiss, A. Soluble Epoxide Hydrolase 2 Expression Is Elevated in Obese Humans and Decreased by Physical Activity. Int. J. Mol. Sci. 2020, 21, 2056. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Alcaraz-Quiles, J.; García-Alonso, V.; Rius, B.; Hwang, S.H.; Titos, E.; Lopategi, A.; Hammock, B.D.; Arroyo, V.; Clària, J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proc. Natl. Acad. Sci. USA 2015, 112, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, D.; Tang, L.; Li, H.; Liu, Z.; Gao, J.; Edin, M.L.; Zhang, H.; Zhang, K.; Chen, J.; et al. Soluble epoxide hydrolase deficiency attenuates lipotoxic cardiomyopathy via upregulation of AMPK-mTORC mediated autophagy. J. Mol. Cell. Cardiol. 2021, 154, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Wang, W.; Sanidad, K.Z.; Cinelli, M.A.; Wan, D.; Hwang, S.H.; Kim, D.; Lee, K.S.S.; Xiao, H.; et al. Soluble epoxide hydrolase is an endogenous regulator of obesity-induced intestinal barrier dysfunction and bacterial translocation. Proc. Natl. Acad. Sci. USA 2020, 117, 8431–8436. [Google Scholar] [CrossRef]
- Overby, H.; Yang, Y.; Xu, X.; Graham, K.; Hildreth, K.; Choi, S.; Wan, D.; Morisseau, C.; Zeldin, D.C.; Hammock, B.D.; et al. Soluble Epoxide Hydrolase Inhibition by t-TUCB Promotes Brown Adipogenesis and Reduces Serum Triglycerides in Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 7039. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, H.; Li, D.; Pang, W.; Hammock, B.D.; Zhu, Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS ONE 2012, 7, e39165. [Google Scholar] [CrossRef]
- Zha, W.; Edin, M.L.; Vendrov, K.C.; Schuck, R.N.; Lih, F.B.; Jat, J.L.; Bradbury, J.A.; DeGraff, L.M.; Hua, K.; Tomer, K.B.; et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J. Lipid Res. 2014, 55, 2124–2136. [Google Scholar] [CrossRef]
- Jamieson, K.L.; Samokhvalov, V.; Akhnokh, M.K.; Lee, K.; Cho, W.J.; Takawale, A.; Wang, X.; Kassiri, Z.; Seubert, J.M. Genetic deletion of soluble epoxide hydrolase provides cardioprotective responses following myocardial infarction in aged mice. Prostaglandins Other Lipid Mediat. 2017, 132, 47–58. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Zordoky, B.N.; Edin, M.L.; Alsaleh, N.; El-Kadi, A.O.; Zeldin, D.C.; Seubert, J.M. Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice. Prostaglandins Other Lipid Mediat. 2013, 104–105, 8–17. [Google Scholar] [CrossRef]
- Zuloaga, K.L.; Zhang, W.; Roese, N.E.; Alkayed, N.J. Soluble epoxide hydrolase gene deletion improves blood flow and reduces infarct size after cerebral ischemia in reproductively senescent female mice. Front. Pharmacol. 2014, 5, 290. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N. Nutritional Psychiatry: Where to Next? EBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Kremer, P.J.; Leslie, E.R.; Berk, M.; Patton, G.C.; Toumbourou, J.W.; Williams, J.W. Associations between diet quality and depressed mood in adolescents: Results from the Australian Healthy Neighbourhoods Study. Aust. New Zealand J. Psychiatry 2010, 44, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.T.; Hao, J.H.; Qian, Q.W.; Cao, H.; Fu, J.L.; Sun, Y.; Huang, L.; Tao, F.B. Is there any relationship between dietary patterns and depression and anxiety in Chinese adolescents? Public Health Nutr. 2012, 15, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, Y.; Chang, L.; Hammock, B.D.; Hashimoto, K. Increased expression of soluble epoxide hydrolase in the brain and liver from patients with major psychiatric disorders: A role of brain—Liver axis. J. Affect. Disord. 2020, 270, 131–134. [Google Scholar] [CrossRef]
- Qin, X.H.; Wu, Z.; Dong, J.H.; Zeng, Y.N.; Xiong, W.C.; Liu, C.; Wang, M.Y.; Zhu, M.Z.; Chen, W.J.; Zhang, Y.; et al. Liver Soluble Epoxide Hydrolase Regulates Behavioral and Cellular Effects of Chronic Stress. Cell Rep. 2019, 29, 3223–3234.e6. [Google Scholar] [CrossRef]
- Xiong, W.; Cao, X.; Zeng, Y.; Qin, X.; Zhu, M.; Ren, J.; Wu, Z.; Huang, Q.; Zhang, Y.; Wang, M.; et al. Astrocytic Epoxyeicosatrienoic Acid Signaling in the Medial Prefrontal Cortex Modulates Depressive-like Behaviors. J. Neurosci. 2019, 39, 4606–4623. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, H.; Song, J.; Chang, Q. The effects of sEH inhibitor on depression-like behavior and neurogenesis in male mice. J. Neurosci. Res. 2017, 95, 2483–2492. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, K.I.; Lin, H.C.; Lee, T.S. Genetic Deletion of Soluble Epoxide Hydroxylase Causes Anxiety-Like Behaviors in Mice. Mol. Neurobiol. 2019, 56, 2495–2507. [Google Scholar] [CrossRef]
- Luria, A.; Morisseau, C.; Tsai, H.J.; Yang, J.; Inceoglu, B.; De Taeye, B.; Watkins, S.M.; Wiest, M.M.; German, J.B.; Hammock, B.D. Alteration in plasma testosterone levels in male mice lacking soluble epoxide hydrolase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E375–E383. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ma, M.; Ishima, T.; Morisseau, C.; Yang, J.; Wagner, K.M.; Zhang, J.C.; Yang, C.; Yao, W.; Dong, C.; et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc. Natl. Acad. Sci. USA 2016, 113, E1944–E1952. [Google Scholar] [CrossRef]
- Spector, A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009, 50, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y.; Hennebelle, M.; Yang, J.; Zamora, D.; Rapoport, S.I.; Hammock, B.D.; Ramsden, C.E. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, V.; Plée-Gautier, E.; Potin, P.; Madec, S.; Salaün, J.P. Human CYP4F3s are the main catalysts in the oxidation of fatty acid epoxides. J. Lipid Res. 2004, 45, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.D.; Morisseau, C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie 2019, 159, 59–65. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, C.; Morisseau, C.; Wagner, K.; Hammock, B. Epoxy Fatty Acids Are Promising Targets for Treatment of Pain, Cardiovascular Disease and Other Indications Characterized by Mitochondrial Dysfunction, Endoplasmic Stress and Inflammation. Adv. Exp. Med. Biol. 2020, 1274, 71–99. [Google Scholar] [CrossRef]
- Anita, N.Z.; Forkan, N.; Kamal, R.; Nguyen, M.M.; Yu, D.; Major-Orfao, C.; Wong, S.K.; Lanctôt, K.L.; Herrmann, N.; Oh, P.I.; et al. Serum soluble epoxide hydrolase related oxylipins and major depression in patients with type 2 diabetes. Psychoneuroendocrinology 2021, 126, 105149. [Google Scholar] [CrossRef]
- Swinbourne, J.; Hunt, C.; Abbott, M.; Russell, J.; St Clare, T.; Touyz, S. The comorbidity between eating disorders and anxiety disorders: Prevalence in an eating disorder sample and anxiety disorder sample. Aust. New Zealand J. Psychiatry 2012, 46, 118–131. [Google Scholar] [CrossRef]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef]
- Godart, N.T.; Flament, M.F.; Lecrubier, Y.; Jeammet, P. Anxiety disorders in anorexia nervosa and bulimia nervosa: Co-morbidity and chronology of appearance. Eur. Psychiatry 2000, 15, 38–45. [Google Scholar] [CrossRef]
- Bulik, C.M.; Sullivan, P.F.; Fear, J.L.; Joyce, P.R. Eating disorders and antecedent anxiety disorders: A controlled study. Acta Psychiatr. Scand. 1997, 96, 101–107. [Google Scholar] [CrossRef]
- Brand-Gothelf, A.; Leor, S.; Apter, A.; Fennig, S. The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. J. Nerv. Ment. Dis. 2014, 202, 759–762. [Google Scholar] [CrossRef][Green Version]
- Zerwas, S.; Lund, B.C.; Von Holle, A.; Thornton, L.M.; Berrettini, W.H.; Brandt, H.; Crawford, S.; Fichter, M.M.; Halmi, K.A.; Johnson, C.; et al. Factors associated with recovery from anorexia nervosa. J. Psychiatr. Res. 2013, 47, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.B.; Keller, M.B.; Sacks, N.R.; Yeh, C.J.; Lavori, P.W. Psychiatric comorbidity in treatment-seeking anorexics and bulimics. J. Am. Acad. Child Adolesc. Psychiatry 1992, 31, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Halmi, K.A.; Eckert, E.; Marchi, P.; Sampugnaro, V.; Apple, R.; Cohen, J. Comorbidity of psychiatric diagnoses in anorexia nervosa. Arch. Gen. Psychiatry 1991, 48, 712–718. [Google Scholar] [CrossRef]
- Samsonoff, W.A.; Reston, J.; McKee, M.; O’Connor, B.; Galivan, J.; Maley, G.; Maley, F. Intracellular location of thymidylate synthase and its state of phosphorylation. J. Biol. Chem. 1997, 272, 13281–13285. [Google Scholar] [CrossRef]
- Chu, E.; Koeller, D.M.; Casey, J.L.; Drake, J.C.; Chabner, B.A.; Elwood, P.C.; Zinn, S.; Allegra, C.J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 8977–8981. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Qi, W.; Yang, H.; Wang, C.; Tan, B.; Hammock, B.D.; Park, Y.; Kim, D.; Zhang, G. Lipidomic profiling of high-fat diet-induced obesity in mice: Importance of cytochrome P450-derived fatty acid epoxides. Obesity 2017, 25, 132–140. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Yang, H.; Sanidad, K.Z.; Hammock, B.D.; Kim, D.; Zhang, G. Effects of high-fat diet on plasma profiles of eicosanoid metabolites in mice. Prostaglandins Other Lipid Mediat. 2016, 127, 9–13. [Google Scholar] [CrossRef]
- Yang, J.; Hammock, B.D.; Halmi, K.; Woodside, B.; German, B.; Schork, N.; Bailer, U.F.; Kaye, W.; Morisseau, C.; Shih, P.-A.B. Substrate-Dependent Postprandial Oxylipin Responses Revealed Evidence of Nutrient-Gene Interaction in Anorexia Nervosa. In Neuropsychopharmacology; Nature Publishing Group Macmillan Building, 4 CRINAN ST; Nature Publishing Group: London, UK, 2015; p. S302. [Google Scholar]
- Micali, N.; Martini, M.G.; Thomas, J.J.; Eddy, K.T.; Kothari, R.; Russell, E.; Bulik, C.M.; Treasure, J. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: A population-based study of diagnoses and risk factors. BMC Med. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Pais, S.M.A.; Nicholls, D.; Chapman, S.; Hudson, L.D. What Are the Effects of Restrictive Eating Disorders on Growth and Puberty and Are Effects Permanent? A Systematic Review and Meta-Analysis. J. Adolesc. Health 2020, 66, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Klibanski, A. Effects of Anorexia Nervosa on Bone Metabolism. Endocr. Rev. 2018, 39, 895–910. [Google Scholar] [CrossRef]
- Huang, A.; Sun, D. Sexually Dimorphic Regulation of EET Synthesis and Metabolism: Roles of Estrogen. Front. Pharmacol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Yang, Y.M.; Sun, D.; Kandhi, S.; Froogh, G.; Zhuge, J.; Huang, W.; Hammock, B.D.; Huang, A. Estrogen-dependent epigenetic regulation of soluble epoxide hydrolase via DNA methylation. Proc. Natl. Acad. Sci. USA 2018, 115, 613–618. [Google Scholar] [CrossRef]
- Koerner, I.P.; Zhang, W.; Cheng, J.; Parker, S.; Hurn, P.D.; Alkayed, N.J. Soluble epoxide hydrolase: Regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front. Biosci. 2008, 13, 2833–2841. [Google Scholar] [CrossRef]
- Scott-Van Zeeland, A.A.; Bloss, C.S.; Tewhey, R.; Bansal, V.; Torkamani, A.; Libiger, O.; Duvvuri, V.; Wineinger, N.; Galvez, L.; Darst, B.F.; et al. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Mol. Psychiatry 2014, 19, 724–732. [Google Scholar] [CrossRef]
- Terlizzi EP, S.J. Estimates of Mental Health Symptomatology, by Month of Interview: United States, 2019; National Center for Health Statistics: Hyattsville, ML, USA, 2021. [Google Scholar]
- U.S. Census Bureau. Household Pulse Survey, 2020–2021; National Center for Health Statistics: Hyattsville, ML, USA, 2021. [Google Scholar]
- Khalsa, S.S.; Hassanpour, M.S.; Strober, M.; Craske, M.G.; Arevian, A.C.; Feusner, J.D. Interoceptive Anxiety and Body Representation in Anorexia Nervosa. Front. Psychiatry 2018, 9, 444. [Google Scholar] [CrossRef]
- Kessing, L.V. Epidemiology of subtypes of depression. Acta Psychiatr. Scand. 2007, 115, 85–89. [Google Scholar] [CrossRef]
- Zhu, X.L.; Wang, L.; Wang, Z.; Chen, S.Z.; Zhang, W.Q.; Ma, M.M. Relationship between EPHX2 gene polymorphisms and essential hypertension in Uygur, Kazakh, and Han. Genet. Mol. Res. 2015, 14, 3474–3480. [Google Scholar] [CrossRef] [PubMed]
- Cresci, S.; Depta, J.P.; Lenzini, P.A.; Li, A.Y.; Lanfear, D.E.; Province, M.A.; Spertus, J.A.; Bach, R.G. Cytochrome p450 gene variants, race, and mortality among clopidogrel-treated patients after acute myocardial infarction. Circ. Cardiovasc. Genet. 2014, 7, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; North, K.E.; Bray, M.S.; Fornage, M.; Seubert, J.M.; Newman, J.W.; Hammock, B.D.; Couper, D.J.; Heiss, G.; Zeldin, D.C. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum. Mol. Genet. 2006, 15, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Sura, P.; Sura, R.; Enayetallah, A.E.; Grant, D.F. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2008, 56, 551–559. [Google Scholar] [CrossRef]
- Lee, A.R.; Pechenino, A.S.; Dong, H.; Hammock, B.D.; Knowlton, A.A. Aging, estrogen loss and epoxyeicosatrienoic acids (EETs). PLoS ONE 2013, 8, e70719. [Google Scholar] [CrossRef] [PubMed]
- Pinot, F.; Grant, D.F.; Spearow, J.L.; Parker, A.G.; Hammock, B.D. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem. Pharmacol. 1995, 50, 501–508. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Morisseau, C.; Gee, S.J.; Bever, C.S.; Liu, X.; Wu, J.; Hammock, B.D.; Ying, Y. Nanobody Based Immunoassay for Human Soluble Epoxide Hydrolase Detection Using Polymeric Horseradish Peroxidase (PolyHRP) for Signal Enhancement: The Rediscovery of PolyHRP? Anal. Chem. 2017, 89, 6248–6256. [Google Scholar] [CrossRef]
- Borhan, B.; Mebrahtu, T.; Nazarian, S.; Kurth, M.J.; Hammock, B.D. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal. Biochem. 1995, 231, 188–200. [Google Scholar] [CrossRef]

| Characteristic | Healthy Women n = 96 | Anorexia Nervosa n = 70 |
|---|---|---|
| Age (years) | 29 ± 8 (95% CI: 27 to 30) | 32 ± 12 (95% CI: 29 to 34) |
| Race: n (%) | White: 48 (50%) Asian: 39 (41%) Black/African American: 2 (2%) More than one race: 7 (7%) | White: 62 (89%) Asian: 7 (10%) Black/African American: 0 (0%) More than one race: 1 (1%) |
| Body mass index (kg/m2) | 22.98 ± 3.16 * (95% CI: 22.34 to 23.62) | 18.66 ± 3.44 (95% CI: 17.84 to 19.48) |
| Beck Depression Inventory score (fasting) | 4.05 ± 6.22 * (95% CI: 2.79 to 5.31) | 22.900 ± 15.75 (95% CI: 19.14 to 26.66) |
| Beck Anxiety Inventory Score (fasting) | 4.74 ± 7.06 * (95% CI: 3.31 to 6.17) | 22.27 ± 13.38 (95% CI: 19.08 to 25.46) |
| Postprandial Beck Anxiety Inventory Score | 3.64 ± 6.50 * (95% CI: 2.31 to 4.97) | 15.82 ± 11.50 (95% CI: 12.03 to 19.60) |
| Postprandial unit change in Beck Anxiety Inventory Score * | −1.20 ± 2.83 (95% CI: −1.78 to −0.62) | −4.32 ± 7.23 (95% CI: −6.69 to −1.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.; Morisseau, C.; Li, D.; Yang, J.; Lam, E.; Woodside, D.B.; Hammock, B.D.; Shih, P.-a.B. Soluble Epoxide Hydrolase Is Associated with Postprandial Anxiety Decrease in Healthy Adult Women. Int. J. Mol. Sci. 2022, 23, 11798. https://doi.org/10.3390/ijms231911798
Nguyen N, Morisseau C, Li D, Yang J, Lam E, Woodside DB, Hammock BD, Shih P-aB. Soluble Epoxide Hydrolase Is Associated with Postprandial Anxiety Decrease in Healthy Adult Women. International Journal of Molecular Sciences. 2022; 23(19):11798. https://doi.org/10.3390/ijms231911798
Chicago/Turabian StyleNguyen, Nhien, Christophe Morisseau, Dongyang Li, Jun Yang, Eileen Lam, D. Blake Woodside, Bruce D. Hammock, and Pei-an Betty Shih. 2022. "Soluble Epoxide Hydrolase Is Associated with Postprandial Anxiety Decrease in Healthy Adult Women" International Journal of Molecular Sciences 23, no. 19: 11798. https://doi.org/10.3390/ijms231911798
APA StyleNguyen, N., Morisseau, C., Li, D., Yang, J., Lam, E., Woodside, D. B., Hammock, B. D., & Shih, P.-a. B. (2022). Soluble Epoxide Hydrolase Is Associated with Postprandial Anxiety Decrease in Healthy Adult Women. International Journal of Molecular Sciences, 23(19), 11798. https://doi.org/10.3390/ijms231911798








