Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn?
Abstract
1. Introduction
2. EDCs Mechanisms of Action
- direct activation of the classical NHRs—EDCs, having similar structure to natural hormones, can enter the cell where some NHRs are kept in an inactive state. Upon EDCs binding, NHRs monomers dimerize and then bind to NHR response elements (NREs), interacting with DNA sequences. The activated dimer can act either as an activator or repressor of gene transcription according to coAct or coRe recruitment to the target gene, respectively
- disturbance of NHRs signaling—EDCs can affect receptor function by interfering with:
- receptor degradation—NHRs degradation may be regulated by the ubiquitin (Ub)–proteasome pathway that also seems to depend on the AhR. The liganded AhR-ARNT heterodimer can represses the hormone-mediated transcription by targeting NHRs to the Ub-ligase complex, promoting the decrease in some NHRs levels, such as ER and AR
- coAct recruitment—the activity of both the NHRs and the AhR depends on transcriptional coAct. Competition between NHRs and AhR for common coAct is a plausible mechanism by which AhR ligands may disturb NHRs signaling
- DNA-binding competition—the liganded AhR-ARNT heterodimer binds to sequences close to unliganded NHRs binding sites, called inhibitory (i)XREs having slightly different composition than XREs. In this way, the activated AhR can bind but cannot activate gene transcription
- dysregulation of hormone metabolism—enzymes induced by activated AhR are involved in metabolism of xenobiotics but also in the catabolism of steroid hormones. AhR can regulate the levels of circulating estradiol (E2) by controlling the gene expression of CYP involved in estrogen production from cholesterol. Many EDCs can interfere with the enzyme aromatase (CYP19), which converts androgens to 17β-E2 by demethylation and has been shown to be a direct AhR target gene. Thus, activation of AhR by EDCs can lead to increased degradation of steroid hormones and higher E2 production as well.
3. EDCs Assessment Methods
4. The Most Common EDCs
4.1. Plastics and Plasticizers
4.1.1. Bisphenols
4.1.2. Phthalates
4.2. Dioxins and Dioxin-Like Polychlorinated Biphenyls
4.3. Pesticides
4.4. Perfluoroalkyl and Polyfluoroalkyl Substances
4.5. Flame Retardants
4.6. Other EDCs
5. EDCs and Endocrine Diseases—Evidence in Humans
5.1. Pre- and Post-Natal Growth
5.1.1. EDCs Mixtures
5.1.2. Bisphenols
5.1.3. Phthalates
5.1.4. Pesticides
5.1.5. Perfluoroalkyl and Polyfluoroalkyl Substances
5.1.6. Brominated Flame Retardants
5.1.7. Arsenic
5.2. Pubertal Development
5.2.1. Bisphenol A
5.2.2. Phthalates
5.2.3. Dioxins
5.2.4. Pesticides
5.2.5. Perfluoroalkyl and Polyfluoroalkyl Substances
5.2.6. Brominated Flame Retardants
5.3. Male Reproductive System
5.4. Thyroid Function
5.5. Metabolic Diseases
6. What We Know
7. What We Need to Learn
8. Materials and Methods
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Iughetti, L.; Lucaccioni, L.; Predieri, B. Childhood Obesity and Environmental Pollutants: A dual Relationship. Acta Biomed. 2015, 86, 5–16. [Google Scholar]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef]
- Iughetti, L.; Lucaccioni, L.; Street, M.E.; Bernasconi, S. Clinical Expression of Endocrine Disruptors in Children. Curr. Opin. Pediatr. 2020, 32, 554–559. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Marrozzini, L.; Bertoncelli, N.; Predieri, B.; Lugli, L.; Berardi, A.; Iughetti, L. Endocrine-Disrupting Chemicals and Their Effects during Female Puberty: A Review of Current Evidence. Int. J. Mol. Sci. 2020, 21, 2078. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C.; Predieri, B.; Iughetti, L. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef]
- Predieri, B.; Alves, C.A.D.; Iughetti, L. New Insights on the Effects of Endocrine-Disrupting Chemicals on Children. J. Pediatr. 2022, 98, S73–S85. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP); The World Health Organization (WHO). The State of the Science of Endocrine Disrupting Chemicals—2012; Bergman, Å., Heindel, J.J., Jobling, S., Kidd, K.A., Zoeller, R.T., Eds.; UNEP/WHO: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/state-of-the-science-of-endocrine-disrupting-chemicals (accessed on 30 April 2022).
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Collet, S.H.; Picard-Hagen, N.; Lacroix, M.Z.; Puel, S.; Viguié, C.; Bousquet-Melou, A.; Toutain, P.L.; Gayrard, V. Allometric Scaling for Predicting Human Clearance of Bisphenol A. Toxicol. Appl. Pharmacol. 2015, 284, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of Bisphenol A in Humans Following a Single Oral Administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An Introduction to the Sources, Fate, Occurrence and Effects of Endocrine Disrupting Chemicals Released into the Environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Antony, S.; Rebello, S.; George, S.; Biju, D.T.; Reshhmy, R.; Madhavan, A.; Binod, P.; Pandey, A.; Sindhu, R.; et al. Bioremediation of Endocrine Disrupting Chemicals—Advancements and Challenges. Environ. Res. 2022, 213, 113509. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT Metabolite p,p’-DDE is a Potent Androgen Receptor Antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid Hormone Action is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.; Park, K. Effects of Consumer Products Chemicals Ingredients and their Mixtures on the Estrogen Receptor/Androgen Receptor Transcriptional Activation. Chemosphere 2022, 302, 134866. [Google Scholar] [CrossRef]
- Messerlian, C.; Martinez, R.M.; Hauser, R.; Baccarelli, A.A. ‘Omics’ and Endocrine-Disrupting Chemicals—New Paths Forward. Nat. Rev. Endocrinol. 2017, 13, 740–748. [Google Scholar] [CrossRef]
- Rhomberg, L.R.; Goodman, J.E. Low-Dose Effects and Nonmonotonic Dose-Responses of Endocrine Disrupting Chemicals: Has the Case Been Made? Regulat. Toxicol. Pharmacol. 2012, 64, 130–133. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Ho, V.; Pelland-St-Pierre, L.; Gravel, S.; Bouchard, M.F.; Verner, M.A.; Labrèche, F. Endocrine Disruptors: Challenges and Future Directions in Epidemiologic Research. Environ. Res. 2022, 204, 111969. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of Endocrine Disrupters from the Contaminated Environment: Public Health Concerns, Treatment Strategies and Future Perspectives—A Review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef] [PubMed]
- European Cluster to Improve Identification of Endocrine Disruptors (EURION). New Testing and Screening Methods to Identify Endocrine Disrupting Chemicals (EDCs). Available online: https://eurion-cluster.eu/ (accessed on 30 August 2022).
- Organization of Economic Cooperation & Development (OECD). Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OECD Series on Testing and Assessment, No. 150; OECD Publishing: Paris, France, 2018; Available online: https://doi.org/10.1787/9789264304741-en (accessed on 30 August 2022).
- Schneider, M.; Pons, J.L.; Labesse, G.; Bourguet, W. In Silico Predictions of Endocrine Disruptors Properties. Endocrinology 2019, 160, 2709–2716. [Google Scholar] [CrossRef]
- Swedenborg, E.; Rüegg, J.; Mäkelä, S.; Pongratz, I. Endocrine Disruptive Chemicals: Mechanisms of Action and Involvement in Metabolic Disorders. J. Mol. Endocrinol. 2009, 43, 1–10. [Google Scholar] [CrossRef]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The Effects of Phthalate Ester Exposure on Human Health: A Review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Häussler, G.; Vogel, M. Endocrine-Disrupting Chemicals and Child Health. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101516. [Google Scholar] [CrossRef]
- Gingrich, J.; Ticiani, E.; Veiga-Lopez, A. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol. Metab. 2020, 31, 508–524. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal-Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, L.; Buerki-Thurnherr, T.; Pastuschek, J.; Aengenheister, L.; Knudsen, L.E. Fetal Exposure to Environmental Chemicals; Insights from Placental Perfusion Studies. Placenta 2021, 106, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Plante, I.; Winn, L.M.; Vaillancourt, C.; Grigorova, P.; Parent, L. Killing Two Birds with One Stone: Pregnancy is a Sensitive Window for Endocrine Effects on both the Mother and the Fetus. Environ. Res. 2022, 205, 112435. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, L.; McCaffery, H.; Chernyak, S.; Lam, S.; Sargis, R.M.; Padmanabhan, V.; Gregg, B. Lactational Exposure to Polychlorinated Biphenyls is Higher in Overweight/Obese Women and Associated with Altered Infant Growth Trajectory: A Pilot Study. Curr. Res. Toxicol. 2020, 1, 133–140. [Google Scholar] [CrossRef]
- Iribarne-Durán, L.M.; Peinado, F.M.; Freire, C.; Castillero-Rosales, I.; Artacho-Cordón, F.; Olea, N. Concentrations of Bisphenols, Parabens, and Benzophenones in Human Breast Milk: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2022, 806, 150437. [Google Scholar] [CrossRef]
- Barker, D.J. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vom Saal, F.S.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma Consensus Statement on Metabolic Disruptors. Environ. Health 2015, 14, 54. [Google Scholar] [CrossRef]
- Barton-Maclaren, T.S.; Wade, M.; Basu, N.; Bayen, S.; Grundy, J.; Marlatt, V.; Moore, R.; Parent, L.; Parrott, J.; Grigorova, P.; et al. Innovation in Regulatory Approaches for Endocrine Disrupting Chemicals: The Journey to Risk Assessment Modernization in Canada. Environ. Res. 2022, 204, 112225. [Google Scholar] [CrossRef]
- Commission of the European Communities. Community Strategy for Endocrine Disrupters. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:1999:0706:FIN:EN:PDF (accessed on 6 September 2022).
- EU Commission. Environment. Available online: https://ec.europa.eu/environment/chemicals/index_en.htm (accessed on 6 September 2022).
- US Environmental Protection Agency (EPA). Endocrine Disruptors. Available online: https://www.epa.gov/endocrine-disruption (accessed on 6 September 2022).
- Wong, K.H.; Durrani, T.S. Exposures to Endocrine Disrupting Chemicals in Consumer Products—A Guide for Pediatricians. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 107–118. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine Disrupting Chemicals and Disease Susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef]
- Lee, H.R.; Jeung, E.B.; Cho, M.H.; Kim, T.H.; Leung, P.C.; Choi, K.C. Molecular Mechanism(s) of Endocrine-Disrupting Chemicals and their Potent Oestrogenicity in Diverse Cells and Tissues that Express Oestrogen Receptors. J. Cell Mol. Med. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Toporova, L.; Balaguer, P. Nuclear Receptors are the Major Targets of Endocrine Disrupting Chemicals. Mol. Cell Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Doan, T.q.; Connolly, L.; Igout, A.; Nott, K.; Muller, M.; Scippo, M.I. In vitro Profiling of the Potential Endocrine Disrupting Activities Affecting Steroid and Aryl Hydrocarbon Receptors of Compounds and Mixtures Prevalent in Human Drinking Water Resources. Chemosphere 2020, 258, 127332. [Google Scholar] [CrossRef]
- Ohtake, F.; Fujii-Kuriyama, Y.; Kawajiri, K.; Kato, S. Cross-Talk of Dioxin and Estrogen Receptor Signals Through the Ubiquitin System. J. Steroid Biochem. Mol. Biol. 2011, 127, 102–107. [Google Scholar] [CrossRef]
- Denison, M.S.; Faber, S.C. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr. Opin. Toxicol. 2017, 2, 124–131. [Google Scholar] [CrossRef]
- Tarnow, P.; Tralau, T.; Luch, A. Chemical Activation of Estrogen and Aryl Hydrocarbon Receptor Signaling Pathways and their Interaction in Toxicology and Metabolism. Expert Opin. Drug Metab. Toxicol. 2019, 15, 219–229. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Martínez, R.; Navarro-Martín, L.; Kamstra, J.H.; Schwendt, A.; Reynaud, S.; Chalifour, L. Emerging Concepts and Opportunities for Endocrine Disruptor Screening of the Non-EATS Modalities. Environ. Res. 2022, 204, 111904. [Google Scholar] [CrossRef]
- Walker, C.L. Minireview: Epigenomic Plasticity and Vulnerability to EDC Exposures. Mol. Endocrinol. 2016, 30, 848–855. [Google Scholar] [CrossRef]
- Crews, D.; McLachlan, J.A. Epigenetics, Evolution, Endocrine Disruption, Health, and Disease. Endocrinology 2006, 147, S4–S10. [Google Scholar] [CrossRef]
- Skinner, M.K. What is an Epigenetic Transgenerational Phenotype? F3 or F2. Reprod. Toxicol. 2008, 25, 2–6. [Google Scholar] [CrossRef]
- Gomes, J.M.; Almeida, T.F.A.; da Silva, T.A.; de Lourdes Cardeal, Z.; Menezes, H.C. Saliva Biomonitoring Using LPME-GC/MS Method to Assess Dentistry Exposure to Plasticizers. Anal. Bioanal. Chem. 2020, 412, 7799–7810. [Google Scholar] [CrossRef]
- Abafe, O.A.; Macheka, L.R.; Olowoyo, J.O. Confirmatory Analysis of Per and Polyfluoroalkyl Substances in Milk and Infant Formula Using UHPLC-MS/MS. Molecules 2021, 26, 3664. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, Y.; An, Q.; Yang, X.; Yin, S.; Ma, L.Q.; Liu, W. Prenatal and Postnatal Exposure to Emerging and Legacy Per-/Polyfluoroalkyl Substances: Levels and Transfer in Maternal Serum, Cord Serum, and Breast Lilk. Sci. Total Environ. 2022, 812, 152446. [Google Scholar] [CrossRef]
- Bianchi, F.; Mattarozzi, M.; Betti, P.; Bisceglie, F.; Careri, M.; Mangia, A.; Sidisky, L.; Ongarato, S.; Dalcanale, E. Innovative Cavitand-Based Sol-Gel Coatings for the Environmental Monitoring of Benzene and Chlorobenzenes Via Solid-Phase Microextraction. Anal. Chem. 2008, 80, 6423–6430. [Google Scholar] [CrossRef]
- Riboni, N.; Fornari, F.; Bianchi, F.; Careri, M. A Simple and Efficient Solid-Phase Microextraction—Gas Chromatography—Mass Spectrometry Method for the Determination of Fragrance Materials at Ultra-Trace Levels in Water Samples Using Multi-Walled Carbon Nanotubes as Innovative Coating. Talanta 2021, 224, 121891. [Google Scholar] [CrossRef]
- Amorini, M.; Riboni, N.; Pesenti, L.; Dini, V.A.; Pedrini, A.; Massera, C.; Gualandi, C.; Bianchi, F.; Pinalli, R.; Dalcanale, E. Reusable Cavitand-Based Electrospun Membranes for the Removal of Polycyclic Aromatic Hydrocarbons from Water. Small 2022, 18, e2104946. [Google Scholar] [CrossRef]
- Dey, A.K.; Kumar, B.; Singh, A.K.; Ranjan, P.; Thiruvengadam, R.; Desiraju, B.K.; Kshetrapal, P.; Wadhwa, N.; Bhatnagar, S.; Rashid, F.; et al. Salivary Proteome Signatures in the Early and Middle Stages of Human Pregnancy with Term Birth Outcome. Sci. Rep. 2020, 10, 8022. [Google Scholar] [CrossRef] [PubMed]
- LaBarre, J.L.; Puttabyatappa, M.; Song, P.X.K.; Goodrich, J.M.; Zhou, L.; Rajendiran, T.M.; Soni, T.; Domino, S.E.; Treadwell, M.C.; Dolinoy, D.C.; et al. Maternal Lipid Levels across Pregnancy Impact the Umbilical Cord Blood Lipidome and Infant Birth Weight. Sci. Rep. 2020, 10, 14209. [Google Scholar] [CrossRef]
- Jeon, B.K.; Jang, Y.; Lee, E.M.; Jung, D.W.; Moon, J.H.; Lee, H.J.; Lee, D.Y. A Systematic Approach to Metabolic Characterization of Thyroid-Disrupting Chemicals and their In Vitro Biotransformants based on Prediction-Assisted Metabolomic Analysis. J. Chromatogr. A 2021, 1649, 462222. [Google Scholar] [CrossRef]
- Audouze, K.; Sarigiannis, D.; Alonso-Magdalena, P.; Brochot, C.; Casas, M.; Vrijheid, M.; Babin, P.J.; Karakitsios, S.; Coumoul, X.; Barouki, R. Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project. Int. J. Mol. Sci. 2020, 21, 2988. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Transcriptomics and Other Omics Approaches to Investigate Effects of Xenobiotics on the Placenta. Front. Cell Dev. Biol. 2021, 9, 723656. [Google Scholar] [CrossRef] [PubMed]
- Günther, K.; Räcker, T.; Böhme, R. An Isomer-Specific Approach to Endocrine-Disrupting Nonylphenol in Infant Food. J. Agric. Food Chem. 2017, 65, 1247–1254. [Google Scholar] [CrossRef]
- Ringbeck, B.; Bury, D.; Hayen, H.; Weiss, T.; Brüning, T.; Koch, H.M. Determination of Specific Urinary Nonylphenol Metabolites by Online-SPE-LC-MS/MS as Novel Human Exposure Biomarkers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1177, 122794. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S. Epigenetic Effects of Environmental Chemicals Bisphenol A and Phthalates. Int. J. Mol. Sci. 2012, 13, 10143–10153. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 10/2011. Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R0010 (accessed on 30 April 2022).
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and Cell Signaling Pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Jørgensen, N.; Andersson, A.M.; Calafat, A.M.; Ye, X.; Redmon, J.B.; Drobnis, E.Z.; Wang, C.; Sparks, A.; Thurston, S.W.; et al. Are environmental Levels of Bisphenol A Associated with Reproductive Function in Fertile Men? Environ. Health Perspect. 2010, 118, 1286–1291. [Google Scholar] [CrossRef]
- Zhou, Q.; Miao, M.; Ran, M.; Ding, L.; Bai, L.; Wu, T.; Yuan, W.; Gao, E.; Wang, J.; Li, G.; et al. Serum Bisphenol-A Concentration and Sex Hormone Levels in Men. Fertil. Steril. 2013, 100, 478–482. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y. Involvement of Suppressor for Gal 1 in the Ubiquitin/Proteasome-Mediated Degradation of Estrogen Receptors. J. Biol. Chem. 2004, 279, 12020–12026. [Google Scholar] [CrossRef]
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.A.; Quesada, I.; Nadal, A. Rapid Insulinotropic Action of Low Doses of Bisphenol-A on Mouse and Human Islets of Langerhans: Role of Estrogen Receptor β. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquié, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic Insulin Content Regulation by the Estrogen Receptor ER alpha. PLoS ONE 2008, 3, e2069. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/2005. Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis (2-ethylhexyl) Phthalate (DEHP), Dibutyl Phthalate (DBP), Benzyl Butyl Phthalate (BBP) and Diisobutyl Phthalate (DIBP). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R2005 (accessed on 30 April 2022).
- EFSA Panel on Food Contact Materials; Enzymes and Processing Aids (CEP); Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; et al. Update of the Risk Assessment of Di-Butylphthalate (DBP), Butyl-benzyl-phthalate (BBP), Bis(2-ethylhexyl)phthalate (DEHP), Di-isononylphthalate (DINP) and Di-isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA J. 2019, 17, e05838. [Google Scholar] [CrossRef]
- Hammel, S.C.; Levasseur, J.L.; Hoffman, K.; Phillips, A.L.; Lorenzo, A.M.; Calafat, A.M.; Webster, T.F.; Stapleton, H.M. Children’s Exposure to Phthalates and Non-Phthalate Plasticizers in the Home: The TESIE Study. Environ. Int. 2019, 132, 105061. [Google Scholar] [CrossRef]
- Stroheker, T.; Cabaton, N.; Nourdin, G.; Régnier, J.F.; Lhuguenot, J.C.; Chagnon, M.C. Evaluation of Anti-Androgenic Activity of Di-(2-ethylhexyl)phthalate. Toxicology 2005, 208, 115–121. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. In Utero Exposure to Di-(2-ethylhexyl) Phthalate Decreases Mineralocorticoid Receptor Expression in the Adult Testis. Endocrinology 2009, 150, 5575–5585. [Google Scholar] [CrossRef]
- Wójtowicz, A.K.; Sitarz-Głownia, A.M.; Szczęsna, M.; Szychowski, K.A. The Action of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon Receptor (AhR) Signaling. Neurotoxic. Res. 2019, 35, 183–195. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Ding, S.; Qi, W.; Zhang, Y.; Xu, Q.; Zhao, T.; Zhang, X.; Li, X.; Wu, F.; et al. The Associations of Urinary DEHP Metabolite Levels, Serum Thyroid Hormones, and Thyroid-Related Genes Among the Adolescent Students from China: A Cross-Sectional Study. Environ. Sci. Pollut. Res. Int. 2022, 29, 19081–19097. [Google Scholar] [CrossRef] [PubMed]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef]
- La Merrill, M.; Emond, C.; Kim, M.J.; Antignac, J.P.; Le Bizec, B.; Clément, K.; Birnbaum, L.S.; Barouki, R. Toxicological Function of Adipose Tissue: Focus on Persistent Organic Pollutants. Environ. Health Perspect. 2013, 121, 162–169. [Google Scholar] [CrossRef] [PubMed]
- You, S.H.; Gauger, K.J.; Bansal, R.; Zoeller, R.T. 4-Hydroxy-PCB106 Acts as a Direct Thyroid Hormone Receptor Agonist in Rat GH3 Cells. Mol. Cell Endocrinol. 2006, 257–258, 26–34. [Google Scholar] [CrossRef]
- Legler, J.; Zeinstra, L.M.; Schuitemaker, F.; Lanser, P.H.; Bogerd, J.; Brouwer, A.; Vethaak, A.D.; De Voogt, P.; Murk, A.J.; Van der Burg, B. Comparison of In Vivo and In Vitro Reporter Gene Assays for Short-Term Screening of Estrogenic Activity. Environ. Sci. Technol. 2002, 36, 4410–4415. [Google Scholar] [CrossRef][Green Version]
- Munier, M.; Grouleff, J.; Gourdin, L.; Fauchard, M.; Chantreau, V.; Henrion, D.; Coutant, R.; Schiøtt, B.; Chabbert, M.; Rodien, P. In Vitro Effects of the Endocrine Disruptor p,p’-DDT on Human Follitropin Receptor. Environ. Health Perspect. 2016, 124, 991–999. [Google Scholar] [CrossRef]
- Picchietti, S.; Belardinelli, M.; Taddei, A.R.; Fausto, A.M.; Pellegrino, M.; Maggio, R.; Rossi, M.; Giorgi, F. Thyroid Disruptor 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) Prevents Internalization of TSH Receptor. Cell Tissue Res. 2009, 336, 31–40. [Google Scholar] [CrossRef]
- Schrader, T.J.; Cooke, G.M. Examination of Selected Food Additives and Organochlorine Food Contaminants for Androgenic Activity In Vitro. Toxicol. Sci. 2000, 53, 278–288. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Fu, Z. Oral Exposure to Atrazine Modulates Hormone Synthesis and the Transcription of Steroidogenic Genes in Male Peripubertal Mice. Gen. Comp. Endocrinol. 2013, 184, 120–127. [Google Scholar] [CrossRef]
- Caron-Beaudoin, É.; Viau, R.; Sanderson, J.T. Effects of Neonicotinoid Pesticides on Promoter-Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF Pathway. Environ. Health Perspect. 2018, 126, 047014. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Weerakoon, D.; Lim, E.; Padhye, L.P. Fate of Environmental Pollutants. Water Environ. Res. 2019, 91, 1294–1325. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, S.; Yu, C.; Cao, R.; Xu, Y.; Fu, L.; Wang, C. Integrated Assessment of Endocrine Disrupting Potential of Four Novel Brominated Flame Retardants. Ecotoxicol. Environ. Saf. 2022, 232, 113206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, X.; Li, D.; Zhao, J.; Zhou, R.; Shu, F.; Jia, W.; Fu, W.; Xia, H.; Liu, G. Prenatal Exposure to Environmentally Relevant Levels of PBDE-99 Leads to Testicular Dysgenesis with Steroidogenesis Disorders. J. Hazard. Mater. 2022, 424, 127547. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Gerónimo, C.A.; León Del Río, A.; Rodríguez-Dorantes, M.; Ostrosky-Wegman, P.; Salazar, A.M. Arsenic-Protein Interactions as a Mechanism of Arsenic Toxicity. Toxicol. Appl. Pharmacol. 2021, 431, 115738. [Google Scholar] [CrossRef] [PubMed]
- Cherry, N.; Moore, H.; McNamee, R.; Pacey, A.; Burgess, G.; Clyma, J.A.; Dippnall, M.; Baillie, H.; Povey, A.; participating centres of Chaps-UK. Occupation and Male Infertility: Glycol Ethers and Other Exposures. Occup. Environ. Med. 2008, 65, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Warembourg, C.; Botton, J.; Lelong, N.; Rouget, F.; Khoshnood, B.; Le Gléau, F.; Monfort, C.; Labat, L.; Pierre, F.; Heude, B.; et al. Prenatal Exposure to Glycol Ethers and Cryptorchidism and Hypospadias: A Nested Case-Control Study. Occup. Environ. Med. 2018, 75, 59–65. [Google Scholar] [CrossRef]
- Kelsey, J.R. Ethylene Oxide Derived Glycol Ethers: A Review of the Alkyl Glycol Ethers Potential to Cause Endocrine Disruption. Regul. Toxicol. Pharmacol. 2022, 129, 105113. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The Effects of Metals as Endocrine Disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Liu, W.; Liu, J. Potential Endocrine-Disrupting Effects of Metals via Interference with Glucocorticoid and Mineralocorticoid Receptors. Environ. Pollut. 2018, 242, 12–18. [Google Scholar] [CrossRef]
- Leff, T.; Stemmer, P.; Tyrrell, J.; Jog, R. Diabetes and Exposure to Environmental Lead (Pb). Toxics 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fu, X.; Zhang, J.; Xu, C.; Hu, Q.; Lin, W. Association Between Blood Lead Level During Pregnancy and Birth Weight: A Meta-Analysis. Am. J. Ind. Med. 2020, 63, 1085–1094. [Google Scholar] [CrossRef]
- Saavedra, S.; Fernández-Recamales, Á.; Sayago, A.; Cervera-Barajas, A.; González-Domínguez, R.; Gonzalez-Sanz, J.D. Impact of Dietary Mercury Intake During Pregnancy on the Health of Neonates and Children: A Systematic Review. Nutr. Rev. 2022, 80, 317–328. [Google Scholar] [CrossRef]
- Niziński, P.; Błażewicz, A.; Kończyk, J.; Michalski, R. Perchlorate—Properties, Toxicity and Human Health Effects: An Updated Review. Rev. Environ. Health 2020, 36, 199–222. [Google Scholar] [CrossRef]
- Acevedo-Barrios, R.; Olivero-Verbel, J. Perchlorate Contamination: Sources, Effects, and Technologies for Remediation. Rev. Environ. Contam. Toxicol. 2021, 256, 103–120. [Google Scholar] [CrossRef]
- Talia, C.; Connolly, L.; Fowler, P.A. The Insulin-Like Growth Factor System: A Target for Endocrine Disruptors? Environ. Int. 2021, 147, 106311. [Google Scholar] [CrossRef] [PubMed]
- Montrose, L.; Padmanabhan, V.; Goodrich, J.M.; Domino, S.E.; Treadwell, M.C.; Meeker, J.D.; Watkins, D.J.; Dolinoy, D.C. Maternal Levels of Endocrine Disrupting Chemicals in the First Trimester of Pregnancy are Associated with Infant Cord Blood DNA Methylation. Epigenetics 2018, 13, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Rager, J.E.; Bangma, J.; Carberry, C.; Chao, A.; Grossman, J.; Lu, K.; Manuck, T.A.; Sobus, J.R.; Szilagyi, J.; Fry, R.C. Review of the Environmental Prenatal Exposome and its Relationship to Maternal and Fetal Health. Reprod. Toxicol. 2020, 98, 1–12. [Google Scholar] [CrossRef]
- Broe, A.; Pottegård, A.; Hallas, J.; Ahern, T.P.; Lamont, R.F.; Damkier, P. Phthalate Exposure from Drugs during Pregnancy and Possible Risk of Preterm Birth and Small for Gestational Age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 293–299. [Google Scholar] [CrossRef]
- Tang, Z.R.; Xu, X.L.; Deng, S.L.; Lian, Z.X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef]
- Shafei, A.E.; Nabih, E.S.; Shehata, K.A.; Abd Elfatah, E.S.M.; Sanad, A.B.A.; Marey, M.Y.; Hammouda, A.A.M.A.; Mohammed, M.M.M.; Mostafa, R.; Ali, M.A. Prenatal Exposure to Endocrine Disruptors and Reprogramming of Adipogenesis: An Early-Life Risk Factor for Childhood Obesity. Child. Obes. 2018, 14, 18–25. [Google Scholar] [CrossRef]
- Birks, L.; Casas, M.; Garcia, A.M.; Alexander, J.; Barros, H.; Bergström, A.; Bonde, J.P.; Burdorf, A.; Costet, N.; Danileviciute, A.; et al. Occupational Exposure to Endocrine-Disrupting Chemicals and Birth Weight and Length of Gestation: A European Meta-Analysis. Environ. Health Perspect. 2016, 124, 1785–1793. [Google Scholar] [CrossRef]
- Bell, G.A.; Perkins, N.; Buck Louis, G.M.; Kannan, K.; Bell, E.M.; Gao, C.; Yeung, E.H. Exposure to Persistent Organic Pollutants and Birth Characteristics: The Upstate KIDS Study. Epidemiology 2019, 30, S94–S100. [Google Scholar] [CrossRef]
- Pearce, J.L.; Neelon, B.; Bloom, M.S.; Buckley, J.P.; Ananth, C.V.; Perera, F.; Vena, J.; Hunt, K.; Program Collaborators for Environmental Influences on Child Health Outcomes. Exploring Associations between Prenatal Exposure to Multiple Endocrine Disruptors and Birth Weight with Exposure Continuum Mapping. Environ. Res. 2021, 200, 111386. [Google Scholar] [CrossRef]
- Lenters, V.; Portengen, L.; Rignell-Hydbom, A.; Jönsson, B.A.; Lindh, C.H.; Piersma, A.H.; Toft, G.; Bonde, J.P.; Heederik, D.; Rylander, L.; et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ. Health Perspect. 2016, 124, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Krönke, A.A.; Jurkutat, A.; Schlingmann, M.; Poulain, T.; Nüchter, M.; Hilbert, A.; Kiviranta, H.; Körner, A.; Vogel, M.; Söder, O.; et al. Persistent Organic Pollutants in Pregnant Women Potentially Affect Child Development and Thyroid Hormone Status. Pediatr. Res. 2022, 91, 690–698. [Google Scholar] [CrossRef]
- Govarts, E.; Portengen, L.; Lambrechts, N.; Bruckers, L.; Den Hond, E.; Covaci, A.; Nelen, V.; Nawrot, T.S.; Loots, I.; Sioen, I.; et al. Early-Life Exposure to Multiple Persistent Organic Pollutants and Metals and Birth Weight: Pooled Analysis in Four Flemish Birth Cohorts. Environ. Int. 2020, 145, 106149. [Google Scholar] [CrossRef]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Sjödin, A.; Calafat, A.M.; Hartman, T.J. Prenatal Exposure to Mixtures of Persistent Endocrine Disrupting Chemicals and Postnatal Body Size in British Girls. Early Hum. Dev. 2021, 161, 105450. [Google Scholar] [CrossRef]
- Song, W.; Puttabyatappa, M.; Zeng, L.; Vazquez, D.; Pennathur, S.; Padmanabhan, V. Developmental Programming: Prenatal Bisphenol A Treatment Disrupts Mediators of Placental Function in Sheep. Chemosphere 2020, 243, 125301. [Google Scholar] [CrossRef]
- Zulkifli, S.; Rahman, A.A.; Kadir, S.H.S.A.; Nor, N.S.M. Bisphenol A and Its Effects on the Systemic Organs of Children. Eur. J. Pediatr. 2021, 180, 3111–3127. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Loukas, N.; Vrachnis, D.; Antonakopoulos, N.; Zygouris, D.; Kοlialexi, A.; Pergaliotis, V.; Iavazzo, C.; Mastorakos, G.; Iliodromiti, Z. A Systematic Review of Bisphenol A from Dietary and Non-Dietary Sources during Pregnancy and Its Possible Connection with Fetal Growth Restriction: Investigating Its Potential Effects and the Window of Fetal Vulnerability. Nutrients 2021, 13, 2426. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Lopez, A.; Kannan, K.; Liao, C.; Ye, W.; Domino, S.E.; Padmanabhan, V. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J. Clin. Endocrinol. Metab. 2015, 100, E1394–E1403. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Hong, Y.C.; Ha, M.; Kim, Y.; Park, H.; Kim, H.S.; Ha, E.H. Prenatal Bisphenol-A Exposure Affects Fetal Length Growth by Maternal Glutathione Transferase Polymorphisms, and Neonatal Exposure Affects Child Volume Growth by Sex: From Multiregional Prospective Birth Cohort MOCEH Study. Sci. Total Environ. 2018, 612, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, H.; Braun, J.M.; Zheng, T.; Zhang, B.; Xia, W.; Zhang, W.; Li, J.; Zhou, Y.; Li, H.; et al. Associations of Trimester-Specific Exposure to Bisphenols with Size at Birth: A Chinese Prenatal Cohort Study. Environ. Health Perspect. 2019, 127, 107001. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lin, B.G.; Zhou, B.; Cao, W.C.; Chen, P.P.; Deng, Y.L.; Hou, J.; Sun, S.Z.; Zheng, T.Z.; Lu, W.Q.; et al. Sex-Specific Associations of Prenatal Exposure to Bisphenol A and Its Alternatives with Fetal Growth Parameters and Gestational Age. Environ. Int. 2021, 146, 106305. [Google Scholar] [CrossRef] [PubMed]
- Sol, C.M.; van Zwol-Janssens, C.; Philips, E.M.; Asimakopoulos, A.G.; Martinez-Moral, M.P.; Kannan, K.; Jaddoe, V.W.V.; Trasande, L.; Santos, S. Maternal Bisphenol Urine Concentrations, Fetal Growth and Adverse Birth Outcomes: A Population-Based Prospective Cohort. Environ. Health 2021, 20, 60. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Tu, X.; Yuan, W.; Zhou, Z.; Jin, L.; Miao, M.; Li, D.K. Bisphenol A and Pubertal Height Growth in School-Aged Children. J. Expo Sci. Environ. Epidemiol. 2019, 29, 109–117. [Google Scholar] [CrossRef]
- Santos, S.; Sol, C.M.; van Zwol-Janssens, C.; Philips, E.M.; Asimakopoulos, A.G.; Martinez-Moral, M.P.; Kannan, K.; Jaddoe, V.W.V.; Trasande, L. Maternal Phthalate Urine Concentrations, Fetal growth and Adverse Birth Outcomes. A Population-Based Prospective Cohort Study. Environ. Int. 2021, 151, 106443. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Wei, Y.; Chen, J.; Kang, L.; Long, C.; Wu, S.; Shen, L.; Wei, G. Maternal Exposure to Endocrine Disrupting Chemicals (EDCs) and Preterm Birth: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Environ. Pollut. 2022, 292, 118264. [Google Scholar] [CrossRef]
- Chen, C.H.; Jiang, S.S.; Chang, I.S.; Wen, H.J.; Sun, C.W.; Wang, S.L. Association between Fetal Exposure to Phthalate Endocrine Disruptor and Genome-Wide DNA Methylation at Birth. Environ. Res. 2018, 162, 261–270. [Google Scholar] [CrossRef]
- Miura, R.; Ikeda-Araki, A.; Ishihara, T.; Miyake, K.; Miyashita, C.; Nakajima, T.; Kobayashi, S.; Ishizuka, M.; Kubota, T.; Kishi, R. Effect of Prenatal Exposure to Phthalates on Epigenome-Wide DNA Methylations in Cord Blood and Implications for Fetal Growth: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 2021, 783, 147035. [Google Scholar] [CrossRef]
- Li, J.; Qian, X.; Zhou, Y.; Li, Y.; Xu, S.; Xia, W.; Cai, Z. Trimester-Specific and Sex-Specific Effects of Prenatal Exposure to Di(2-ethylhexyl) Phthalate on Fetal Growth, Birth Size, and Early-Childhood Growth: A Longitudinal Prospective Cohort Study. Sci. Total Environ. 2021, 777, 146146. [Google Scholar] [CrossRef]
- Harley, K.G.; Berger, K.; Rauch, S.; Kogut, K.; Claus Henn, B.; Calafat, A.M.; Huen, K.; Eskenazi, B.; Holland, N. Association of Prenatal Urinary Phthalate Metabolite Concentrations and Childhood BMI and Obesity. Pediatr. Res. 2017, 82, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Berman, Y.E.; Doherty, D.A.; Main, K.M.; Frederiksen, H.; Hickey, M.; Keelan, J.A.; Newnham, J.P.; Hart, R.J. Associations between Prenatal Exposure to Phthalates and Timing of Menarche and Growth and Adiposity into Adulthood: A Twenty-Years Birth Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4725. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, H.M.; Lee, J.Y.; Min, K.B.; Shin, C.H.; Lee, Y.A.; Hong, Y.C. Prenatal Exposure to Phthalate and Decreased Body Mass Index of Children: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 8961. [Google Scholar] [CrossRef] [PubMed]
- Gemmill, A.; Gunier, R.B.; Bradman, A.; Eskenazi, B.; Harley, K.G. Residential Proximity to Methyl Bromide Use and Birth Outcomes in an Agricultural Population in California. Environ. Health Perspect. 2013, 121, 737–743. [Google Scholar] [CrossRef]
- Migeot, V.; Albouy-Llaty, M.; Carles, C.; Limousi, F.; Strezlec, S.; Dupuis, A.; Rabouan, S. Drinking-Water Exposure to a Mixture of Nitrate and Low-Dose Atrazine Metabolites and Small-For-Gestational age (SGA) Babies: A Historic Cohort Study. Environ. Res. 2013, 122, 58–64. [Google Scholar] [CrossRef]
- Béranger, R.; Hardy, E.M.; Binter, A.C.; Charles, M.A.; Zaros, C.; Appenzeller, B.M.R.; Chevrier, C. Multiple Pesticides in Mothers’ Hair Samples and Children’s Measurements at Birth: Results from the French National Birth Cohort (ELFE). Int. J. Hyg. Environ. Health 2020, 223, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Ebara, T.; Tamada, H.; Ito, Y.; Yamada, Y.; Kano, H.; Kurihara, T.; Sato, H.; Kato, S.; Saitoh, S.; et al. Association between Prenatal Exposure to Household Pesticides and Neonatal Weight and Length Growth in the Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 2020, 17, 4608. [Google Scholar] [CrossRef]
- Kartini, A.; Subagio, H.W.; Hadisaputro, S.; Kartasurya, M.I.; Suhartono, S.; Budiyono, B. Pesticide Exposure and Stunting among Children in Agricultural Areas. Int. J. Occup. Environ. Med. 2019, 10, 17–29. [Google Scholar] [CrossRef]
- Mendez, M.A.; Garcia-Esteban, R.; Guxens, M.; Vrijheid, M.; Kogevinas, M.; Goñi, F.; Fochs, S.; Sunyer, J. Prenatal Organochlorine Compound Exposure, Rapid Weight Gain, and Overweight in Infancy. Environ. Health Perspect. 2011, 119, 272–278. [Google Scholar] [CrossRef]
- Valvi, D.; Mendez, M.A.; Garcia-Esteban, R.; Ballester, F.; Ibarluzea, J.; Goñi, F.; Grimalt, J.O.; Llop, S.; Marina, L.S.; Vizcaino, E.; et al. Prenatal Exposure to Persistent Organic Pollutants and Rapid Weight Gain and Overweight in Infancy. Obesity 2014, 22, 488–496. [Google Scholar] [CrossRef]
- Agay-Shay, K.; Martinez, D.; Valvi, D.; Garcia-Esteban, R.; Basagaña, X.; Robinson, O.; Casas, M.; Sunyer, J.; Vrijheid, M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ. Health Perspect. 2015, 123, 1030–1037. [Google Scholar] [CrossRef]
- Warner, M.; Ye, M.; Harley, K.; Kogut, K.; Bradman, A.; Eskenazi, B. Prenatal DDT Exposure and Child Adiposity at Age 12: The CHAMACOS Study. Environ. Res. 2017, 159, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadi, M.; Georgiou, V.; Chalkiadaki, G.; Rantakokko, P.; Kiviranta, H.; Karachaliou, M.; Fthenou, E.; Venihaki, M.; Sarri, K.; Vassilaki, M.; et al. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother-Child Cohort (Crete, Greece). Environ. Health Perspect. 2015, 123, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Güil-Oumrait, N.; Valvi, D.; Garcia-Esteban, R.; Guxens, M.; Sunyer, J.; Torrent, M.; Casas, M.; Vrijheid, M. Prenatal Exposure to Persistent Organic Pollutants and Markers of Obesity and Cardiometabolic Risk in Spanish Adolescents. Environ. Int. 2021, 151, 106469. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Goudarzi, H.; Oulhote, Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr. Environ. Health Rep. 2018, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Deji, Z.; Liu, P.; Wang, X.; Zhang, X.; Luo, Y.; Huang, Z. Association between Maternal Exposure to Perfluoroalkyl and Polyfluoroalkyl Substances and Risks of Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2021, 783, 146984. [Google Scholar] [CrossRef]
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and Polyfluoroalkyl Substances and Human Fetal Growth: A Systematic Review. Crit. Rev. Toxicol. 2015, 45, 53–67. [Google Scholar] [CrossRef]
- Caserta, D.; Pegoraro, S.; Mallozzi, M.; Di Benedetto, L.; Colicino, E.; Lionetto, L.; Simmaco, M. Maternal Exposure to Endocrine Disruptors and Placental Transmission: A Pilot Study. Gynecol. Endocrinol. 2018, 34, 1001–1004. [Google Scholar] [CrossRef]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.Ø.; van de Bor, M.; Jacobsen, G.W. Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines and Indices of Fetal Growth: A Scandinavian Case-Cohort Study. Pediatr. Res. 2017, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.S.; Ghassabian, A.; Vandyousefi, S.; Messito, M.J.; Gao, C.; Kannan, K.; Trasande, L. Persistent Organic Pollutants Exposure in Newborn Dried Blood Spots and Infant Weight Status: A Case-Control Study of Low-Income Hispanic Mother-Infant Pairs. Environ. Pollut. 2020, 267, 115427. [Google Scholar] [CrossRef]
- Wikström, S.; Lin, P.I.; Lindh, C.H.; Shu, H.; Bornehag, C.G. Maternal Serum Levels of Perfluoroalkyl Substances in Early Pregnancy and Offspring Birth Weight. Pediatr. Res. 2020, 87, 1093–1099. [Google Scholar] [CrossRef]
- Shoaff, J.; Papandonatos, G.D.; Calafat, A.M.; Chen, A.; Lanphear, B.P.; Ehrlich, S.; Kelsey, K.T.; Braun, J.M. Prenatal Exposure to Perfluoroalkyl Substances: Infant Birth Weight and Early Life Growth. Environ. Epidemiol. 2018, 2, e010. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Vaughan, O.R.; Forhead, A.J.; Fowden, A.L. Hormonal and Nutritional Drivers of Intrauterine Growth. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Shy, C.G.; Huang, H.L.; Chao, H.R.; Chang-Chien, G.P. Cord Blood Levels of Thyroid Hormones and IGF-1 Weakly Correlate with Breast Milk Levels of PBDEs in Taiwan. Int. J. Hyg. Environ. Health 2012, 215, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 Expression Correlate with Umbilical Cord Blood PAH and PBDE Levels from Prenatal Exposure to Electronic Waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.G.; Chevrier, J.; Aguilar Schall, R.; Sjödin, A.; Bradman, A.; Eskenazi, B. Association of Prenatal Exposure to Polybrominated Diphenyl Ethers and Infant Birth Weight. Am. J. Epidemiol. 2011, 174, 885–892. [Google Scholar] [CrossRef]
- Lopez-Espinosa, M.J.; Costa, O.; Vizcaino, E.; Murcia, M.; Fernandez-Somoano, A.; Iñiguez, C.; Llop, S.; Grimalt, J.O.; Ballester, F.; Tardon, A. Prenatal Exposure to Polybrominated Flame Retardants and Fetal Growth in the INMA Cohort (Spain). Environ. Sci. Technol. 2015, 49, 10108–10116. [Google Scholar] [CrossRef] [PubMed]
- Serme-Gbedo, Y.K.; Abdelouahab, N.; Pasquier, J.C.; Cohen, A.A.; Takser, L. Maternal Levels of Endocrine Disruptors, Polybrominated Diphenyl Ethers, in Early Pregnancy are not Associated with Lower Birth Weight in the Canadian Birth Cohort GESTE. Environ. Health 2016, 15, 49. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xie, Y.; Tian, Y.K.; Liu, H.; He, C.D.; An, S.L.; Chen, W.; Zhou, Y.Z.; Zhong, X.N. Associations between Polybrominated Diphenyl Ethers Concentrations in Human Placenta and Small for Gestational Age in Southwest China. Front. Public Health 2022, 10, 812268. [Google Scholar] [CrossRef]
- Jin, Y.T.; Deng, X.K.; Zhao, Y.Y.; Li, J.L.; Song, Q.; Zhang, Y.H.; Yang, Q.; Chen, S.Q. Concentrations of Polybrominated Diphenyl Ethers in Maternal Blood, Placental Size, and Risk for Fetal Growth Restriction: A Nested Case-control Study. Biomed. Environ. Sci. 2020, 33, 821–828. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Q.; Ge, W.; Jin, Y.; Chen, S.; Zhao, Y.; Xiao, X.; Zhang, Y. Associations between in Utero Exposure to Polybrominated Diphenyl Ethers, Pathophysiological State of Fetal Growth and Placental DNA Methylation Changes. Environ. Int. 2019, 133, 105255. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Miao, W.; Wu, C.; Zhang, J.; Qi, X.; Yu, H.; Chang, X.; Zhang, Y.; Zhou, Z. Umbilical Cord Serum PBDE Concentrations and Child Adiposity Measures at 7 years. Ecotoxicol. Environ. Saf. 2020, 203, 111009. [Google Scholar] [CrossRef]
- Zhong, Q.; Cui, Y.; Wu, H.; Niu, Q.; Lu, X.; Wang, L.; Huang, F. Association of Maternal Arsenic Exposure with Birth Size: A Systematic Review and Meta-Analysis. Environ. Toxicol. Pharmacol. 2019, 69, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Predieri, B.; Ferrari, M.; Gallo, C.; Livio, L.; Milioli, S.; Forese, S.; Bernasconi, S. Diagnosis of Central Precocious Puberty: Endocrine Assessment. J. Pediatr. Endocrinol. Metab. 2000, 13, 709–715. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Gray, L.E., Jr.; Marcus, M.; Ojeda, S.R.; Pescovitz, O.H.; Witchel, S.F.; Sippell, W.; Abbott, D.H.; Soto, A.; Tyl, R.W.; et al. Environmental Factors and Puberty Timing: Expert Panel Research Needs. Pediatrics 2008, 121, S192–S207. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.P.; Juul, A.; Franssen, D.; Fudvoye, J.; Pinson, A.; Parent, A.S. Contribution of the Endocrine Perspective in the Evaluation of Endocrine Disrupting Chemical Effects: The Case Study of Pubertal Timing. Horm. Res. Paediatr. 2016, 86, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the Literature on Current Changes in the Timing of Pubertal Development and the Incomplete Forms of Early Puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef]
- Livadas, S.; Chrousos, G.P. Molecular and Environmental Mechanisms Regulating Puberty Initiation: An Integrated Approach. Front. Endocrinol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Scippo, M.L.; Argiris, C.; Van De Weerdt, C.; Muller, M.; Willemsen, P.; Martial, J.; Maghuin-Rogister, G. Recombinant Human Estrogen, Androgen and Progesterone Receptors for Detection of Potential Endocrine Disruptors. Anal. Bioanal. Chem. 2004, 378, 664–669. [Google Scholar] [CrossRef]
- Caserta, D.; Maranghi, L.; Mantovani, A.; Marci, R.; Maranghi, F.; Moscarini, M. Impact of Endocrine Disruptor Chemicals in Gynaecology. Hum. Reprod. Update 2008, 14, 59–72. [Google Scholar] [CrossRef]
- Brannick, K.E.; Craig, Z.R.; Himes, A.D.; Peretz, J.R.; Wang, W.; Flaws, J.A.; Raetzman, L.T. Prenatal Exposure to Low Doses of Bisphenol A Increases Pituitary Proliferation and Gonadotroph Number in Female Mice Offspring at Birth. Biol. Reprod. 2012, 87, 82. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Bianchi, M.; Lux-Lantos, V.; Libertun, C. Neonatal Exposure to Bisphenol A Alters Reproductive Parameters and Gonadotropin Releasing Hormone Signaling in Female Rats. Environ. Health Perspect. 2009, 117, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Aşçı, A.; Erkekoğlu, P.; Akçurin, S.; Gümüşel, B.K.; Bircan, I. Urinary Bisphenol A Levels in Girls with Idiopathic Central Precocious Puberty. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 16–21. [Google Scholar] [CrossRef]
- Supornsilchai, V.; Jantarat, C.; Nosoognoen, W.; Pornkunwilai, S.; Wacharasindhu, S.; Soder, O. Increased Levels of Bisphenol A (BPA) in Thai Girls with Precocious Puberty. J. Pediatr. Endocrinol. Metab. 2016, 29, 1233–1239. [Google Scholar] [CrossRef]
- Durmaz, E.; Asci, A.; Erkekoglu, P.; Balcı, A.; Bircan, I.; Koçer-Gumusel, B. Urinary Bisphenol A Levels in Turkish Girls with Premature Thelarche. Hum. Exp. Toxicol. 2018, 37, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- McGuinn, L.A.; Ghazarian, A.A.; Joseph Su, L.; Ellison, G.L. Urinary Bisphenol A and Age at Menarche among Adolescent Girls: Evidence from NHANES 2003-2010. Environ. Res. 2015, 136, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Wang, Z.; Liu, X.; Liang, H.; Zhou, Z.; Tan, H.; Yuan, W.; Li, D.K. Urinary Bisphenol A and Pubertal Development in Chinese School-Aged Girls: A Cross-Sectional Study. Environ. Health 2017, 16, 80. [Google Scholar] [CrossRef]
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of Phthalate Exposure with Precocious and Delayed Pubertal Timing in Girls and Boys: A Systematic Review and Meta-Analysis. Environ. Sci. Process. Impacts 2020, 22, 873–894. [Google Scholar] [CrossRef]
- Larriuz-Serrano, M.C.; Pérez-Cardona, C.M.; Ramos-Valencia, G.; Bourdony, C.J. Natural History and Incidence of Premature Thelarche in Puerto Rican Girls Aged 6 Months to 8 Years Diagnosed between 1990 and 1995. P R Health Sci. J. 2001, 20, 13–18. [Google Scholar]
- Colón, I.; Caro, D.; Bourdony, C.J.; Rosario, O. Identification of Phthalate Esters in the Serum of Young Puerto Rican Girls with Premature Breast Development. Environ. Health Perspect. 2000, 108, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Erkekoglu, P.; Asci, A.; Akçurin, S.; Bircan, İ.; Kocer-Gumusel, B. Urinary Phthalate Metabolite Concentrations in Girls with Premature Thelarche. Environ. Toxicol. Pharmacol. 2018, 59, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Petersen, J.H.; Skakkebaek, N.E.; Andersson, A.M.; Juul, A. High Urinary Phthalate Concentration Associated with Delayed Pubarche in Girls. Int. J. Androl. 2012, 35, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, A.; Frederiksen, H.; Sørensen, K.; Aksglaede, L.; Hagen, C.; Skakkebaek, N.E.; Main, K.M.; Andersson, A.M.; Juul, A. Urinary Phthalates from 168 Girls and Boys Measured Twice a Year during a 5-Year Period: Associations with Adrenal Androgen Levels and Puberty. J. Clin. Endocrinol. Metab. 2013, 98, 3755–3764. [Google Scholar] [CrossRef]
- Wolff, M.S.; Pajak, A.; Pinney, S.M.; Windham, G.C.; Galvez, M.; Rybak, M.; Silva, M.J.; Ye, X.; Calafat, A.M.; Kushi, L.H.; et al. Associations of Urinary Phthalate and Phenol Biomarkers with Menarche in a Multiethnic Cohort of Young Girls. Reprod. Toxicol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.M.; Corvalan, C.; Calafat, A.M.; Ye, X.; Mericq, V.; Pereira, A.; Michels, K.B. Childhood and Adolescent Phenol and Phthalate Exposure and the Age of Menarche in Latina Girls. Environ. Health 2018, 17, 32. [Google Scholar] [CrossRef]
- Jung, M.K.; Choi, H.S.; Suh, J.; Kwon, A.; Chae, H.W.; Lee, W.J.; Yoo, E.G.; Kim, H.S. The Analysis of Endocrine Disruptors in Patients with Central Precocious Puberty. BMC Pediatr. 2019, 19, 323. [Google Scholar] [CrossRef] [PubMed]
- Buluş, A.D.; Aşci, A.; Erkekoglu, P.; Balci, A.; Andiran, N.; Koçer-Gümüşel, B. The Evaluation of Possible Role of Endocrine Disruptors in Central and Peripheral Precocious Puberty. Toxicol. Mech. Methods 2016, 26, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Srilanchakon, K.; Thadsri, T.; Jantarat, C.; Thengyai, S.; Nosoognoen, W.; Supornsilchai, V. Higher Phthalate Concentrations are Associated with Precocious Puberty in Normal Weight Thai Girls. J. Pediatr. Endocrinol. Metab. 2017, 30, 1293–1298. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 97004. [Google Scholar] [CrossRef]
- Guo, Y.L.; Lambert, G.H.; Hsu, C.C.; Hsu, M.M. Yucheng: Health Effects of Prenatal Exposure to Polychlorinated Biphenyls and Dibenzofurans. Int. Arch. Occup. Environ. Health 2004, 77, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; Koppe, J.G.; Olie, K.; van Aalderen, W.M.; Voogt, P.; Vulsma, T.; Westra, M.; ten Tusscher, G.W. Delayed Initiation of Breast Development in Girls with Higher Prenatal Dioxin Exposure; a Longitudinal Cohort Study. Chemosphere 2008, 73, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Roels, H.A.; Hoppenbrouwers, K.; Nawrot, T.; Thijs, L.; Vandermeulen, C.; Winneke, G.; Vanderschueren, D.; Staessen, J.A. Sexual Maturation in Relation to Polychlorinated Aromatic Hydrocarbons: Sharpe and Skakkebaek’s Hypothesis Revisited. Environ. Health Perspect. 2002, 110, 771–776. [Google Scholar] [CrossRef]
- Warner, M.; Samuels, S.; Mocarelli, P.; Gerthoux, P.M.; Needham, L.; Patterson, D.G., Jr.; Eskenazi, B. Serum Dioxin Concentrations and Age at Menarche. Environ. Health Perspect. 2004, 112, 1289–1292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krstevska-Konstantinova, M.; Charlier, C.; Craen, M.; Du Caju, M.; Heinrichs, C.; de Beaufort, C.; Plomteux, G.; Bourguignon, J.P. Sexual Precocity after Immigration from Developing Countries to Belgium: Evidence of Previous Exposure to Organochlorine Pesticides. Hum. Reprod. 2001, 16, 1020–1026. [Google Scholar] [CrossRef]
- Parent, A.S.; Franssen, D.; Fudvoye, J.; Gérard, A.; Bourguignon, J.P. Developmental Variations in Environmental Influences Including Endocrine Disruptors on Pubertal Timing and Neuroendocrine Control: Revision of Human Observations and Mechanistic Insight from Rodents. Front. Neuroendocrinol. 2015, 38, 12–36. [Google Scholar] [CrossRef]
- Vasiliu, O.; Muttineni, J.; Karmaus, W. In Utero Exposure to Organochlorines and Age at Menarche. Hum. Reprod. 2004, 19, 1506–1512. [Google Scholar] [CrossRef]
- Ouyang, F.; Perry, M.J.; Venners, S.A.; Chen, C.; Wang, B.; Yang, F.; Fang, Z.; Zang, T.; Wang, L.; Xu, X.; et al. Serum DDT, Age at Menarche, and Abnormal Menstrual Cycle Length. Occup. Environ. Med. 2005, 62, 878–884. [Google Scholar] [CrossRef]
- Namulanda, G.; Maisonet, M.; Taylor, E.; Flanders, W.D.; Olson, D.; Sjodin, A.; Qualters, J.R.; Vena, J.; Northstone, K.; Naeher, L. In Utero Exposure to Organochlorine Pesticides and Early Menarche in the Avon Longitudinal Study of Parents and Children. Environ. Int. 2016, 94, 467–472. [Google Scholar] [CrossRef]
- Coppola, L.; Tait, S.; Ciferri, L.; Frustagli, G.; Merola, C.; Perugini, M.; Fabbrizi, E.; La Rocca, C. Integrated Approach to Evaluate the Association between Exposure to Pesticides and Idiopathic Premature Thelarche in Girls: The PEACH Project. Int. J. Mol. Sci. 2020, 21, 3282. [Google Scholar] [CrossRef]
- Sergeyev, O.; Burns, J.S.; Williams, P.L.; Korrick, S.A.; Lee, M.M.; Revich, B.; Hauser, R. The Association of Peripubertal Serum Concentrations of Organochlorine Chemicals and Blood Lead with Growth and Pubertal Development in a Longitudinal Cohort of Boys: A Review of Published Results from the Russian Children’s Study. Rev. Environ. Health 2017, 32, 83–92. [Google Scholar] [CrossRef]
- Ernst, A.; Brix, N.; Lauridsen, L.L.B.; Olsen, J.; Parner, E.T.; Liew, Z.; Olsen, L.H.; Ramlau-Hansen, C.H. Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, 17004. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ramlau-Hansen, C.H.; Ernst, E.; Olsen, S.F.; Bonde, J.P.; Vested, A.; Halldorsson, T.I.; Becher, G.; Haug, L.S.; Toft, G. Long-term Effects of Prenatal Exposure to Perfluoroalkyl Substances on Female Reproduction. Hum. Reprod. 2013, 28, 3337–3348. [Google Scholar] [CrossRef]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Calafat, A.M.; Sjödin, A.; Marcus, M.; Hartman, T.J. Prenatal Exposure to Mixtures of Persistent Endocrine Disrupting Chemicals and Early Menarche in a Population-Based Cohort of British Girls. Environ. Pollut. 2021, 276, 116705. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, H.W.; Kim, H.Y.; Choi, Y.-J.; Lee, Y.A. Early-life Exposure to Per- and Poly-fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front. Endocrinol. 2021, 12, 683297. [Google Scholar] [CrossRef]
- Chen, A.; Chung, E.; DeFranco, E.A.; Pinney, S.M.; Dietrich, K.N. Serum PBDEs and Age at Menarche in Adolescent Girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ. Res. 2011, 111, 831–837. [Google Scholar] [CrossRef]
- Deodati, A.; Sallemi, A.; Maranghi, F.; Germani, D.; Puglianiello, A.; Baldari, F.; Busani, L.; Mancini, F.R.; Tassinari, R.; Mantovani, A.; et al. Serum Levels of Polybrominated Diphenyl Ethers in Girls with Premature Thelarche. Horm. Res. Paediatr. 2016, 86, 233–239. [Google Scholar] [CrossRef]
- Blanck, H.M.; Marcus, M.; Tolbert, P.E.; Rubin, C.; Henderson, A.K.; Hertzberg, V.S.; Zhang, R.H.; Cameron, L. Age at Menarche and Tanner Stage in Girls Exposed In Utero and Postnatally to Polybrominated Biphenyl. Epidemiology 2000, 11, 641–647. [Google Scholar] [CrossRef]
- Harley, K.G.; Rauch, S.A.; Chevrier, J.; Kogut, K.; Parra, K.L.; Trujillo, C.; Lustig, R.H.; Greenspan, L.C.; Sjödin, A.; Bradman, A.; et al. Association of Prenatal and Childhood PBDE Exposure with Timing of Puberty in Boys and Girls. Environ. Int. 2017, 100, 132–138. [Google Scholar] [CrossRef]
- Henley, D.V.; Lipson, N.; Korach, K.S.; Bloch, C.A. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med. 2007, 356, 479–485. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; Coons, L.A.; Diaz, A.; Korach, K.S. Lavender Products Associated with Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Aljaber, D.; Au, D.; Thai, C.; Sanchez, A.; Nunez, A.; Resto, C.; Chavez, T.; Jankowska, M.M.; Benmarhnia, T.; et al. Environmental Exposures during Puberty: Window of Breast Cancer Risk and Epigenetic Damage. Int. J. Environ. Res. Public Health 2020, 17, 493. [Google Scholar] [CrossRef]
- Kehm, R.D.; Oskar, S.; Tehranifar, P.; Zeinomar, N.; Rundle, A.G.; Herbstman, J.B.; Perera, F.; Miller, R.L.; Terry, M.B. Associations of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons with Pubertal Timing and Body Composition in Adolescent Girls: Implications for Breast Cancer Risk. Environ. Res. 2021, 196, 110369. [Google Scholar] [CrossRef]
- Macon, M.B.; Fenton, S.E. Endocrine Disruptors and the Breast: Early Life Effects and Later Life Disease. J. Mammary Gland Biol. Neoplasia 2013, 18, 43–61. [Google Scholar] [CrossRef]
- Williams, G.P.; Darbre, P.D. Low-dose Environmental Endocrine Disruptors, Increase Aromatase Activity, Estradiol Biosynthesis and Cell Proliferation in Human Breast Cells. Mol. Cell Endocrinol. 2019, 486, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B.M.V.; Boberg, J.; Christiansen, S.; Connolly, L.; Damdimopoulou, P.; Filis, P.; Fowler, P.A.; Gadella, B.M.; Holte, J.; Jääger, K.; et al. Safeguarding Female Reproductive Health against Endocrine Disrupting Chemicals-The FREIA Project. Int. J. Mol. Sci. 2020, 21, 3215. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.S.; Bai, Z.M. Is Testicular Dysgenesis Syndrome a Genetic, Endocrine, or Environmental Disease, or an Unexplained Reproductive Disorder? Life Sci. 2018, 194, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.; Liu, D.Y.; Yang, Y.; Zhong, Z.H.; Wang, Y.X.; Ding, Y.B. In Utero Exposure to Persistent and Nonpersistent Endocrine-Disrupting Chemicals and Anogenital distance. A Systematic Review of Epidemiological Studies. Biol. Reprod. 2020, 102, 276–291. [Google Scholar] [CrossRef]
- Fisher, B.G.; Thankamony, A.; Mendiola, J.; Petry, C.J.; Frederiksen, H.; Andersson, A.M.; Juul, A.; Ong, K.K.; Dunger, D.B.; Hughes, I.A.; et al. Maternal Serum Concentrations of Bisphenol A and Propyl Paraben in Early Pregnancy are Associated with Male Infant Genital Development. Hum. Reprod. 2020, 35, 913–928. [Google Scholar] [CrossRef]
- Qian, Y.; Shao, H.; Ying, X.; Huang, W.; Hua, Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Front. Public Health 2020, 8, 366. [Google Scholar] [CrossRef]
- Ormond, G.; Nieuwenhuijsen, M.J.; Nelson, P.; Toledano, M.B.; Iszatt, N.; Geneletti, S.; Elliott, P. Endocrine Disruptors in the Workplace, Hair Spray, Folate Supplementation, and Risk of Hypospadias: Case-Control Study. Environ. Health Perspect. 2009, 117, 303–307. [Google Scholar] [CrossRef]
- Nassar, N.; Abeywardana, P.; Barker, A.; Bower, C. Parental Occupational Exposure to Potential Endocrine Disrupting Chemicals and Risk of Hypospadias in Infants. Occup. Environ. Med. 2010, 67, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Anand-Ivell, R.; Nørgaard-Pedersen, B.; Jönsson, B.A.; Bonde, J.P.; Hougaard, D.M.; Cohen, A.; Lindh, C.H.; Ivell, R.; Toft, G. Amniotic Fluid Phthalate Levels and Male Fetal Gonad Function. Epidemiology 2015, 26, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y.; Serizawa, S.; Shiraishi, H. Foetal Exposure to Phthalate Esters and Anogenital Distance in Male Newborns. Int. J. Androl. 2012, 35, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.; Redmon, J.B.; TIDES Study Team. First Trimester Phthalate Exposure and Anogenital Distance in Newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef]
- Jensen, T.K.; Frederiksen, H.; Kyhl, H.B.; Lassen, T.H.; Swan, S.H.; Bornehag, C.G.; Skakkebaek, N.E.; Main, K.M.; Lind, D.V.; Husby, S.; et al. Prenatal Exposure to Phthalates and Anogenital Distance in Male Infants from a Low-Exposed Danish Cohort (2010–2012). Environ. Health Perspect. 2016, 124, 1107–1113. [Google Scholar] [CrossRef]
- Schultz, R.; Suominen, J.; Värre, T.; Hakovirta, H.; Parvinen, M.; Toppari, J.; Pelto-Huikko, M. Expression of Aryl Hydrocarbon Receptor and Aryl Hydrocarbon Receptor Nuclear Translocator Messenger Ribonucleic Acids and Proteins in Rat and Human Testis. Endocrinology 2003, 144, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.; Koskenniemi, J.J.; Sundqvist, E.; Main, K.M.; Kiviranta, H.; Tuomisto, J.T.; Tuomisto, J.; Viluksela, M.; Vartiainen, T.; Skakkebaek, N.E.; et al. Associations between Congenital Cryptorchidism in Newborn Boys and Levels of Dioxins and PCBs in Placenta. Int. J. Androl. 2012, 35, 283–293. [Google Scholar] [CrossRef]
- Mocarelli, P.; Gerthoux, P.M.; Needham, L.L.; Patterson, D.G., Jr.; Limonta, G.; Falbo, R.; Signorini, S.; Bertona, M.; Crespi, C.; Sarto, C.; et al. Perinatal Exposure to Low Doses of Dioxin can Permanently Impair Human Semen Quality. Environ. Health Perspect. 2011, 119, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, I.N.; Skakkebaek, N.E.; Toppari, J.; Virtanen, H.E.; Shen, H.; Schramm, K.W.; Petersen, J.H.; Jensen, T.K.; Main, K.M.; Nordic Cryptorchidism Study Group. Persistent Pesticides in Human Breast Milk and Cryptorchidism. Environ. Health Perspect. 2006, 114, 1133–1138. [Google Scholar] [CrossRef]
- Brucker-Davis, F.; Wagner-Mahler, K.; Delattre, I.; Ducot, B.; Ferrari, P.; Bongain, A.; Kurzenne, J.Y.; Mas, J.C.; Fénichel, P.; Cryptorchidism Study Group from Nice Area. Cryptorchidism at Birth in Nice Area (France) is Associated with Higher Prenatal Exposure to PCBs and DDE, as Assessed by Colostrum Concentrations. Hum. Reprod. 2008, 23, 1708–1718. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Lindh, C.H.; Dillner, J.; Jonsson, B.A.; Rylander, L. A Nested Case-Control Study of Intrauterine Exposure to Persistent Organochlorine Pollutants and the Risk of Hypospadias. PLoS ONE 2012, 7, e44767. [Google Scholar] [CrossRef]
- Trabert, B.; Longnecker, M.P.; Brock, J.W.; Klebanoff, M.A.; McGlynn, K.A. Maternal Pregnancy Levels of Trans-nonachlor and Oxychlordane and Prevalence of Cryptorchidism and Hypospadias in Boys. Environ. Health Perspect. 2012, 120, 478–482. [Google Scholar] [CrossRef]
- Winston, J.J.; Emch, M.; Meyer, R.E.; Langlois, P.; Weyer, P.; Mosley, B.; Olshan, A.F.; Band, L.E.; Luben, T.J.; National Birth Defects Prevention Study. Hypospadias and Maternal Exposure to Atrazine Via Drinking Water in the National Birth Defects Prevention Study. Environ. Health 2016, 15, 76. [Google Scholar] [CrossRef]
- Tian, Y.; Liang, H.; Miao, M.; Yang, F.; Ji, H.; Cao, W.; Liu, X.; Zhang, X.; Chen, A.; Xiao, H.; et al. Maternal Plasma Concentrations of Perfluoroalkyl and Polyfluoroalkyl Substances during Pregnancy and Anogenital Distance in Male Infants. Hum. Reprod. 2019, 34, 1356–1368. [Google Scholar] [CrossRef]
- Mughal, B.B.; Fini, J.B.; Demeneix, B.A. Thyroid-Disrupting Chemicals and Brain Development: An Update. Endocr Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T. Endocrine Disrupting Chemicals and Thyroid Hormone Action. Adv. Pharmacol. 2021, 92, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J.; Frädrich, C. Thyroid Hormone System Disrupting Chemicals. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101562. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Axelstad, M.; Baig, A.H.; Bergman, Å.; Bornehag, C.G.; Cenijn, P.; Christiansen, S.; Demeneix, B.; Derakhshan, A.; Fini, J.B.; et al. Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals-The ATHENA Project. Int. J. Mol. Sci. 2020, 21, 3123. [Google Scholar] [CrossRef] [PubMed]
- Holbech, H.; Matthiessen, P.; Hansen, M.; Schüürmann, G.; Knapen, D.; Reuver, M.; Flamant, F.; Sachs, L.; Kloas, W.; Hilscherova, K.; et al. ERGO: Breaking Down the Wall between Human Health and Environmental Testing of Endocrine Disrupters. Int. J. Mol. Sci. 2020, 21, 2954. [Google Scholar] [CrossRef]
- Moroni, L.; Barbaro, F.; Caiment, F.; Coleman, O.; Costagliola, S.; Conza, G.D.; Elviri, L.; Giselbrecht, S.; Krause, C.; Mota, C.; et al. SCREENED: A Multistage Model of Thyroid Gland Function for Screening Endocrine-Disrupting Chemicals in a Biologically Sex-Specific Manner. Int. J. Mol. Sci. 2020, 21, 3648. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as Environmental Triggers of Thyroid Dysfunction: Clues and Evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Philips, E.M.; Ghassabian, A.; Santos, S.; Asimakopoulos, A.G.; Kannan, K.; Kortenkamp, A.; Jaddoe, V.W.V.; Trasande, L.; Peeters, R.P.; et al. Association of Urinary Bisphenols during Pregnancy with Maternal, Cord Blood and Childhood Thyroid Function. Environ. Int. 2021, 146, 106160. [Google Scholar] [CrossRef] [PubMed]
- Koutaki, D.; Paltoglou, G.; Vourdoumpa, A.; Charmandari, E. The Impact of Bisphenol A on Thyroid Function in Neonates and Children: A Systematic Review of the Literature. Nutrients 2021, 14, 168. [Google Scholar] [CrossRef]
- Romano, M.E.; Eliot, M.N.; Zoeller, R.T.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Chen, A.; Lanphear, B.P.; Braun, J.M. Maternal Urinary Phthalate Metabolites during Pregnancy and Thyroid Hormone Concentrations in Maternal and Cord Sera: The HOME Study. Int. J. Hyg. Environ. Health 2018, 221, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Kuo, P.L.; Chang, W.H.; Shih, S.F.; Chang, W.T.; Lee, C.C. Prenatal Phthalates Exposure and Cord Thyroid Hormones: A Birth Cohort Study in Southern Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 4323. [Google Scholar] [CrossRef]
- Warner, M.; Rauch, S.; Ames, J.; Mocarelli, P.; Brambilla, P.; Signorini, S.; Eskenazi, B. Prenatal Dioxin Exposure and Thyroid Hormone Levels in the Seveso Second Generation Study. Environ. Res. 2020, 183, 109280. [Google Scholar] [CrossRef]
- De Cock, M.; de Boer, M.R.; Govarts, E.; Iszatt, N.; Palkovicova, L.; Lamoree, M.H.; Schoeters, G.; Eggesbø, M.; Trnovec, T.; Legler, J.; et al. Thyroid-Stimulating Hormone Levels in Newborns and Early Life Exposure to Endocrine-Disrupting Chemicals: Analysis of Three European Mother-Child Cohorts. Pediatr. Res. 2017, 82, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Baba, T.; Yuasa, M.; Miyashita, C.; Kobayashi, S.; Araki, A.; Sasaki, S.; Kajiwara, J.; Hori, T.; Todaka, T.; et al. Association of Maternal Serum Concentration of Hydroxylated Polychlorinated Biphenyls with Maternal and Neonatal Thyroid Hormones: The Hokkaido Birth Cohort Study. Environ. Res. 2018, 167, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Webster, T.F.; Claus Henn, B.; McClean, M.D.; Gennings, C.; Oken, E.; Rifas-Shiman, S.L.; Pearce, E.N.; Calafat, A.M.; Fleisch, A.F.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances and Maternal and Neonatal Thyroid Function in the Project Viva Cohort: A Mixtures Approach. Environ. Int. 2020, 139, 105728. [Google Scholar] [CrossRef]
- Cowell, W.J.; Sjödin, A.; Jones, R.; Wang, Y.; Wang, S.; Whyatt, R.M.; Factor-Litvak, P.; Bradwin, G.; Hassoun, A.; Oberfield, S.; et al. Pre- and Postnatal Polybrominated Diphenyl Ether Concentrations in Relation to Thyroid Parameters Measured During Early Childhood. Thyroid 2019, 29, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Endocrine Disrupters as Obesogens. Mol. Cell Endocrinol. 2009, 304, 19–29. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Gutiérrez-Torres, D.S.; Barraza-Villarreal, A.; Hernandez-Cadena, L.; Escamilla-Nuñez, C.; Romieu, I. Prenatal Exposure to Endocrine Disruptors and Cardiometabolic Risk in Preschoolers: A Systematic Review Based on Cohort Studies. Ann. Glob. Health 2018, 84, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Shioda, T.; Blumberg, B. Transgenerational Inheritance of Prenatal Obesogen Exposure. Mol. Cell Endocrinol. 2014, 398, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Blumberg, B. Obesogens: An Emerging Threat to Public Health. Am. J. Obstet. Gynecol. 2016, 214, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing Causal Links between Chemical Exposures and Obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine Disruptors in the Etiology of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A Enhances Adipogenic Differentiation of Human Adipose Stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353. [Google Scholar] [CrossRef]
- Braun, J.M.; Li, N.; Arbuckle, T.E.; Dodds, L.; Massarelli, I.; Fraser, W.D.; Lanphear, B.P.; Muckle, G. Association between Gestational Urinary Bisphenol A Concentrations and Adiposity in Young Children: The MIREC Study. Environ. Res. 2019, 172, 454–461. [Google Scholar] [CrossRef]
- Mustieles, V.; Casas, M.; Ferrando-Marco, P.; Ocón-Hernández, O.; Reina-Pérez, I.; Rodríguez-Carrillo, A.; Vela-Soria, F.; Pérez-Lobato, R.; Navarrete-Muñoz, E.M.; Freire, C.; et al. Bisphenol A and Adiposity Measures in Peripubertal Boys from the INMA-Granada Cohort. Environ. Res. 2019, 173, 443–451. [Google Scholar] [CrossRef]
- Robles-Aguilera, V.; Gálvez-Ontiveros, Y.; Rodrigo, L.; Salcedo-Bellido, I.; Aguilera, M.; Zafra-Gómez, A.; Monteagudo, C.; Rivas, A. Factors Associated with Exposure to Dietary Bisphenols in Adolescents. Nutrients 2021, 13, 1553. [Google Scholar] [CrossRef]
- Menale, C.; Grandone, A.; Nicolucci, C.; Cirillo, G.; Crispi, S.; Di Sessa, A.; Marzuillo, P.; Rossi, S.; Mita, D.G.; Perrone, L.; et al. Bisphenol A is Associated with Insulin Resistance and Modulates Adiponectin and Resistin Gene Expression in Obese Children. Pediatr. Obes. 2017, 12, 380–387. [Google Scholar] [CrossRef]
- Ouyang, F.; Zhang, G.H.; Du, K.; Shen, L.; Ma, R.; Wang, X.; Wang, X.; Zhang, J. Maternal Prenatal Urinary Bisphenol A Level and Child Cardio-Metabolic Risk Factors: A Prospective Cohort Study. Environ. Pollut. 2020, 265, 115008. [Google Scholar] [CrossRef]
- Akgül, S.; Sur, Ü.; Düzçeker, Y.; Balcı, A.; Kızılkan, M.P.; Kanbur, N.; Bozdağ, G.; Erkekoğlu, P.; Gümüş, E.; Kocer-Gumusel, B.; et al. Bisphenol A and Phthalate Levels in Adolescents with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2019, 35, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of Exposure to Phthalates with Cardiometabolic Risk Factors in Children and Adolescents: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 35670–35686. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Hyland, C.; Ames, J.L.; Mora, A.M.; Huen, K.; Eskenazi, B.; Holland, N.; Harley, K.G. Prenatal Exposure to Mixtures of Phthalates, Parabens, and Other Phenols and Obesity in Five-Year-Olds in the CHAMACOS Cohort. Int. J. Environ. Res. Public Health 2021, 18, 1796. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Soheimi, S.S.; Abdul Rahman, A.; Abd Latip, N.; Ibrahim, E.; Sheikh Abdul Kadir, S.H. Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and their Risk Factors: A Meta-Analysis Study. Int. J. Environ. Res. Public Health 2021, 18, 8345. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.Ø.; van de Bor, M.; Jacobsen, G.W. Prenatal Exposure to Persistent Organic Pollutants and Child Overweight/Obesity at 5-Year Follow-Up: A Prospective Cohort Study. Environ. Health 2018, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Iughetti, L.; Guerranti, C.; Bruzzi, P.; Perra, G.; Focardi, S.E. High Levels of Perfluorooctane Sulfonate in Children at the Onset of Diabetes. Int. J. Endocrinol. 2015, 2015, 234358. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, A.; Sinioja, T.; Lamichhane, S.; Sen, P.; Bodin, J.; Siljander, H.; Dickens, A.M.; Geng, D.; Carlsson, C.; Duberg, D.; et al. Prenatal Exposure to Perfluoroalkyl Substances Modulates Neonatal Serum Phospholipids, Increasing Risk of Type 1 Diabetes. Environ. Int. 2020, 143, 105935. [Google Scholar] [CrossRef] [PubMed]
- Küblbeck, J.; Vuorio, T.; Niskanen, J.; Fortino, V.; Braeuning, A.; Abass, K.; Rautio, A.; Hakkola, J.; Honkakoski, P.; Levonen, A.L. The EDCMET Project: Metabolic Effects of Endocrine Disruptors. Int. J. Mol. Sci. 2020, 21, 3021. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Zalko, D.; Jourdan, F.; Jacobs, M.; Fromenty, B.; Balaguer, P.; Bourguet, W.; Munic Kos, V.; Nadal, A.; Beausoleil, C.; et al. The GOLIATH Project: Towards an Internationally Harmonised Approach for Testing Metabolism Disrupting Compounds. Int. J. Mol. Sci. 2020, 21, 3480. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]

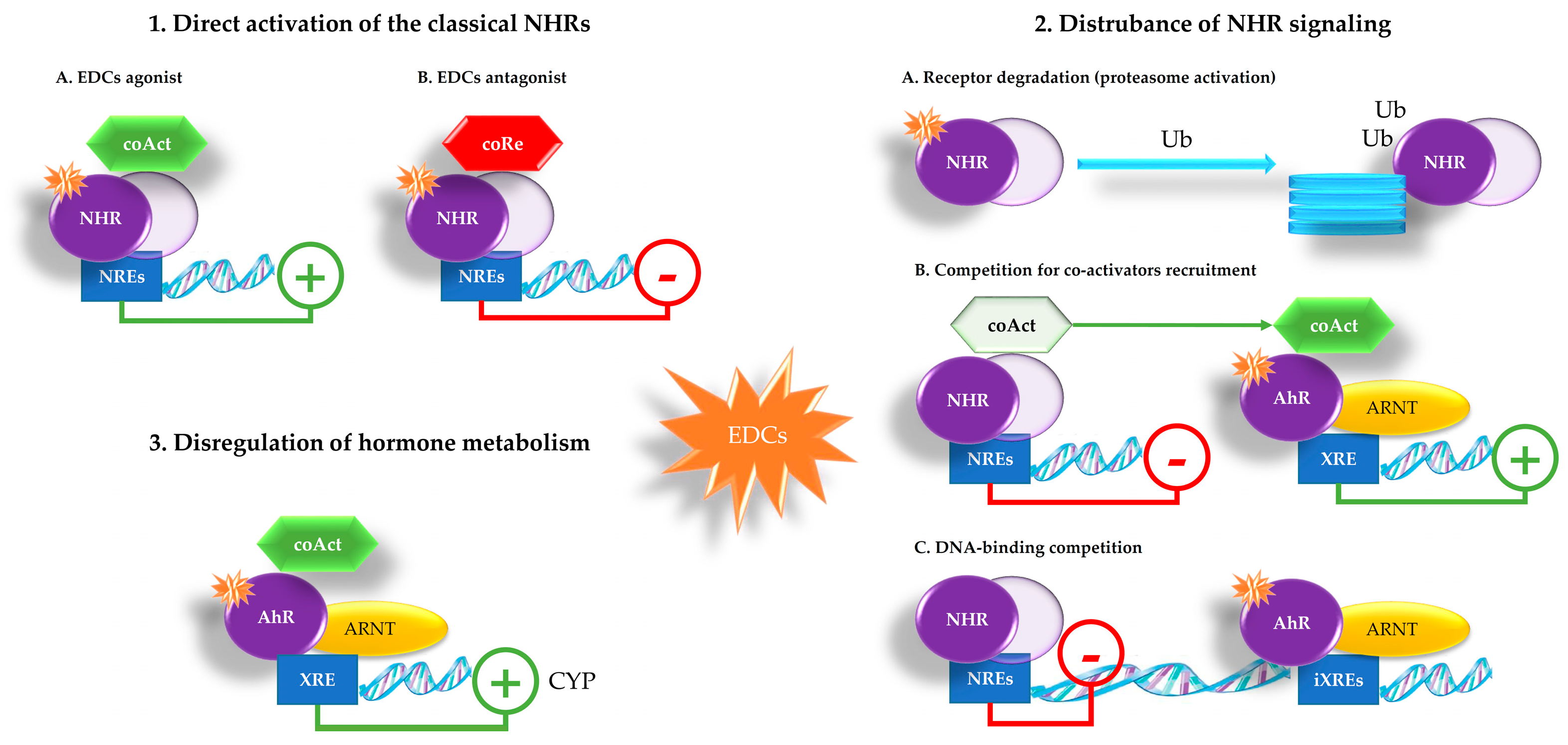
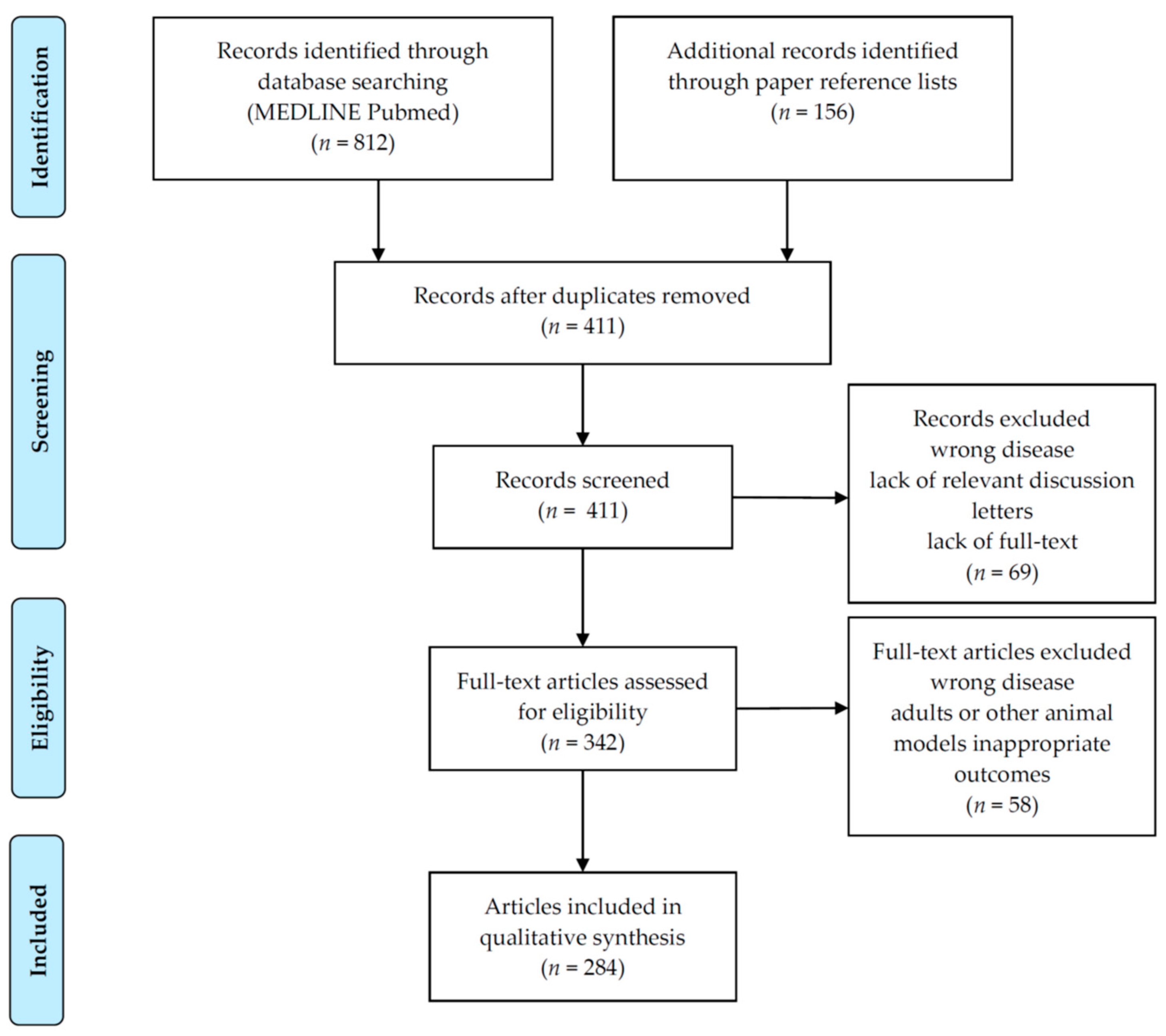
It is preferable to opt for:
|
| Avoid plastic containers for food and beverages. Food in plastic containers should not be heated in a microwave oven. Plastic containers can be replaced by glass or ceramic ones. |
| The consumption of fat dairy or meat products should be reduced. |
| Avoid handling receipts. |
| Personal care products should be free of phthalates, parabens, triclosan and other chemicals. |
| Avoid home cleaning products that are “antibacterial” or carry a fragrance. Regularly clean floors and windowsills to prevent buildup of dust. Indoors environments should be regularly ventilated. |
| Alternatives to plastic toys are preferred. |
| Replace or repair furniture that have torn or exposed foam. Flame retardant treated furniture should be avoided |
| Analytical Techniques | Advantages | Limitations |
|---|---|---|
Liquid chromatography methods
|
|
|
| Gas chromatography-MS |
|
|
| High-resolution gas chromatography-negative chemical ionization-MS |
|
|
| Food Production | The Most Common EDCs | |
|---|---|---|
| Agricultural chemicals Food additives Packaging materials | Bisphenols Organochlorines (OCs) PFASs Pesticides Phthalates |
|
| Industrial Activity | ||
| Air pollutants Industrial chemical and by-products Water contaminants | Dioxins Fracking fluids Nitrogen oxides Ozone Particulate matter Polyaromatic hydrocarbons (PAHs) PCBs Toxic metals | |
| Edcs Exposure | Study Population | Summary of the Main Results | Ref. |
|---|---|---|---|
| EDCs mixtures | |||
| Maternal occupational exposure to PAHs, PCBs, pesticides, phthalates, organic solvents, BPA, BFRs, metals, and miscellaneous | 133,957 mother-child pairs from 11 European cohorts | Higher risk of low BW for exposure to ≥1 EDCs group (OR = 1.25) and to ≥4 EDCs groups (OR = 2.11). The most specific EDCs were pesticides, phthalates, BFRs, and metals | [119] |
| Newborn dried blood spots concentrations of 11 PCBs, PBDE-47, and p,p′-DDE | 2065 newborn, US | Higher concentrations of PCB-52 and PCB-95 were associated with a slightly higher odds of LGA birth (OR = 1.02 and OR = 1.03, respectively) | [120] |
| Maternal blood samples concentrations for 4 pesticides, 4 PBDEs, 4 PCBs, and 4 PFAS (1st-trimester) | 604 pregnant women and their newborns’, US | Higher levels of PBDEs and p,p′-DDE were associated with lower BW and combinations with higher levels of PCBs and PFAS were associated with increased BW | [121] |
| Maternal serum samples concentrations of six secondary metabolites of phthalates, eight PFASs, PCB-153, and p,p′-DDE | 1250 newborns from Greenland, Poland, and Ukraine | MECPP, MeOHP, PFOA, and p,p′-DDE were predictors for lower BW, while exposure to mono(oxo-isononyl) phthalate was associated with higher BW | [122] |
| Maternal serum samples concentrations of 10 PCB congeners, p,p′-DDT and p,p′-DDE (24th or 36th week of pregnancy) | 324 pregnant women and their child, Germany | Significant negative association between the concentration of PCB-183 and length at birth. Concentrations of p,p′-DDE and several PCBs were positively correlated with weight gain in the first 2 years of life | [123] |
| Cord blood samples concentrations of four PCB congeners, p,p′-DDE, HCB, and heavy metals | 1579 mother-newborn pairs, four Flemish birth cohorts | p,p′-DDE and PCB-180 were most consistently associated with BW. An inverse association with BW was found for the PCBs congeners, while an increased BW was observed for elevated levels of p,p′-DDE | [124] |
| Maternal serum samples concentrations of 8 PFASs, 35 PCBs, and 9 pesticides (median of 15 weeks’ gestation) | 425 pregnant mother-daughter pairs, UK | EDCs mixture at the 75th centile compared to the 50th centile was associated with 0.15 lower weight-for-age z-score. At mean EDCs values for 19 months of age, a 0.15 lowering of the weight-for-age z-score corresponds to 0.18 kg lower weight. Weakly inverse associations were also seen for height-for-age and BMI-for-age z-scores | [125] |
| Bisphenols | |||
| Maternal blood (8–14 weeks’ gestation and delivery) and umbilical cord blood (delivery) samples concentrations of BPA | 80 pregnant women and their newborns’, US | Higher levels of unconjugated BPA both during the 1st-trimester and the end of gestation were associated with a gender-specific reduction in BW and an increase in length of pregnancy | [129] |
| Maternal urine (3rd-trimester) and neonatal urine samples concentrations of BPA | 788 mother-child couples at the 3rd-trimester of pregnancy and 366 mother-child couples during the neonatal period, Korea | BPA exposure was negatively associated with intrauterine linear growth. Maternal urinary BPA levels and birth outcomes were positively correlated | [130] |
| Maternal urine samples concentrations of BPA, BPF, and BPS (1st-, 2nd-, and 3rd-trimesters) | 845 pregnant women and their newborns’, China | Urinary BPS concentrations in the 1st-trimester were significantly associated with reduced BW and ponderal index while concentrations in the 2nd-trimester were significantly associated with reduced BW and birth length. Maternal exposure levels of BPF and BPS for newborns in the 10th percentile of BW and birth length were higher than the ones in the 90th percentile across the period of 10–36 weeks’ gestation | [131] |
| Maternal urine samples concentrations of BPA, BPF, and BPS | 1197 pregnant women and their newborns’, China | Maternal urinary BPA and BPF were negatively related to birth length and positively related to ponderal index. These associations were more pronounced in girls | [132] |
| Maternal urine samples concentrations of BPA, BPF, and BPS (1st-, 2nd-, and 3rd-trimesters) | 1379 pregnant women, Netherlands | Maternal BPS urine concentrations, especially during the 1st-trimester, were related with larger fetal head circumference, higher weight, and lower risk of being SGA at birth | [133] |
| Children spot urine samples concentrations of BPA | 754 children, China | Inverse association between urine BPA levels and height was observed in boys. Height z-score at enrolment decreased by 0.49 for the highest BPA exposure levels (90th centile), compared with the lowest ones (25th centile). The inverse association was confirmed considering data 19 months after the enrolment. | [134] |
| Phthalates | |||
| Maternal urine samples concentrations of DEHP metabolites (1st, 2nd, and 3rd trimesters) | 814 mother-offspring pairs, China | Among males, DEHP levels were negatively related to fetal growth at the 1st trimester, negatively related to both the BW and the birth length at the 2nd trimester, and positively associated with BW at the 3rd trimester. Among females, the 1st-trimester DEHP levels were associated with increased birth length. Data were longitudinally confirmed. Among males, DEHP levels were positively related to 24-month BMI at the 1st trimester, weight gain rates from birth to 24 months at the 2nd trimester, and BMI at 6 and 12 months at the 3rd trimester. Among females, the 2nd-trimester DEHP was negatively associated with BMI at 6 and 12 months | [139] |
| Maternal urine samples concentrations of 11 phthalates’ metabolites | 345 pregnant women and their child, US | Some metabolites resulted strongly associated with BMI z-scores, waist circumference z-scores, and body fat% in children of different ages. Specifically, in the 12-year-old children, in utero levels of DEP, DBP, and DEHP metabolites were positively associated with being overweight or obesity | [140] |
| Maternal blood samples concentrations of 32 metabolites of 15 phthalates diesters (18–34 weeks’ gestation) | 1342 females, Australia | During infancy, a weak negative association was observed between height z-score changes and MHBP, MCiOP, and MEP concentrations. From 2 to 10 years of age, a weak positive relationship between height z-score and higher exposure to MBzP and MECPP was detected. At 20 years of follow-up, associations between phthalates levels and deviation from mid-parental height were not found. Similar results were reported for weight z-score | [141] |
| Pesticides | |||
| Estimated agricultural use of methyl bromide near each woman’s for at least one trimester of pregnancy | 442 pregnant women and their newborns’, US | Pesticide exposure during the 2nd trimester of pregnancy was negatively associated with weight, length, and head circumference at birth | [142] |
| Drinking-water exposure to atrazine metabolites and nitrates mixture during 2nd trimester | 11,446 pregnant woman-neonate couples, France | Incidence of SGA newborns was increased in exposed mothers. At the 2nd trimester, exposure to 2nd tercile of nitrates without atrazine significantly increased the risk of SGA (OR = 1.74) | [144] |
| Maternal hair concentrations of 64 pesticides | 311 women and their newborns’, France | A significantly higher BW was found for a medium but not a high level of exposure to fipronil sulfone, compared to the lowest exposure level | [145] |
| History of pesticides exposure in perinatal period, infancy, and childhood | 48 children with stunting and 112 controls, Indonesia | Median IGF-1 levels were significantly lower in cases compared to controls. The high level of pesticide exposure and the low IGF-1 levels were significantly associated with stunting | [147] |
| Cord blood samples concentrations of p,p′-DDT, p,p′-DDE, HCB, and 7 PCBs congeners | 379 children, Spain | HCB exposure in the 3rd tertile, compared to the 1st tertile, was associated with higher BMI and WHtR z-score. A continuous increase in HCB levels was associated with higher body fat%, blood pressure z-score (across all ages), cardiometabolic-risk score, and lipid biomarkers (at 14 years). p,p′-DDT exposure was associated with increased cardiometabolic-risk score | [153] |
| Per- and polyfluoroalkyl substances | |||
| Maternal and cord blood samples concentrations of PFOS, PFOA, DEHP, and MEHP | 29 mother-newborn pairs, Italy | High exposure to PFASs, especially the PFOA, was associated with low BW in newborns | [157] |
| Maternal blood samples concentrations of PFASs and five OCs | 424 mother-child pairs, Norway and Sweden | Prenatal exposure to PFOA, PCB-153 and HCB were associated with higher odds for SGA birth among Swedish mothers. The associations between PFOA and SGA birth were stronger among male offspring | [158] |
| Newborn dried blood spots concentrations of PFOA, PFOS, PFNA, PFHxS, 3 PBDE, and p,p′-DDE | 52 infants with overweight status (at 18 months) and 46 infants with healthy weight status (at 18 months), US | High concentrations of PFOS and PFHxS were associated with lower BW z-score compared to those with low concentrations (more prominent in males than in females). Associations with infant overweight status were not found | [159] |
| Maternal blood serum samples concentrations of eight PFASs (3–27 weeks’ gestation) | 1533 mother–newborn pairs, Sweden | Increased concentrations of PFOS, PFOA, PFNA, PFDA, and PFUnDA were significantly associated with lower BW and BW z-score. Prenatal exposure for PFOS, PFOA, PFNA, and PFDA was also significantly associated with being born SGA. Associations were significant only in girls | [160] |
| Maternal blood samples concentrations of PFOA, PFOS, PFNA, and PFHxS (16 or 26 weeks’ gestation, or within 48 h from delivery) | 345 pregnant women and their child, US | PFASs concentrations, particularly PFOA, were inversely associated with weight and length/height measurements of infant/child from 4 weeks to 2 years of age | [161] |
| Brominated flame retardants | |||
| Maternal blood samples concentrations of 10 PBDE congeners (near 26th weeks’ gestation) | 286 pregnant women and their newborns, US | Negative associations with BW were seen for BDE-47, BDE-99, and BDE-100. Each 10-fold increase in their concentrations was associated with an approximately 115 g decrease in BW | [165] |
| Maternal blood (near 12th week gestation) and umbilical cord samples concentrations of 14 PBDEs | 686 pregnant women and their newborns, Spain | Inverse associations between BDE-99 and BW and birth head circumference | [166] |
| Maternal blood samples concentrations of PBDEs and PCBs (mean 12 week gestation) | 349 pregnant women and their newborns, Canada | No associations between PBDEs exposure and birth outcomes | [167] |
| Placental samples concentrations of eight PBDEs congeners | 996 pregnant women, China | Prenatal exposure to high level of PBDEs was associated with increased risk of SGA (OR = 2.20) | [168] |
| Maternal serum samples concentrations of 19 PBDEs congeners (3rd-trimester) | 202 maternal-infant pairs (101 with fetal growth restriction cases and 101 controls), China | Concentrations of BDE-207, -208, -209, and the sum of 19 PBDEs were higher in newborns with fetal growth restriction compared with the healthy ones. Increased PBDEs levels were related to decreased placental length, breadth, surface area, BW, birth length, gestational age, and Quetelet index of newborns | [169] |
| Edcs Exposure | Study Population | Summary of the Main Results | Ref. |
|---|---|---|---|
| Bisphenol A | |||
| Urine samples | 28 girls with idiopathic CPP and 25 healthy girls, Turkey | BPA levels were significantly higher in the idiopathic CPP group compared with the control group, but they did not correlate with basal serum LH, FSH, and E2 levels | [182] |
| Urine samples | 41 girls with advanced puberty and 47 age-matched controls, Thailand | BPA levels were significantly higher in the advanced puberty group compared with the control group. The median adjust-BPA concentration in girls with advanced puberty who were overweight/obese was greater than in the normal pubertal overweight/obese girls | [183] |
| Urine samples | 25 girls with premature thelarche and 25 healthy age-matched girls, Turkey | BPA levels were significantly higher in girls with premature thelarche compared to the health control group. Weak positive correlations with uterus volume, E2, and LH levels were found | [184] |
| Urine samples | 987 adolescents, US | BPA levels appear to be associated with delayed menarche, particularly for moderate levels of BPA exposure | [185] |
| Urine samples | 655 adolescents, China | Girls with intermediate and high levels of BPA were more likely to have delayed menarche compared to the ones with undetectable levels | [186] |
| Phthalates | |||
| Blood samples | 41 girls with premature thelarche and 35 healthy controls, US | Higher levels of phthalates were demonstrated in girls with premature thelarche; specifically, measurable values of phthalates were found in 68% of girls with premature thelarche compared to 14% of healthy controls | [189] |
| Urine samples | 29 girls with premature thelarche and 25 healthy age-matched girls, Turkey | MEHP concentrations in girls with premature thelarche were significantly higher (~2 times) than in the control group | [190] |
| Urine samples | 725 girls, Denmark | The highest quartile of urinary phthalates excretion was associated with pubarche delay. No association between phthalates and breast development was found. No difference in urinary phthalate metabolite levels was demonstrated between girls with precocious puberty and controls | [191] |
| Urine samples | 168 children (84 girls), Denmark | Early pubarche was shown in most exposed boys who also had higher testosterone and lower adrenal hormone levels. Pubarche was not altered in most exposed girls | [192] |
| Urine samples | 1051 girls, US | The earlier menarche age, the higher levels of urinary high molecular weight phthalates measured several years before | [193] |
| Maternal blood serum (18 and 34–36 weeks’ gestation) | 369 girls, US | The age at menarche, despite still within the normal range, was slightly delayed in girls exposed at the middle tertile concentration compared to the ones exposed at the lowest tertile | [140] |
| Urine samples | 200 girls, Chile | Higher phthalate concentrations were reported to be associated with earlier menarche among overweight/obese girls | [194] |
| Maternal urine samples (twice during pregnancy) | 338 children (179 girls), US | In utero exposure to phthalates was associated with delayed puberty in females, especially in normal weight ones, and with early puberty in males, especially in overweight/obese ones | [198] |
| Pesticides | |||
| Maternal blood samples concentrations of PCBs, p,p′-DDE and other OCs | 259 pregnant women and their 213 daughters, US | The earlier was the onset of menarche in girls, the higher was the in utero exposure to p,p′-DDE. The menarche was advanced by about one year for each increase in in utero exposure of 15 g/L | [205] |
| Serum samples concentrations of p,p′-DDT and its major metabolites | 466 women, China | A significant dose–response association between serum p,p′-DDT concentrations and earlier menarche was demonstrated. The mean age at menarche was younger (−1.11 years) in women in the 4th p,p′-DDT quartile compared to the ones in the lowest quartile | [206] |
| Maternal serum samples concentrations of 9 OCs (pregnancy) | 218 girls (case—menarche < 11.5 years) and 230 girls (controls—menarche ≥ 11.5 years), England | No association between in utero exposure to OCs pesticides and early menarche was found | [207] |
| Blood samples concentrations of 2 pesticides, p,p′-DDE 7 dioxins, 41 PCBs, and 10 furans | 516 boys, Russia | PCBs, OCs pesticides, and Pb may delay puberty in boys which pubertal staging and testicular volume were annually evaluated (from 8–9 years until 18–19 years) | [209] |
| Brominated flame retardants | |||
| Serum samples concentrations of 6 PBDEs congeners | 271 adolescent girls, US | Higher serum PBDEs levels were associated with earlier menarche age | [214] |
| Serum samples concentrations of PBDEs | 124 girls (37 with idiopathic CPP, 56 with premature thelarche, and 31 controls), Italy | Serum PBDEs levels were significantly higher in girls with premature thelarche than in controls | [215] |
| Maternal serum (near the time of birth) and breast milk samples concentrations of PBBs and PCBs | 327 girls, US | Menarche was found to be 1 year earlier in girls who were exposed to high PBBs concentrations in utero and in early infancy through breastfeeding than in girls not exposed or exposed only in utero | [216] |
| Maternal blood (during pregnancy) and children (9 years old) samples concentrations of 4 PBDEs | 623 children (314 girls), US | Prenatal PBDEs concentrations were associated with delayed menarche in girls and early pubarche in boys. No association was demonstrated with breast and pubic hair (in girls) and genitals (boys) development. PBDEs concentrations measured during childhood were not associated with alterations in pubertal timing | [217] |
| EDCs Exposure | Study Population | Summary of the Main Results | Ref. |
|---|---|---|---|
| Bisphenol A | |||
| Maternal serum (10–17 weeks’ gestation) | 334 infants, UK | BPA concentrations were positively associated with risk of congenital or acquired cryptorchidism | [227] |
| Phthalates | |||
| Interview on maternal occupational exposure during the 1st trimester of pregnancy | 471 hypospadias cases and 490 controls, UK | High rate of hypospadias was reported in children of mothers who were exposed to phthalates at work compared with those with no exposure (OR = 3.65) | [229] |
| Maternal occupational exposure | 1202 cases of hypospadias and 2583 controls, Australia | Increased risk of hypospadias in children whose mothers worked in the hairdressing, beauty, or cleaning industry | [230] |
| Amniotic fluid samples concentrations of DEHP and DiNP (2nd trimester pregnancy) | 270 cryptorchidism cases, 75 hypospadias cases, and 300 controls, Denmark | DiNP metabolites were associated with an increased likelihood of hypospadias (OR = 1.69). Concentrations of DEHP were not associated with hypospadias | [231] |
| Maternal urine samples concentrations of seven phthalates | 111 pregnant women, Japan | An inverse association between maternal urine phthalates concentrations and AGD was found in boys, but not in girls | [232] |
| Maternal urine samples concentrations of 11 phthalate (1st-trimester) | 753 pregnant women and their children (380 boys), US | MEHP, MEOHP, and MEHHP concentrations were significantly and inversely associated with measures of boys’ AGD | [233] |
| Maternal urine samples concentrations of 12 phthalate (28 weeks’ gestation) | 245 mother–son pairs, Denmark | No association between phthalates exposure in late pregnancy and AGD in infants at 3 months of age | [234] |
| Dioxins | |||
| Placenta samples concentrations of 17 dioxins and 37 PCBs | 95 cryptorchidism cases and 185 controls, Finland and Denmark | No association between placenta levels of dioxins or PCBs and congenital cryptorchidism was found at birth and after 3 months | [236] |
| Maternal serum samples concentrations of TCDD (extrapolation from the concentrations measured soon after the explosion) | 39 men (born to mothers exposed to dioxin) and 58 controls, Italy | Men exposed to relatively low dioxin doses in utero and through breastfeeding had a permanently reduced sperm quality (lower sperm concentration, total count, progressive motility, and total motile count) | [237] |
| Pesticides | |||
| Maternal breast milk samples concentrations of eight pesticides (1–3 months postpartum) | 62 boys with cryptorchidism and 68 controls, Finland and Denmark | Pesticides were found in higher concentrations in 3 months-old boys with cryptorchidism than in controls; no individual compound was significantly correlated with the disease | [238] |
| Maternal serum and breast milk samples concentrations of seven PCBs, p,p′-DDE, and DBP (3–5 days post-partum) | 164 mother-infant pairs (78 cryptorchid and 86 controls), France | Children exposed to high prenatal concentrations of PCBs and possibly also p,p′-DDE have a higher risk for congenital cryptorchidism | [239] |
| Maternal serum samples concentrations of PCB-153, p,p′-DDE, and HCB (14 weeks gestation) | 237 children with hypospadias and 237 controls, Sweden | Increased risk of hypospadias among children from women with p,p′-DDE concentrations at the highest quartile (compared to those in the 1st quartile) and with the highest exposure quartile of HCB (compared to the three lowest quartile) (OR = 1.65) | [240] |
| Maternal serum (3rd trimester) samples concentrations of p,p′-DDE and 11 PCBs | 217 children with cryptorchidism, 197 with hypospadias, and 557 controls, US | No association was found between EDCs’ levels and cryptorchidism/hypospadias | [241] |
| Questionnaire to estimate total maternal consumption of atrazine via drinking water (6–16 post-conception) | 343 children with hypospadias and 1422 controls, US | A weak association between hypospadias and maternal consumption of atrazine was found | [242] |
| EDCs and Sample | Study Population | Summary of the Main Results | Ref. |
|---|---|---|---|
| Bisphenols | |||
| Maternal urine samples concentrations of bisphenols (<18, 18–25, >25 weeks’ gestation) | 1267 pregnant women, 853 newborns and 882 children, Netherlands | Higher late pregnancy maternal BPA levels were associated with higher TSH levels in female newborns and higher FT4 levels in males during childhood | [252] |
| Phthalate | |||
| Maternal urine samples concentrations of nine phthalate (16 and 26 weeks’ gestation) | 389 pregnant women and their newborns, US | Alterations of thyroid hormone levels were demonstrated in both the maternal serum and the umbilical cord blood. Specifically, at 16 weeks of pregnancy for each 10-fold increase in maternal urinary MEP, maternal serum total thyroxine decreased by 0.52 μg/dL. At 16- and 26-weeks’ gestation, for each 10-fold increase in average of maternal urinary MBzP, cord serum TSH decreased by 19% | [254] |
| Maternal urine (3rd-trimester) and cord blood samples concentrations of five phthalate | 61 pregnant women and their newborns, Taiwan | High MBP levels in umbilical cord blood levels were significantly and negatively associated with cord serum TSH and FT4 | [255] |
| Dioxins | |||
| Prenatal TCDD exposure as: (1) maternal initial TCDD concentration measured in serum samples collected soon after exposure and (2) maternal TCDD estimated at pregnancy | 570 children (288 girls), Italy | Compared to the lowest quartile, maternal serum TCDD concentrations at higher quartiles were associated with lower FT3. Children with high thyroid antibodies had significantly inverse associations between maternal serum TCDD and both the TSH and the FT3 levels were stronger than in subjects with normal antibody status. Similar results were found for TCDD estimated at pregnancy | [256] |
| Pesticides | |||
| Cord blood and breast milk (14 days after delivery) samples concentrations of PCB-153, p,p′-DDE, and HCB | 1784 mother–child pairs, Belgium, Norway, and Slovakia | Early-life exposure to PCB-153 and p,p′-DDE was demonstrate to impact newborns’ TSH levels. Newborns in the 3rd-exposure quartile had TSH lower by 12–15% | [257] |
| Maternal blood samples concentrations of PCBs (3rd trimester) | 497 mother-newborn pairs, Japan | Exposure to PCBs during pregnancy was demonstrated to increase maternal and neonatal FT4 levels | [258] |
| Per- and polyfluoroalkyl substances | |||
| Maternal plasma samples concentrations of six PFASs (early pregnancy) | 726 pregnant women and 465 neonates, US | In infants, higher concentrations of PFHxS were associated with lower total thyroxine levels, mainly in males | [259] |
| Brominated flame retardants | |||
| Cord blood and blood (age 2–3 years) samples concentrations of PBDEs | 158 children, US | Children with high exposure to BDE-47 during the prenatal period or toddler age had significantly lower mean TSH levels compared to the ones with low exposure throughout early life. Associations with postnatal exposure may be stronger among boys compared to girls | [260] |
| EDCs and Sample | Study Population | Summary of the Main Results | Ref. |
|---|---|---|---|
| Bisphenols | |||
| Maternal urine samples concentrations of BPA (6.3–15 weeks’ gestation) | 719 mother–child pairs, Canada | Higher levels of BPA concentrations were associated with increased central adiposity outcomes among girls during early childhood | [271] |
| Urine samples concentrations of BPA | 210 boys, Spain | BPA concentrations were associated with increased BMI z-scores and a higher risk of overweight/obesity. Children with higher urinary BPA concentrations also had higher WHtR values and a greater risk of abdominal obesity | [272] |
| Food concentrations of BPA and BPS and estimation of dietary exposure | 585 adolescents (46.6% girls), Spain | Girls <14 years old compared to the older ones had a greater risk for high dietary exposure (3rd tercile) to bisphenols overall (OR = 4.77), as well as BPS (OR = 4.24). Overweight/obese girls were at a greater risk of having high dietary exposure to total bisphenols (OR = 2.81) and BPA (OR = 3.38) than normal weight ones. The more sedentary boys had a greater risk of being included in the 3rd-tercile with regard to bisphenols dietary exposure (OR = 1.10) | [273] |
| Maternal urine sample concentrations of BPA | 218 pregnant women and their children 2 years old, China | BPA exposure during prenatal period was associated with increased blood pressure in girls and blood glucose in boys | [275] |
| Urine sample concentrations of BPA | 62 girls with PCOS and 33 controls, Turkey | Adolescents with PCOS had significantly increased levels of BPA when compared with the control group. BPA levels were significantly correlated with polycystic morphology on ultrasound but not with obesity androgen levels, or other metabolic parameters | [276] |
| Phthalates | |||
| Maternal urine samples concentrations of 11 phthalate and 9 phenols (14.0 and 26.9 weeks’ gestation) | 309 pregnant women and their children 5 years old, US | Prenatal urinary concentrations of MEP, MCNP, and cumulative mixture were associated with an increased BMI z-score and overweight/obesity status at age 5 | [278] |
| Per- and polyfluoroalkyl substances | |||
| Maternal serum samples concentrations of two PFASs and five OCs (17–20 weeks’ gestation) | 412 pregnant women and their children with overweight/obesity, Norway and Sweden | In children at 5-year follow-up, both the BMI z-score and the triceps skinfold z-score were found to be increased per logarithmic-unit increase in maternal serum PFOS concentrations. Increased odds for child overweight/obesity for each logarithmic-unit increase in maternal serum PFOS and PFOA levels were found | [280] |
| Serum samples concentrations of PFOS and PFOA | 25 children at the onset of T1D and 19 controls, Italy | PFOS levels were significantly higher in patients at the onset of T1D compared to healthy controls | [281] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predieri, B.; Iughetti, L.; Bernasconi, S.; Street, M.E. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? Int. J. Mol. Sci. 2022, 23, 11899. https://doi.org/10.3390/ijms231911899
Predieri B, Iughetti L, Bernasconi S, Street ME. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? International Journal of Molecular Sciences. 2022; 23(19):11899. https://doi.org/10.3390/ijms231911899
Chicago/Turabian StylePredieri, Barbara, Lorenzo Iughetti, Sergio Bernasconi, and Maria Elisabeth Street. 2022. "Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn?" International Journal of Molecular Sciences 23, no. 19: 11899. https://doi.org/10.3390/ijms231911899
APA StylePredieri, B., Iughetti, L., Bernasconi, S., & Street, M. E. (2022). Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? International Journal of Molecular Sciences, 23(19), 11899. https://doi.org/10.3390/ijms231911899









