NOD2 Signaling Circuitry during Allergen Sensitization Does Not Worsen Experimental Neutrophilic Asthma but Promotes a Th2/Th17 Profile in Asthma Patients but Not Healthy Subjects
Abstract
1. Introduction
2. Results
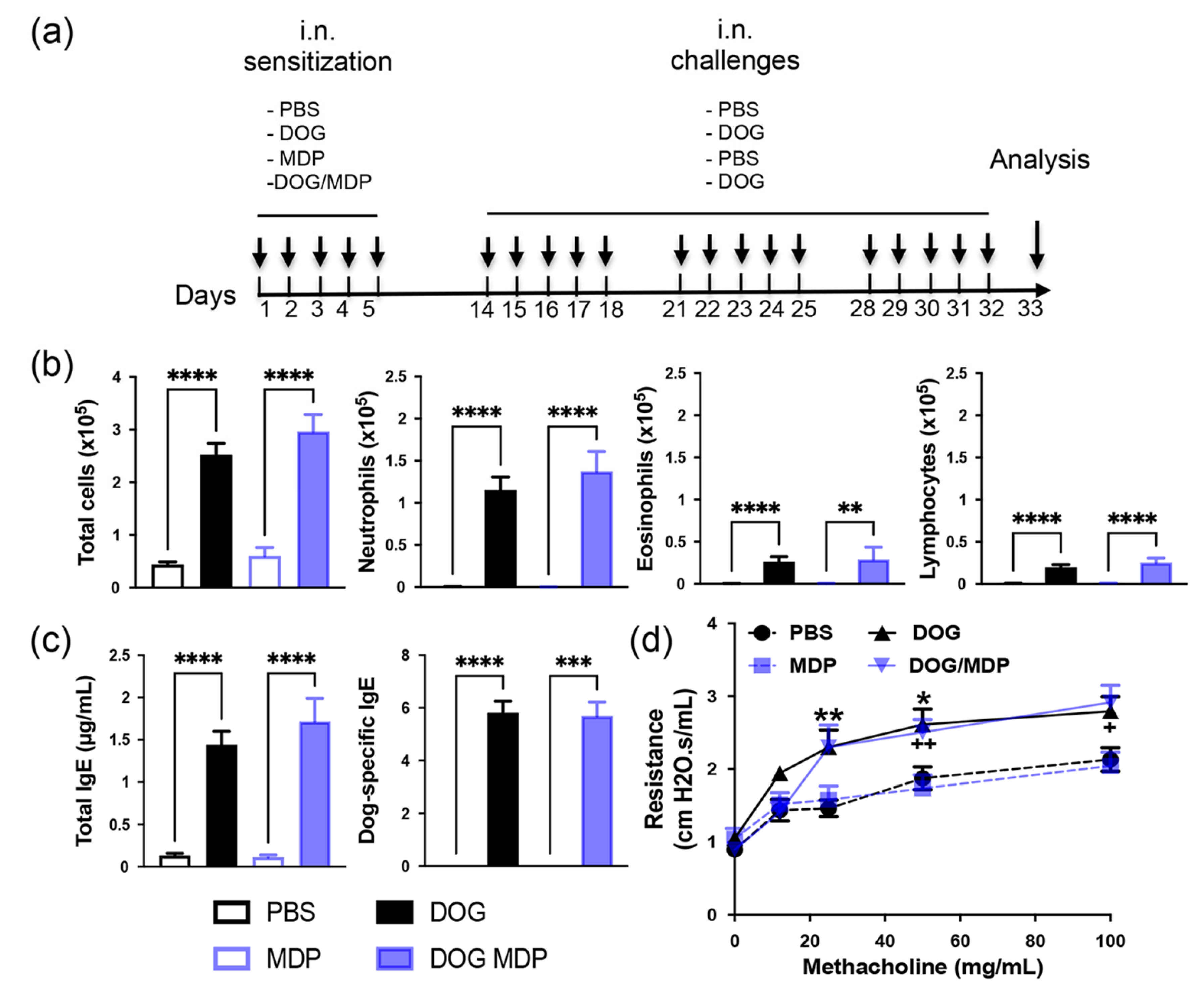
2.1. NOD2 Ligand Co-Administration Does Not Aggravate Allergen-Induced Airway Inflammation, Humoral Response, and AHR
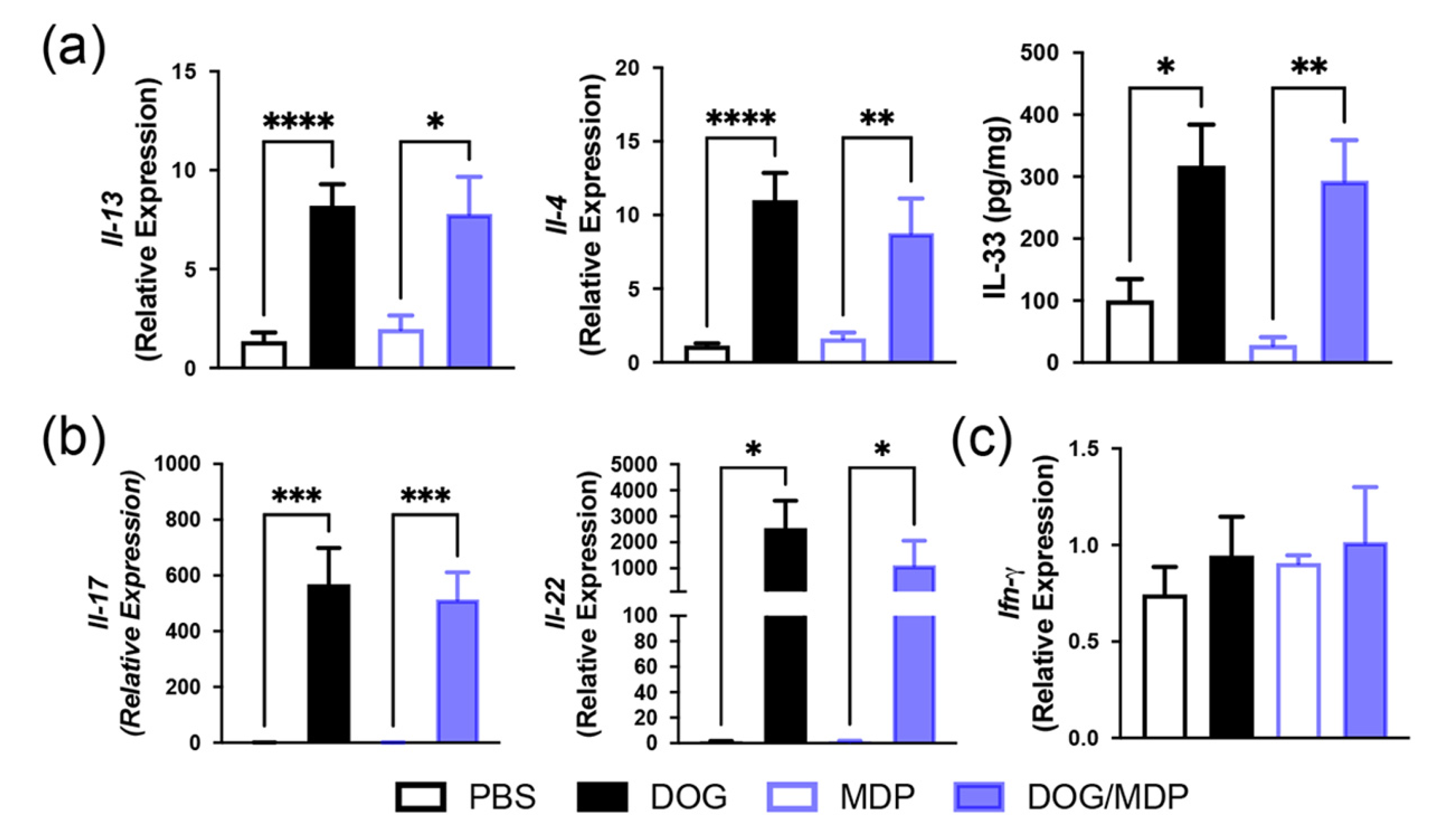
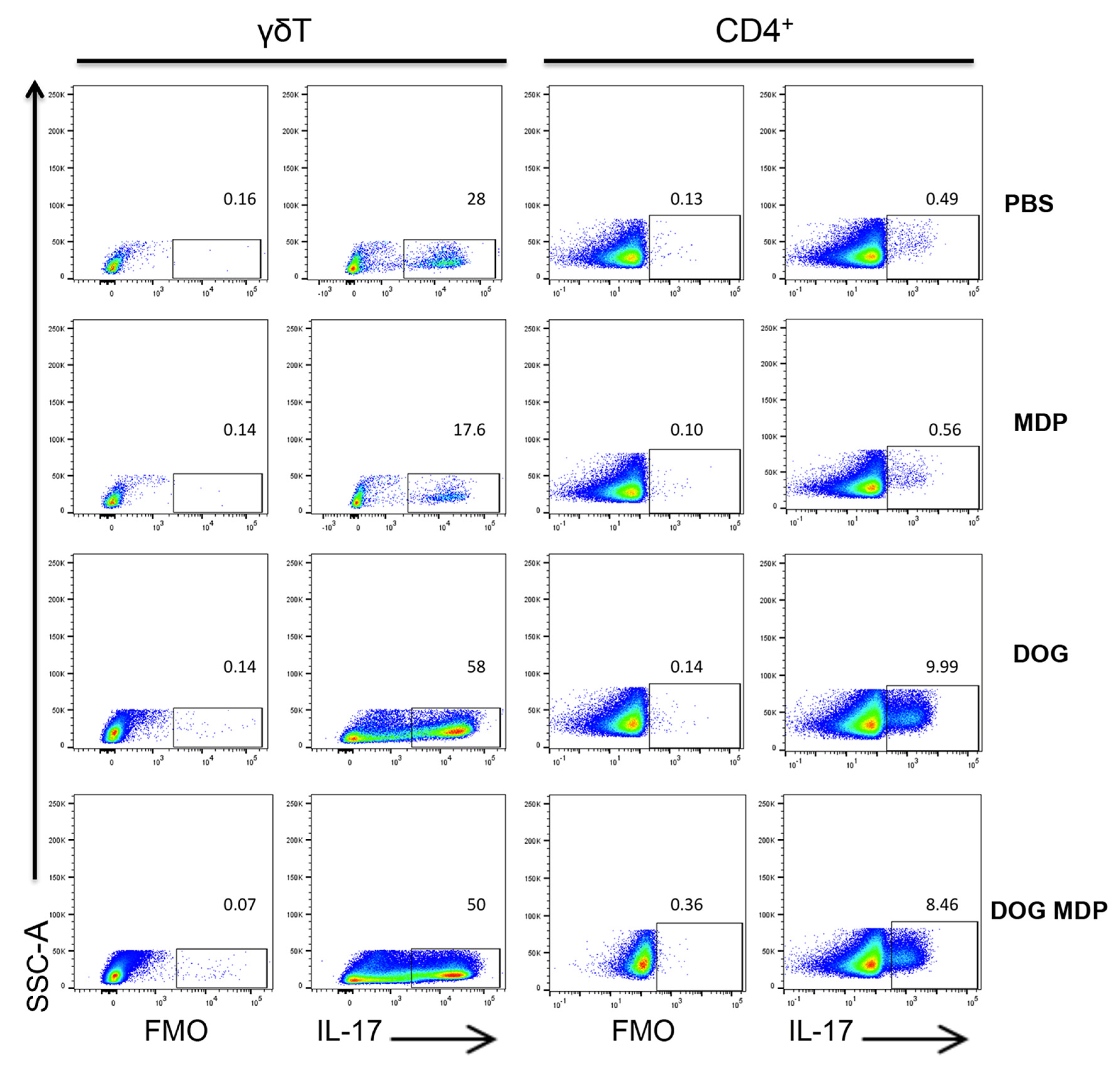
2.2. Mixed Th2/Th17-Type Endotypic Profile Induced by Dog Allergen Remains Unchanged under the Effect of NOD2 Ligand
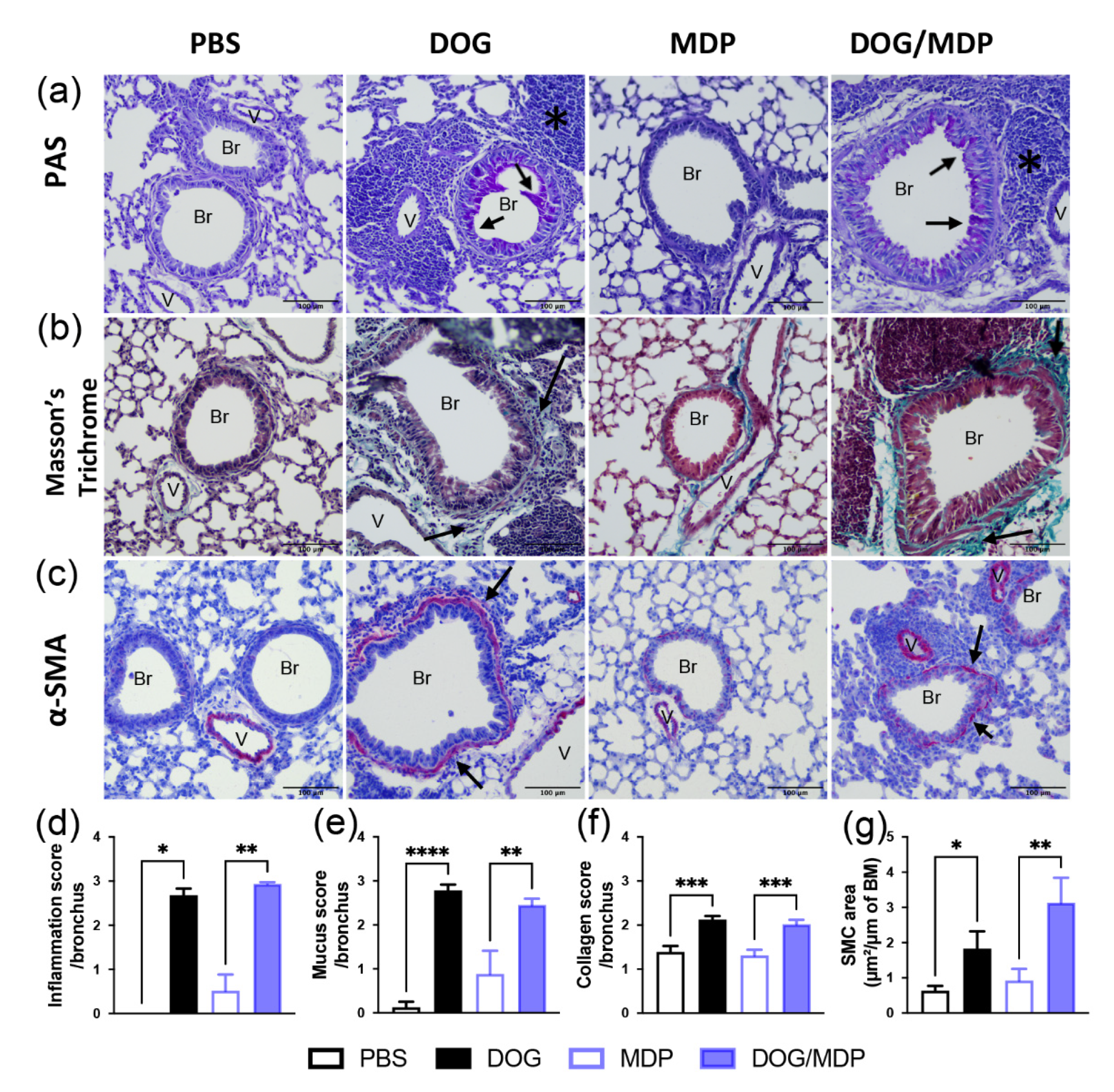
2.3. Dog-Allergen-Induced AAI Is Associated with Bronchial Remodeling without Additional Effect of NOD2 Ligand
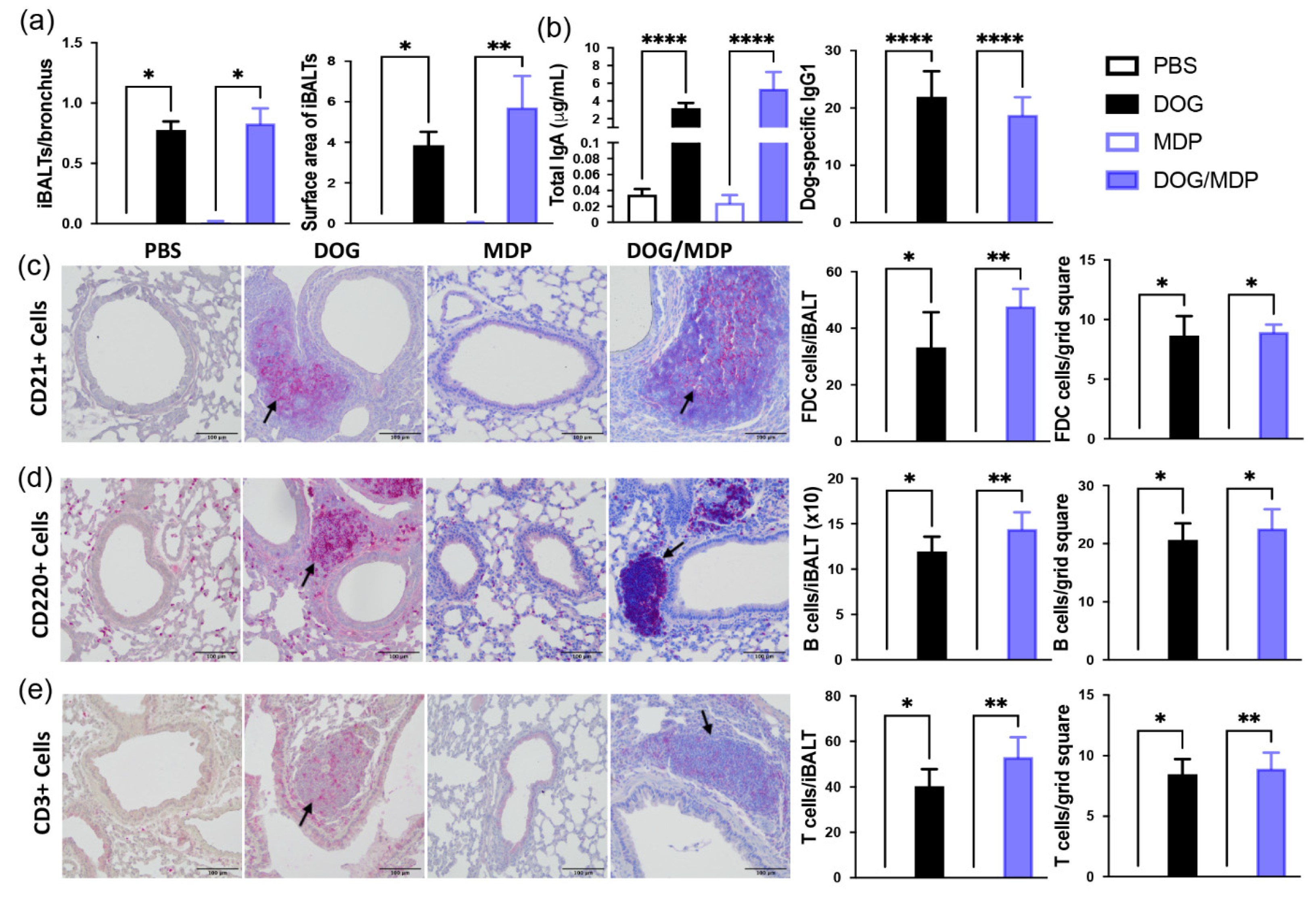
2.4. NOD2 Ligand and Allergen Co-Administration Does Not Change the Formation of iBALT in Mice
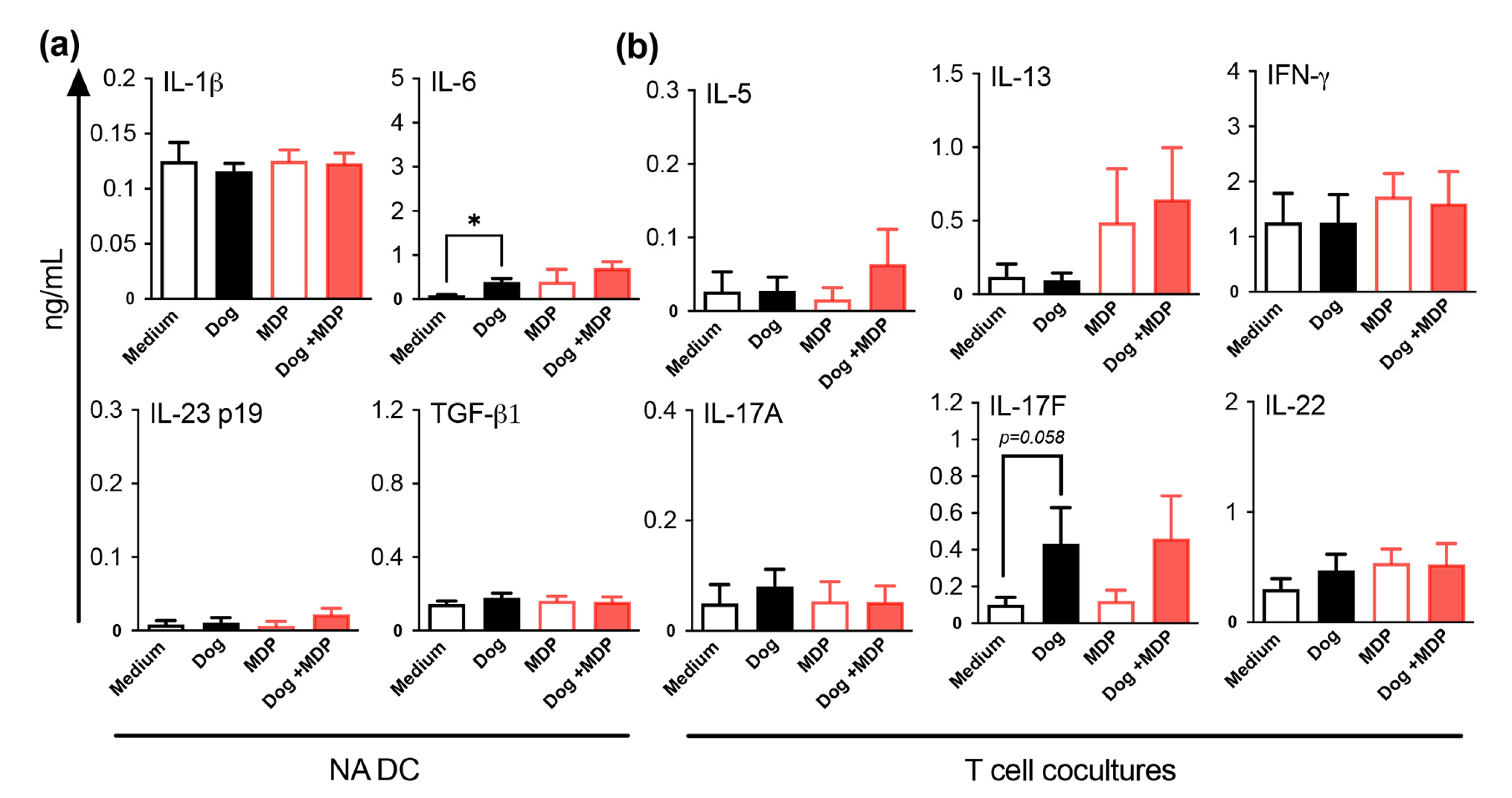
2.5. NOD2 Ligand and Allergen Co-Stimulation of DC from Healthy Subjects Does Not Alter DC Cytokine and T Cell Polarization Profiles
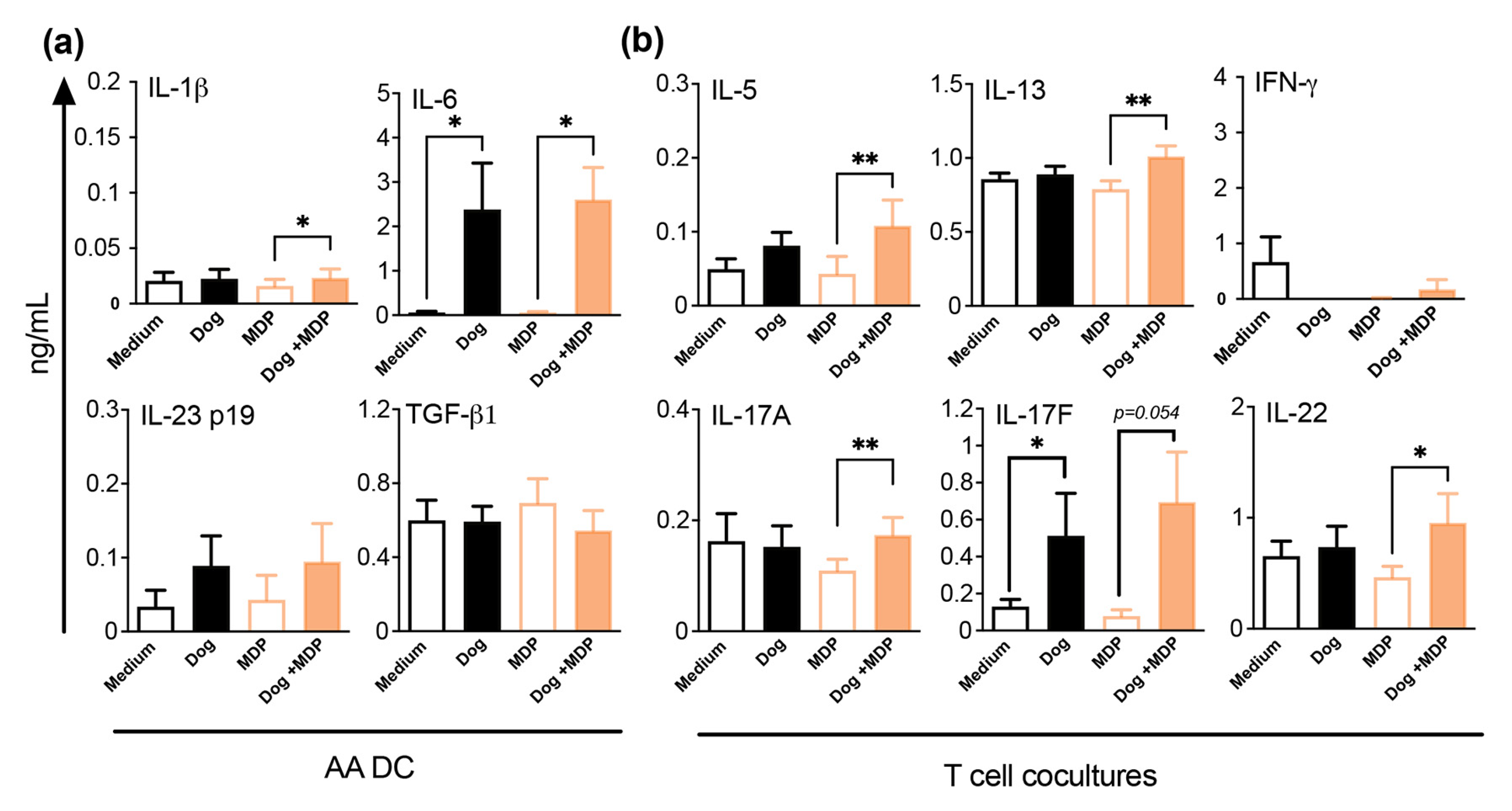
2.6. NOD2 Ligand and Allergen Co-Stimulation of DC from Allergic Asthmatic Patients Promotes a Th2/Th17 Profile
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Allergen Sensitization and Challenge
4.3. Airway Responsiveness Measurement
4.4. BAL Analysis
4.5. Serum and BAL Antibodies Measurement
4.6. RNA Isolation and Quantitative RT-PCR
4.7. Lung Protein Extracts
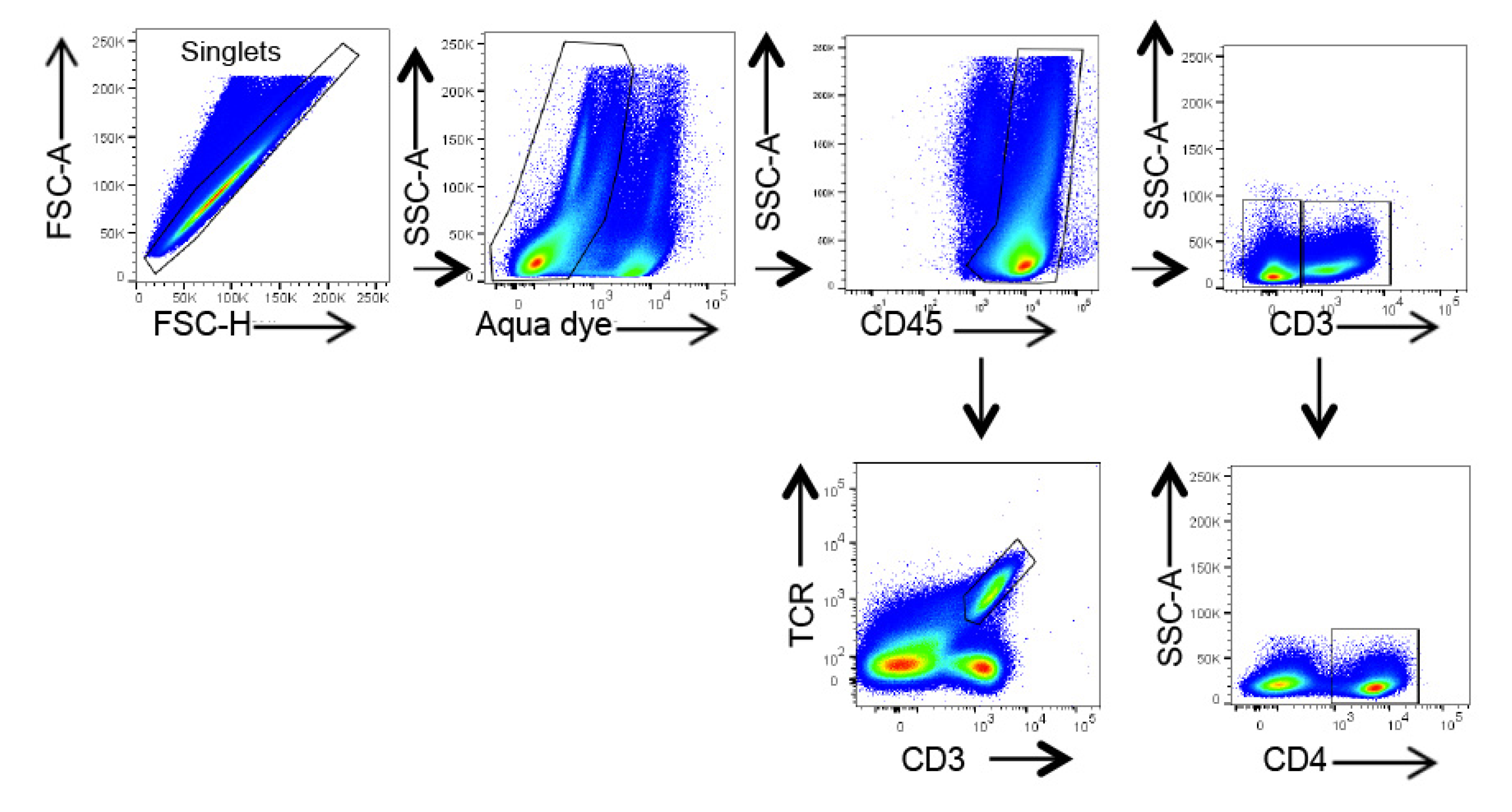
4.8. Flow Cytometry
4.9. Histology and Immunohistochemistry
4.10. Human Donors
4.11. Generation of DC
4.12. Isolation of CD4+CD45RA+ Naive T Cells and DC/T Cell Co-Cultures
4.13. Cytokine and Chemokine Protein Assessment
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- The Global Asthma Report 2018 n.d. Available online: https://globalasthmareport.org/ (accessed on 3 May 2022).
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2013, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Raundhal, M.; Oriss, T.B.; Ray, P.; Wenzel, S.E. Current concepts of severe asthma. J. Clin. Investig. 2016, 126, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- McKinley, L.; Alcorn, J.F.; Peterson, A.; DuPont, R.B.; Kapadia, S.; Logar, A.; Henry, A.; Irvin, C.G.; Piganelli, J.D.; Ray, A.; et al. TH17 Cells Mediate Steroid-Resistant Airway Inflammation and Airway Hyperresponsiveness in Mice. J. Immunol. 2008, 181, 4089–4097. [Google Scholar] [CrossRef]
- Castillo, J.M.L.; Yoon, J.; Geha, R.S. IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice. J. Allergy Clin. Immunol. 2018, 143, 619–630.e7. [Google Scholar] [CrossRef]
- Kudo, M.; Melton, A.C.; Chen, C.; Engler, M.B.; E Huang, K.; Ren, X.; Wang, Y.; Bernstein, X.; Li, J.T.; Atabai, K.; et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012, 18, 547–554. [Google Scholar] [CrossRef]
- Zhao, J.; Lloyd, C.; Noble, A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2012, 6, 335–346. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood Asthma after Bacterial Colonization of the Airway in Neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef]
- Elambert, L.; Sagfors, A.M.; Openshaw, P.J.M.; Culley, F.J. Immunity to RSV in Early-Life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Opitz, B.; van Laak, V.; Eitel, J.; Suttorp, N. Innate Immune Recognition in Infectious and Noninfectious Diseases of the Lung. Am. J. Respir. Crit. Care Med. 2010, 181, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Munks, M.W.; Marrack, P. How Do Adjuvants Work? Important Considerations for New Generation Adjuvants. Immunity 2007, 27, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23, Erratum in 2014, 14, 131. [Google Scholar] [CrossRef]
- Alvarez-Simon, D.; Yahia, S.A.; de Nadai, P.; Audousset, C.; Chamaillard, M.; Boneca, I.G.; Tsicopoulos, A. NOD-like receptors in asthma. Front. Immunol. 2022, 13, 928886. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Ciorbaru, R.; Ellouz, F.; Petit, J.-F.; Lederer, E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem. Biophys. Res. Commun. 1974, 56, 561–567. [Google Scholar] [CrossRef]
- Ellouz, F.; Adam, A.; Ciorbaru, R.; Lederer, E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem. Biophys. Res. Commun. 1974, 59, 1317–1325. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Levitz, S.M.; Golenbock, D.T. Beyond Empiricism: Informing Vaccine Development through Innate Immunity Research. Cell 2012, 148, 1284–1292. [Google Scholar] [CrossRef]
- Gutjahr, A.; Papagno, L.; Vernejoul, F.; Lioux, T.; Jospin, F.; Chanut, B.; Perouzel, E.; Rochereau, N.; Appay, V.; Verrier, B.; et al. New chimeric TLR7/NOD2 agonist is a potent adjuvant to induce mucosal immune responses. eBioMedicine 2020, 58, 102922. [Google Scholar] [CrossRef]
- Jackson, E.M.; Herbst-Kralovetz, M.M. Intranasal Vaccination with Murabutide Enhances Humoral and Mucosal Immune Responses to a Virus-Like Particle Vaccine. PLoS ONE 2012, 7, e41529. [Google Scholar] [CrossRef]
- Pavot, V.; Rochereau, N.; Primard, C.; Genin, C.; Perouzel, E.; Lioux, T.; Paul, S.; Verrier, B. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J. Control. Release 2013, 167, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Climent, N.; Rochereau, N.; García, F.; Genin, C.; Tiraby, G.; Vernejoul, F.; Perouzel, E.; Lioux, T.; Verrier, B.; et al. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials 2016, 75, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Van Beelen, A.J.; Zelinkova, Z.; Taanman-Kueter, E.W.; Muller, F.J.; Hommes, D.W.; Zaat, S.A.; Kapsenberg, M.L.; de Jong, E.C. Stimulation of the Intracellular Bacterial Sensor NOD2 Programs Dendritic Cells to Promote Interleukin-17 Production in Human Memory T Cells. Immunity 2007, 27, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; Le Bourhis, L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J.; et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 2011, 17, 837–844. [Google Scholar] [CrossRef]
- Fritz, J.H.; Le Bourhis, L.; Sellge, G.; Magalhaes, J.G.; Fsihi, H.; Kufer, T.A.; Collins, C.; Viala, J.; Ferrero, R.; Girardin, S.E.; et al. Nod1-Mediated Innate Immune Recognition of Peptidoglycan Contributes to the Onset of Adaptive Immunity. Immunity 2007, 26, 445–459. [Google Scholar] [CrossRef]
- Opitz, B.; Püschel, A.; Schmeck, B.; Hocke, A.C.; Rosseau, S.; Hammerschmidt, S.; Schumann, R.R.; Suttorp, N.; Hippenstiel, S. Nucleotide-binding Oligomerization Domain Proteins Are Innate Immune Receptors for Internalized Streptococcus pneumoniae. J. Biol. Chem. 2004, 279, 36426–36432. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Weidinger, S.; Klopp, N.; Rummler, L.; Wagenpfeil, S.; Baurecht, H.J.; Gauger, A.; Darsow, U.; Jakob, T.; Novak, N.; Schafer, T.; et al. Association of CARD15 polymorphisms with atopy-related traits in a population-based cohort of Caucasian adults. Clin. Exp. Allergy 2005, 35, 866–872. [Google Scholar] [CrossRef]
- Cai, X.; Xu, Q.; Zhou, C.; Zhou, L.; Dai, W.; Ji, G. The association of nucleotide-binding oligomerization domain 2 gene polymorphisms with the risk of asthma in the Chinese Han population. Mol. Genet. Genom. Med. 2019, 7, e00675. [Google Scholar] [CrossRef]
- Kabesch, M.; Peters, W.; Carr, D.; Leupold, W.; Weiland, S.K.; von Mutius, E. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J. Allergy Clin. Immunol. 2003, 111, 813–817. [Google Scholar] [CrossRef]
- Ferreira, M.A.R.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; Lu, Y.; et al. Age-of-onset information helps identify 76 genetic variants associated with allergic disease. PLoS Genet. 2020, 16, e1008725. [Google Scholar] [CrossRef] [PubMed]
- Yahia, S.A.; Azzaoui, I.; Everaere, L.; Vorng, H.; Chenivesse, C.; Marquillies, P.; Duez, C.; Delacre, M.; Grandjean, T.; Balsamelli, J.; et al. CCL17 Production by Dendritic Cells Is Required for NOD1-mediated Exacerbation of Allergic Asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Bouté, M.; Yahia, S.A.; Nanou, J.; Alvarez-Simon, D.; Audousset, C.; Vorng, H.; Balsamelli, J.; Ying, F.; Marquillies, P.; Werkmeister, E.; et al. Direct activation of the aryl hydrocarbon receptor by dog allergen participates in airway neutrophilic inflammation. Allergy 2021, 76, 2245–2249. [Google Scholar] [CrossRef]
- Tschernig, T.; Pabst, R.; Prenzler, F.; Rittinghausen, S.; Braun, A.; Hohlfeld, J.M. Isolated aggregates of lymphoid cells in the inner bronchial wall in asthma patients. Cell Tissue Res. 2018, 374, 423–425. [Google Scholar] [CrossRef]
- Heier, I.; Malmstrom, K.; Pelkonen, A.S.; Malmberg, L.P.; Kajosaari, M.; Turpeinen, M.; Lindahl, H.; Brandtzaeg, P.; Jahnsen, F.L.; Makela, M.J. Bronchial response pattern of antigen presenting cells and regulatory T cells in children less than 2 years of age. Thorax 2008, 63, 703–709. [Google Scholar] [CrossRef][Green Version]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.J.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Wong, C.K.; Hu, S.; Leung, K.M.-L.; Dong, J.; He, L.; Chu, Y.J.; Chu, I.M.-T.; Qiu, H.-N.; Liu, K.Y.-P.; Lam, C.W.-K. NOD-like receptors mediated activation of eosinophils interacting with bronchial epithelial cells: A link between innate immunity and allergic asthma. Cell. Mol. Immunol. 2013, 10, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Mehta, A.K.; Magalhaes, J.G.; Ziegler, S.F.; Dong, C.; Philpott, D.J.; Croft, M. Innate signals from Nod2 block respiratory tolerance and program TH2-driven allergic inflammation. J. Allergy Clin. Immunol. 2010, 126, 1284–1293.e10. [Google Scholar] [CrossRef]
- Yahia, S.A.; Audousset, C.; Alvarez-Simon, D.; Vorng, H.; Togbe, D.; Marquillies, P.; Delacre, M.; Rose, S.; Bouscayrol, H.; Rifflet, A.; et al. NOD1 sensing of house dust mite–derived microbiota promotes allergic experimental asthma. J. Allergy Clin. Immunol. 2021, 148, 394–406. [Google Scholar] [CrossRef]
- Shu, H.-J.; Takeda, H.; Shinzawa, H.; Takahashi, T.; Kawata, S. Effect of Lipopolysaccharide on Peptide Transporter 1 Expression in Rat Small Intestine and Its Attenuation by Dexamethasone. Digestion 2002, 65, 21–29. [Google Scholar] [CrossRef]
- Ni, G.; Chen, Y.; Wu, F.; Zhu, P.; Song, L. NOD2 promotes cell proliferation and inflammatory response by mediating expression of TSLP in human airway smooth muscle cells. Cell. Immunol. 2017, 312, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.-G.; Seo, S.-U.; Kim, D.-J.; Kamada, N.; Prescott, D.; Philpott, D.J.; Rosenstiel, P.; Inohara, N.; Núñez, G.; et al. Erratum: Corrigendum: Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 2016, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- Stamatos, N.M.; Carubelli, I.; van de Vlekkert, D.; Bonten, E.J.; Papini, N.; Feng, C.; Venerando, B.; D’azzo, A.; Cross, A.S.; Wang, L.-X.; et al. LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J. Leukoc. Biol. 2010, 88, 1227–1239. [Google Scholar] [CrossRef]
- Fritz, J.H.; Girardin, S.E.; Fitting, C.; Werts, C.; Mengin-Lecreulx, D.; Caroff, M.; Cavaillon, J.-M.; Philpott, D.J.; Adib-Conquy, M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 2005, 35, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Aiba, S.; Shibata, K.-I.; Ohteki, T.; Takada, H. Synergistic Effect of Nod1 and Nod2 Agonists with Toll-Like Receptor Agonists on Human Dendritic Cells To Generate Interleukin-12 and T Helper Type 1 Cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef]
- Magalhaes, J.G.; Fritz, J.H.; Le Bourhis, L.; Sellge, G.; Travassos, L.; Selvanantham, T.; Girardin, S.E.; Gommerman, J.; Philpott, D.J. Nod2-Dependent Th2 Polarization of Antigen-Specific Immunity. J. Immunol. 2008, 181, 7925–7935. [Google Scholar] [CrossRef]
- Volpe, E.; Servant, N.; Zollinger, R.; I Bogiatzi, S.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef]
- Dong, C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008, 8, 337–348. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.-H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef]
- Pavot, V.; Rochereau, N.; Rességuier, J.; Gutjahr, A.; Genin, C.; Tiraby, G.; Perouzel, E.; Lioux, T.; Vernejoul, F.; Verrier, B.; et al. Cutting Edge: New Chimeric NOD2/TLR2 Adjuvant Drastically Increases Vaccine Immunogenicity. J. Immunol. 2014, 193, 5781–5785. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Posselt, G.; Wurm, P.; Ulbing, M.; Duschl, A.; Horejs-Hoeck, J. TLR8 and NOD signaling synergistically induce the production of IL-1β and IL-23 in monocyte-derived DCs and enhance the expression of the feedback inhibitor SOCS2. Immunobiology 2013, 218, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iijima, K.; Checkel, J.L.; Kita, H. IL-1 Family Cytokines Drive Th2 and Th17 Cells to Innocuous Airborne Antigens. Am. J. Respir. Cell Mol. Biol. 2013, 49, 989–998. [Google Scholar] [CrossRef]
- Caucheteux, S.M.; Hu-Li, J.; Guo, L.; Bhattacharyya, N.; Crank, M.; Collins, M.T.; Paul, W.E. IL-1β enhances inflammatory T H 2 differentiation. J. Allergy Clin. Immunol. 2016, 138, 898–901.e4. [Google Scholar] [CrossRef] [PubMed]
- Paulissen, G.; Rocks, N.; Guéders, M.M.; Bedoret, D.; Crahay, C.; Quesada-Calvo, F.; Hacha, J.; Bekaert, S.; Desmet, C.; Foidart, J.-M.; et al. ADAM-8, a metalloproteinase, drives acute allergen-induced airway inflammation. Eur. J. Immunol. 2010, 41, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of Inflammation and Airway Hyperresponsiveness in Immune-sensitized Mice by Dominant-Negative Phosphoinositide 3-Kinase–TAT. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Teeters, K.; Miyasato, S.; Choi, J.; Nakamatsu, G.; Richardson, J.A.; Starcher, B.; Davis, E.C.; Tam, E.K.; Saux, C.J.-L. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L1007–L1017. [Google Scholar] [CrossRef]
- Randall, T.D. Bronchus-Associated Lymphoid Tissue (BALT) structure and function. Adv. Immunol. 2010, 107, 187–241. [Google Scholar] [CrossRef]
- Fleige, H.; Ravens, S.; Moschovakis, G.L.; Bölter, J.; Willenzon, S.; Sutter, G.; Häussler, S.; Kalinke, U.; Prinz, I.; Förster, R. IL-17–induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J. Exp. Med. 2014, 211, 643–651. [Google Scholar] [CrossRef]
- Azzaoui, I.; Yahia, S.A.; Chang, Y.; Vorng, H.; Morales, O.; Fan, Y.; Delhem, N.; Ple, C.; Tonnel, A.-B.; Wallaert, B.; et al. CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood 2011, 118, 3549–3558. [Google Scholar] [CrossRef]
- Chenivesse, C.; Chang, Y.; Azzaoui, I.; Yahia, S.A.; Morales, O.; Plé, C.; Foussat, A.; Tonnel, A.-B.; Delhem, N.; Yssel, H.; et al. Pulmonary CCL18 Recruits Human Regulatory T Cells. J. Immunol. 2012, 189, 128–137. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Catalog Number | Provider | Quantity (µg) |
|---|---|---|---|
| TCRγδ PE-Cy7 | 25-5711-82 | Invitrogen | 0.3 |
| CD3 Alexa Fluor 700 | 561388 | BD | 0.3 |
| CD4 Brilliant Violet 605 | 100547 | Biolegend | 0.25 |
| CD45 APC Cy7 | 557659 | BD | 0.3 |
| IL-17A Brilliant Violet 421 | 506925 | Biolegend | 0.25 |
| Sex (F/M) | 8/2 |
| Age (y) | 37.42 ± 3.36 |
| FEV1 (% predicted) | 88.13 ± 3.16 |
| FEV1/FVC ratio (%) | 74.14 ± 3.27 |
| Specific IgE (KU/L) | 10.33 ± 4.74 |
| μg Inhaled CS (equivalent beclometasone dipropionate) | 614.6 ± 215 |
| Smokers (n) | 2/10 |
| BMI above 25 (n) | 1/10 |
| ACT ≥ 20 (%) | 80 |
| Intermittent (n) | 3/10 |
| Moderate (n) | 5/10 |
| Severe (n) | 2/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouté, M.; Ait Yahia, S.; Fan, Y.; Alvarez-Simon, D.; Vorng, H.; Balsamelli, J.; Nanou, J.; de Nadai, P.; Chenivesse, C.; Tsicopoulos, A. NOD2 Signaling Circuitry during Allergen Sensitization Does Not Worsen Experimental Neutrophilic Asthma but Promotes a Th2/Th17 Profile in Asthma Patients but Not Healthy Subjects. Int. J. Mol. Sci. 2022, 23, 11894. https://doi.org/10.3390/ijms231911894
Bouté M, Ait Yahia S, Fan Y, Alvarez-Simon D, Vorng H, Balsamelli J, Nanou J, de Nadai P, Chenivesse C, Tsicopoulos A. NOD2 Signaling Circuitry during Allergen Sensitization Does Not Worsen Experimental Neutrophilic Asthma but Promotes a Th2/Th17 Profile in Asthma Patients but Not Healthy Subjects. International Journal of Molecular Sciences. 2022; 23(19):11894. https://doi.org/10.3390/ijms231911894
Chicago/Turabian StyleBouté, Mélodie, Saliha Ait Yahia, Ying Fan, Daniel Alvarez-Simon, Han Vorng, Joanne Balsamelli, Julie Nanou, Patricia de Nadai, Cécile Chenivesse, and Anne Tsicopoulos. 2022. "NOD2 Signaling Circuitry during Allergen Sensitization Does Not Worsen Experimental Neutrophilic Asthma but Promotes a Th2/Th17 Profile in Asthma Patients but Not Healthy Subjects" International Journal of Molecular Sciences 23, no. 19: 11894. https://doi.org/10.3390/ijms231911894
APA StyleBouté, M., Ait Yahia, S., Fan, Y., Alvarez-Simon, D., Vorng, H., Balsamelli, J., Nanou, J., de Nadai, P., Chenivesse, C., & Tsicopoulos, A. (2022). NOD2 Signaling Circuitry during Allergen Sensitization Does Not Worsen Experimental Neutrophilic Asthma but Promotes a Th2/Th17 Profile in Asthma Patients but Not Healthy Subjects. International Journal of Molecular Sciences, 23(19), 11894. https://doi.org/10.3390/ijms231911894






