Current Development and Future Application Prospects of Plants-Derived Polyphenol Bioactive Substance Curcumin as a Novel Feed Additive in Livestock and Poultry
Abstract
:1. Introduction
2. Methods
3. Results
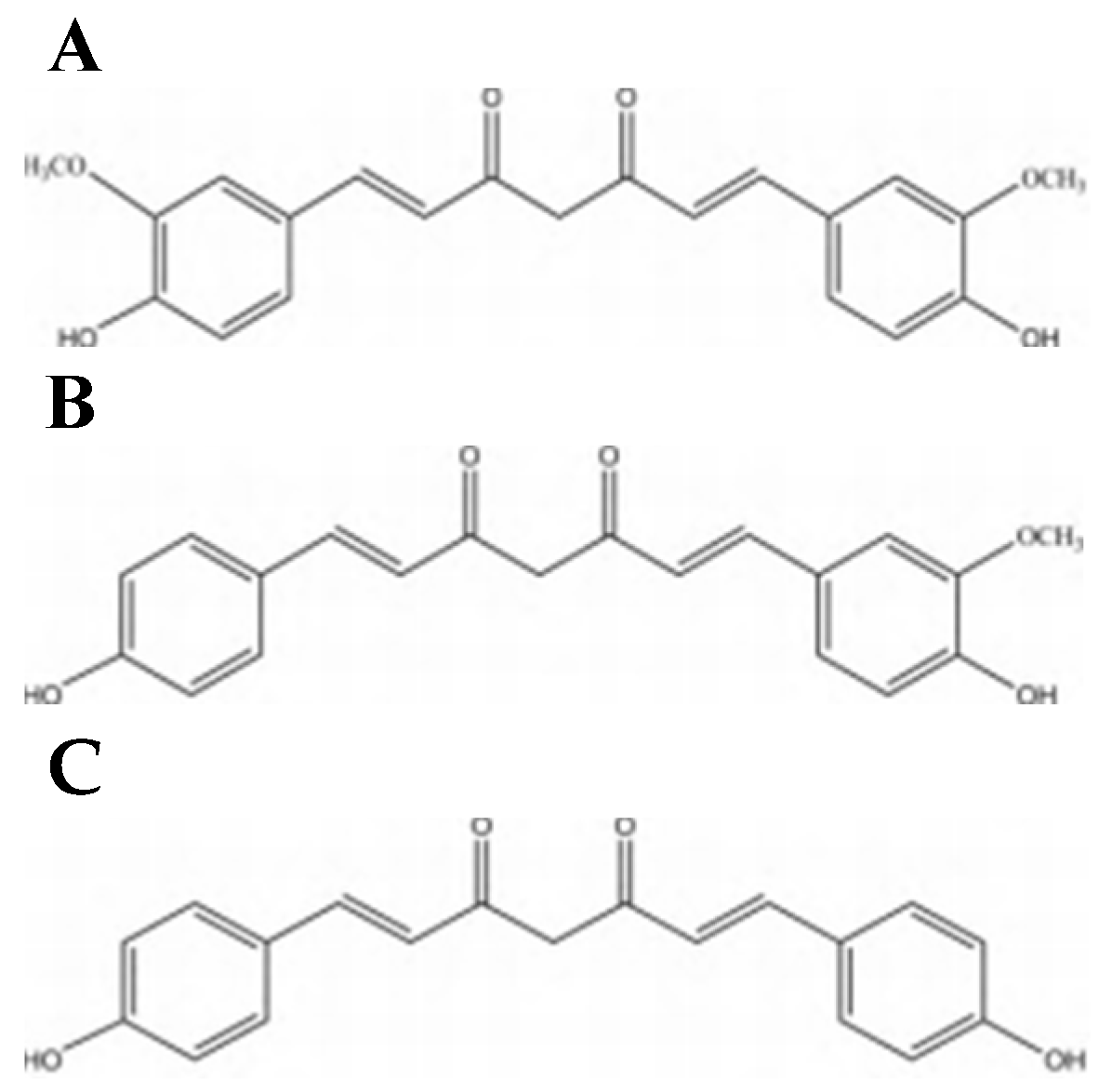
3.1. Physical and Chemical Properties of CUR
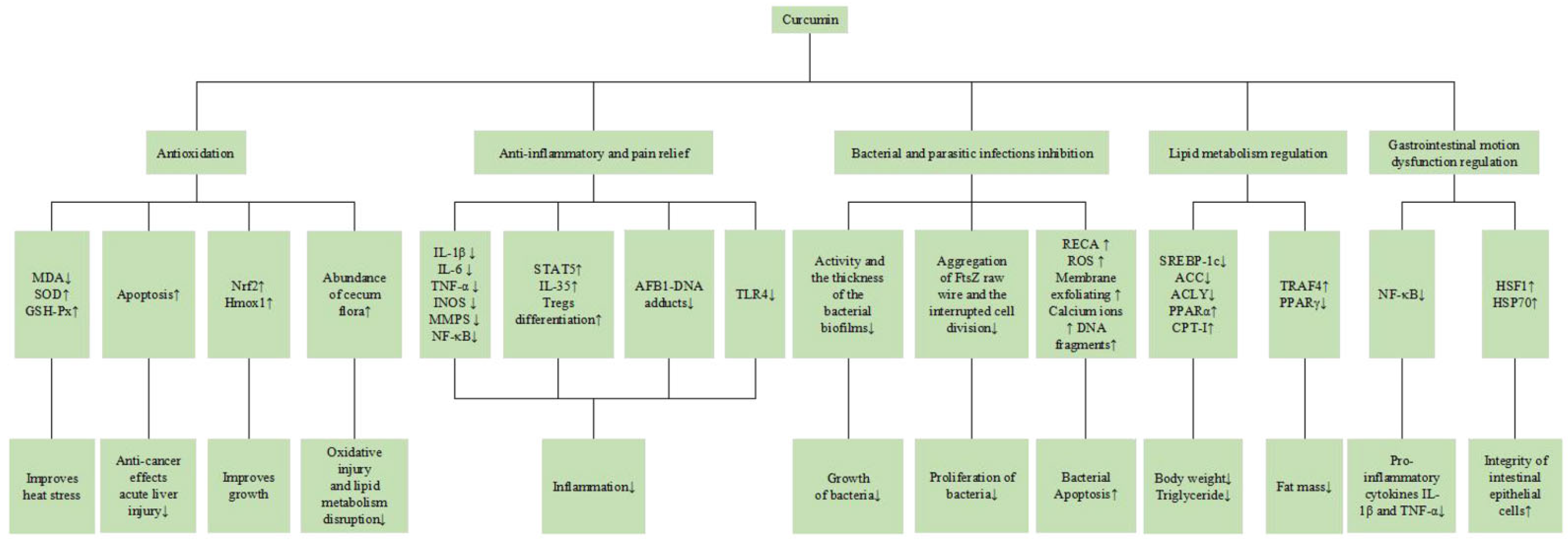
3.2. Biological Functions of CUR
3.2.1. Antioxidation
3.2.2. Anti-Inflammatory and Pain Relief
3.2.3. Bacterial and Parasitic Infections Inhibition
3.2.4. Lipid Metabolism Regulation
3.2.5. Gastrointestinal Motion Dysfunction Regulation
3.3. Application Research Progress of CUR in the Livestock and Poultry Production
3.3.1. Application of CUR in Poultry Production
3.3.2. Application of CUR in Pig Production
3.3.3. Application of CUR in the Ruminant Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Haulisah, N.A.; Hassan, L.; Bejo, S.K.; Jajere, S.M.; Ahmad, N.I. High levels of antibiotic resistance in isolates from diseased livestock. Front. Vet. Sci. 2021, 8, 652351. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Mk, U.; Ke, V.; Mukhopadhyay, C.; U, D.A.; M, S.R.; V, R. Antimicrobial resistance and the post antibiotic era: Better late than never effort. Expert Opin. Drug Saf. 2021, 20, 1375–1390. [Google Scholar] [CrossRef]
- Khin, M.; Jones, A.M.; Cech, N.B.; Caesar, L.K. Phytochemical analysis and antimicrobial efficacy of macleaya cordata against extensively drug-resistant staphylococcus aureus. Nat. Prod. Commun. 2018, 13, 1934578X1801301117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simó-Mirabet, P.; Piazzon, M.C.; Calduch-Giner, J.A.; Ortiz, Á.; Puyalto, M.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Sodium salt medium-chain fatty acids and Bacillus-based probiotic strategies to improve growth and intestinal health of gilthead sea bream (Sparus aurata). PeerJ 2017, 5, e4001. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.T.; Hwang, J.A.; Hoon, J.; Mun, H.S.; Yang, C.J. Comparison of single and blend acidifiers as alternative to antibiotics on growth performance, fecal microflora, and humoral immunity in weaned piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 93–100. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lv, J.; Dou, X.; Zhang, Y. Effects of dietary supplementation with clostridium butyricum on the amelioration of growth performance, rumen fermentation, and rumen microbiota of holstein heifers. Front. Nutr. 2021, 8, 763700. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Ugolini, L.; Scarafile, D.; Matteo, R.; Pagnotta, E.; Malaguti, L.; Lazzeri, L.; Modesto, M.; Checcucci, A.; Mattarelli, P.; Braschi, I. Effect of bioactive compounds released from Brassicaceae defatted seed meals on bacterial load in pig manure. Environ. Sci. Pollut. Res. Int. 2021, 28, 62353–62367. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Camacho, D.; Vinyeta, E.; Pérez, J.F.; Aumiller, T.; Criado, L.; Palade, L.M.; Taranu, I.; Folch, J.M.; Calvo, M.A.; Van der Klis, J.D.; et al. Phytogenic actives supplemented in hyperprolific sows: Effects on maternal transfer of phytogenic compounds, colostrum and milk features, performance and antioxidant status of sows and their offspring, and piglet intestinal gene expression. J. Anim. Sci. 2020, 98, skz390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual two-way interactions of curcumin and gut microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [Green Version]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Faehnrich, B.; Lukas, B.; Humer, E.; Zebeli, Q. Phytogenic pigments in animal nutrition: Potentials and risks. J. Sci. Food Agric. 2016, 96, 1420–1430. [Google Scholar] [CrossRef]
- Nawab, A.; Tang, S.; Li, G.; An, L.; Wu, J.; Liu, W.; Xiao, M. Dietary curcumin supplementation effects on blood immunological profile and liver enzymatic activity of laying hens after exposure to high temperature conditions. J. Therm. Biol. 2020, 90, 102573. [Google Scholar]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.; Okoth, S.A.; Sultanbawa, Y. Curcumin-based photosensitization inactivates Aspergillus flavus and reduces aflatoxin B1 in maize kernels. Food Microbiol. 2019, 82, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and intestinal inflammatory diseases: Molecular mechanisms of protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, X.; Shu, S.; Zhang, W.; Fang, B.; Chen, X.; Zhao, Y.; Liu, Z.; Liang, G. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI). Eur. J. Med. Chem. 2019, 167, 414–425. [Google Scholar] [CrossRef]
- Kumar, P.; Saha, T.; Behera, S.; Gupta, S.; Das, S.; Mukhopadhyay, K. Enhanced efficacy of a Cu(2+) complex of curcumin against Gram-positive and Gram-negative bacteria: Attributes of complex formation. J. Inorg. Biochem. 2021, 222, 111494. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; Jiang, X.; Yang, J.; Cao, Z.; He, Y.; Feng, D. Curcumin supplementation ameliorates bile cholesterol supersaturation in hamsters by modulating gut microbiota and cholesterol absorption. Nutrients 2022, 14, 1828. [Google Scholar] [CrossRef]
- Hong, S.; Dia, V.P.; Zhong, Q. Synergistic anti-inflammatory activity of apigenin and curcumin co-encapsulated in caseins assessed with lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 193, 702–712. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Lüer, S.C.; Goette, J.; Troller, R.; Aebi, C. Synthetic versus natural curcumin: Bioequivalence in an in vitro oral mucositis model. BMC Complement. Altern. Med. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopresti, A.L. The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila) 2013, 6, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Gupta, A.K.; Chávez-González, M.L.; Aguilar, C.N.; Chakravorty, N.; et al. Curcumin extraction, isolation, quantification and its application in functional foods: A review with a focus on immune enhancement activities and COVID-19. Front. Nutr. 2021, 8, 747956. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Bunsuwansakul, C.; Leonard, T.E.; Laohaprapanon, S.; Hounkong, K.; Bunluepuech, K.; Kaewjai, C.; Mahboob, T.; Sumudi Raju, C.; Dhobi, M.; et al. Curcuma longa ethanol extract and Curcumin inhibit the growth of acanthamoeba triangularis trophozoites and cysts isolated from water reservoirs at walailak university, Thailand. Pathog. Glob. Health. 2020, 114, 194–204. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, L.; Zhang, L.; Ying, Z.; Su, W.; Wang, T. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 2014, 144, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; He, K.; Cao, T.; Song, D.; Yang, H.; Li, L.; Lin, J. The apoptosis of liver cancer cells promoted by curcumin/TPP-CZL nanomicelles with mitochondrial targeting function. Front. Bioeng. Biotechnol. 2022, 10, 804513. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, Y.; Gao, P.; Xie, X.; Li, D.; Yu, D.; Yu, M. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult. Sci. 2020, 99, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Da Silva, A.S.; Biazus, A.H.; Reis, J.H.; Boiago, M.M.; Topazio, J.P.; Migliorini, M.J.; Guarda, N.S.; Moresco, R.N.; Ourique, A.F.; et al. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res. Vet. Sci. 2018, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Dietary curcumin supplementation increases antioxidant capacity, upregulates Nrf2 and Hmox1 levels in the liver of piglet model with intrauterine growth retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.S.; Ruan, D.; Zhu, Y.W.; Li, M.C.; Ye, H.; Wang, W.C.; Yang, L. Protective effect of curcumin on ochratoxin A-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020, 99, 1124–1134. [Google Scholar] [CrossRef]
- Béguin, E.P.; van den Eshof, B.L.; Hoogendijk, A.J.; Nota, B.; Mertens, K.; Meijer, A.B.; van den Biggelaar, M. Integrated proteomic analysis of tumor necrosis factor α and interleukin 1β-induced endothelial inflammation. J. Proteom. 2019, 192, 89–101. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chai, Y.S.; Xie, K.; Yu, F.; Wang, C.J.; Lin, S.H.; Yang, Y.Z.; Xu, F. Curcumin promotes the expression of IL-35 by regulating regulatory T cell differentiation and restrains uncontrolled inflammation and lung injury in mice. Inflammation 2020, 43, 1913–1924. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary curcumin alleviated acute ileum damage of ducks (anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef] [PubMed]
- Eke-Okoro, U.J.; Raffa, R.B.; Pergolizzi, J.V.; Breve, F.; Taylor, R. For the NEMA Research Group Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia. J. Clin. Pharm. Ther. 2018, 43, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Ortega, L.A.; Acosta-Osorio, A.A.; Grube-Pagola, P.; Palmeros-Exsome, C.; Cano-Sarmiento, C.; García-Varela, R.; García, H.S. Anti-inflammatory activity of curcumin in gel carriers on mice with atrial edema. J. Oleo Sci. 2020, 69, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yin, L.; Ramage, G.; Li, B.; Tao, Y.; Zhi, Q.; Lin, H.; Zhou, Y. Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. MicrobiologyOpen 2019, 8, e937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial mechanism of curcumin: A review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.C.; Simon, S.D.; Jin, Z.; Gui, Y.; Xu, B.; Xu, Z.; Rosado-de-Castro, P.H.; Silveira Braghirolli, A.M.; Barbosa da Fonseca, L.M.; Inoue, T.; et al. Efficient synthesis of glutamate peptide-estradiol conjugate for imaging estrogen receptor-positive diseases. BioMed. Res. Int. 2018, 2018, 5208964. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 14204. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Souza Dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Disbanchong, P.; Punmanee, W.; Srithanasuwan, A.; Pangprasit, N.; Wongsawan, K.; Suriyasathaporn, W.; Chuammitri, P. Immunomodulatory effects of herbal compounds quercetin and curcumin on cellular and molecular functions of bovine-milk-isolated neutrophils toward streptococcus agalactiae infection. Animals 2021, 11, 3286. [Google Scholar] [CrossRef]
- Laorodphun, P.; Cherngwelling, R.; Panya, A.; Arjinajarn, P. Curcumin protects rats against gentamicin-induced nephrotoxicity by amelioration of oxidative stress, endoplasmic reticulum stress and apoptosis. Pharm. Biol. 2022, 60, 491–500. [Google Scholar] [CrossRef]
- Erental, A.; Kalderon, Z.; Saada, A.; Smith, Y.; Engelberg-Kulka, H. Apoptosis-like death, an extreme SOS response in escherichia coli. Mbio 2014, 5, e01426. [Google Scholar] [CrossRef]
- Erental, A.; Kalderon, Z.; Saada, A.; Smith, Y.; Engelberg-Kulka, H. Correction for Erental et al., Apoptosis-like death, an extreme SOS response in escherichia coli. Mbio 2020, 11, e03040. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.Y.; Tang, J.; Tian, H.T.; Shi, Y.Y.; Jia, J. Adipocyte-secreted microvesicle-derived miR-148a regulates adipogenic and osteogenic differentiation by targeting Wnt5a/Ror2 pathway. Life Sci. 2021, 278, 119548. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019, 98, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, R.; Chen, W.; Liu, Y.; Liao, X.; Zeng, B.; Guo, G.; Lou, F.; Xiang, Y.; Wang, Y.; et al. Curcumin prevents obesity by targeting TRAF4-induced ubiquitylation in m(6) A-dependent manner. EMBO Rep. 2021, 22, e52146. [Google Scholar] [CrossRef]
- Reisinger, N.; Emsenhuber, C.; Doupovec, B.; Mayer, E.; Schatzmayr, G.; Nagl, V.; Grenier, B. Endotoxin translocation and gut inflammation are increased in broiler chickens receiving an oral lipopolysaccharide (LPS) bolus during heat stress. Toxins 2020, 12, 622. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, R.; Xiong, S.; Zhang, C.; Zhang, Y. Protective effects of curcumin against rat intestinal inflammation-related motility disorders. Mol. Med. Rep. 2021, 23, 391. [Google Scholar] [CrossRef]
- Guo, M.; Xu, W.; Yamamoto, Y.; Suzuki, T. Curcumin increases heat shock protein 70 expression via different signaling pathways in intestinal epithelial cells. Arch. Biochem. Biophys. 2021, 707, 108938. [Google Scholar] [CrossRef]
- Yu, J.; Xu, W.H.; Sun, W.; Sun, Y.; Guo, Z.L.; Yu, X.L. Curcumin alleviates the functional gastrointestinal disorders of mice in vivo. J. Med. Food. 2017, 20, 1176–1183. [Google Scholar] [CrossRef]
- Salah, A.S.; Ahmed-Farid, O.A.; Nassan, M.A.; El-Tarabany, M.S. Dietary curcumin improves energy metabolism, brain monoamines, carcass traits, muscle oxidative stability and fatty acid profile in heat-stressed broiler chickens. Antioxidants 2021, 10, 1265. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Z.; Lang, W.Y.; Lan, H.N.; Fan, Y.Y.; Wang, T.P.; Chu, Q.R.; Wang, J.H.; Li, D.; Zheng, X.; Wu, M. Effects of laying breeder hens dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on the jejunal microbiota and immune response of their offspring chicks. Poult. Sci. 2020, 99, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, H.; Liu, F.; Pang, Q.; Shan, A.; Feng, X. Effect of dietary curcumin supplementation on duck growth performance, antioxidant capacity and breast meat quality. Foods 2021, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Jarriyawattanachaikul, W.; Girolami, F.; Longobardi, C.; Nebbia, C.; Andretta, E.; Lauritano, C.; Dabbou, S.; Avantaggiato, G.; Schiavone, A.; et al. Curcumin supplementation protects broiler chickens against the renal oxidative stress induced by the dietary exposure to low levels of aflatoxin B1. Front. Vet. Sci. 2021, 8, 822227. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef]
- Ashry, A.; Taha, N.M.; Lebda, M.A.; Abdo, W.; El-Diasty, E.M.; Fadl, S.E.; Elkamshishi, M.M. Ameliorative effect of nanocurcumin and Saccharomyces cell wall alone and in combination against aflatoxicosis in broilers. BMC Vet. Res. 2022, 18, 178. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T. The effect of using acidified turmeric on some productive parameters and intestinal bacterial counts in broilers at high stocking density pens. J. Adv. Vet. Anim. Res. 2022, 9, 87–94. [Google Scholar] [CrossRef]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Anim. Int. J. Anim. Biosci. 2019, 13, 42–52. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin attenuates lipopolysaccharide-induced hepatic lipid metabolism disorder by modification of m(6) A RNA methylation in piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.H.; Liang, X.D.; Chen, J.; Liu, C.C.; Liu, Y.Y.; Cheng, Y.; Go, Y.Y.; Zhou, B. Curcumin inhibits classical swine fever virus replication by interfering with lipid metabolism. Vet. Microbiol. 2021, 259, 109152. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.R.; Lunney, J.; Dekkers, J. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front. Genet. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yan, E.; Zhang, L.; Wang, T.; Wang, C. Curcumin reduces oxidative stress and fat deposition in longissimus dorsi muscle of intrauterine growth-retarded finishing pigs. Anim. Sci. J. 2022, 93, e13741. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2015, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chanapiwat, P.; Kaeoket, K. The effect of curcuma longa extracted (curcumin) on the quality of cryopreserved boar semen. Anim. Sci. J. 2015, 86, 863–868. [Google Scholar] [PubMed]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Chen, J.; Wang, F.; Mao, D. Effect of curcumin supplement in summer diet on blood metabolites, antioxidant status, immune response, and testicular gene expression in Hu Sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaguezeski, A.M.; Gündel, S.S.; Favarin, F.R.; Gündel, A.; Souza, C.F.; Baldissera, M.D.; Cazarotto, C.C.; Volpato, A.; Fortuoso, B.F.; Ourique, A.F.; et al. Low-dose curcumin-loaded eudragit L-100-nanocapsules in the diet of dairy sheep increases antioxidant levels and reduces lipid peroxidation in milk. J. Food Biochem. 2019, 43, e12942. [Google Scholar] [CrossRef]
- Li, R.; Fang, H.; Shen, J.; Jin, Y.; Zhao, Y.; Wang, R.; Fu, Y.; Tian, Y.; Yu, H.; Zhang, J. Curcumin alleviates lps-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Toxins 2021, 13, 208. [Google Scholar] [CrossRef]
- Salman, A.; Caamaño, J.N.; Fernández-Alegre, E.; Hidalgo, C.O.; Nadri, T.; Tamargo, C.; Fueyo, C.; Fernández, Á.; Merino, M.J.; Martínez-Pastor, F. Supplementation of the BIOXcell extender with the antioxidants crocin, curcumin and GSH for freezing bull semen. Res. Vet. Sci. 2021, 136, 444–452. [Google Scholar] [CrossRef]
- Marcon, H.; Griss, L.G.; Molosse, V.L.; Cecere, B.G.; Alba, D.F.; Leal, K.W.; Galli, G.M.; Souza, C.F.; Baldissera, M.D.; Gundel, S.; et al. Dietary supplementation with curcumin-loaded nanocapsules in lambs: Nanotechnology as a new tool for nutrition. Anim. Nutr. 2021, 7, 521–529. [Google Scholar] [CrossRef]
- Shah, S.A.H.; Andrabi, S.M.H.; Qureshi, I.Z. Freezability of water buffalo bull (Bubalus bubalis) spermatozoa is improved with the addition of curcumin (diferuoyl methane) in semen extender. Andrologia 2017, 49, e12713. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Experimental Animal | Supplementation Dose | Major Findings | References |
|---|---|---|---|
| Hy-Line brown hens | Dietary supplementation with 100 mg/Kg, 150 mg/kg, 200 mg/kg of CUR | Supplementation with CUR dose-dependently improved egg production by 8.67%, 11.58% and 1.56%, respectively, while the feed conversion ratios decreased by 9.50%, 10.74%, and 2.07%, respectively. Furthermore, the eggshell strength greatly improved by 22.22%, 23.22%, and 26.74%, respectively, and the eggshell thickness improved by 61.49%, 76.40%, and 90.06%, respectively. Antioxidative capability, reproductive hormones and immune parameters, etc. were all significantly increased. | [43] |
| Broiler chickens | Dietary supplementation with 100 mg/kg, 200 mg/kg of CUR | CUR exhibited some positive responses on antioxidant capacity, lesion score and oocyst shedding, based on the increased growth performance and intestinal permeability, and reduced the lesion scores of duodenum, jejunum and cecum and oocyst shedding. Furthermore, CUR treated chickens had numerically lower oocyst count of Eimeria maxima. | [58] |
| Hy-line brown layers | Dietary supplementation with 250 mg/kg of CUR | Alpha- and beta-diversity of iejunal microbial communities were significantly increased, while Proteobacteria and Bacteroidetes were significantly decreased. NF-κB in jejunums, and TNF-α in jejunums, the expression of jejunal IL-12 and IL-4 genes were all upregulated. The genes expression of jejunal proteasome activator subunit 3 and 4 (PSME3 and PSME4) was both significantly upregulated. | [72] |
| Rooster (Ross) | Dietary supplementation with 100 mg/kg of CUR | The dietary CUR supplementation significantly increased the breast yield, but reduced the percentage of abdominal fat. Furthermore, the levels of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) in breast and thigh muscles were both increased. In addition, the dietary CUR supplementation significantly improved the levels of ATP and CoQ10 in liver tissue and brain serotonin. | [70] |
| Rooster (Ross 308) | Dietary supplementation with 400 mg/kg of CUR | Dietary CUR supplementation was able to almost completely counteract AFB1 induced impairment of SOD, CAT, and GPx. Furthermore, CUR was able to attenuate all the AFB1 modified oxidative stress parameters in the kidney of chicken. | [74] |
| Broiler chickens | Dietary supplementation with 100 mg/kg, 200 mg/kg of CUR | Growth performance, behavioral patterns, and immunity were enhanced after dietary CUR supplementation by reducing oxidative stress and increasing growth-related gene expression of HSD broilers. | [71] |
| Broiler chickens (AA) | Dietary supplementation with 300 mg/kg of CUR | CUR significantly decreased the levels of ROS and MDA and increased the activities of SOD, CAT, GSH and ATPase activity, and thus alleviated AFB1-induced liver necrosis by regulating the TLR4/RIPK pathway in broilers. | [75] |
| Broiler chickens (Cobb) | Dietary supplementation with 200 mg/kg, 400 mg/kg of CUR | Dietary supplementation with nano-CUR significantly attenuated aflatoxin impaired growth performance, blood and serum parameters, carcass traits, and aflatoxin residue in the liver and muscle of broilers. | [76] |
| Broiler chickens | Dietary supplementation with 1% CUR, 1% acidified CUR | CUR treatment significantly decreased erythrocytes, hematocrit, hemoglobin, ileal coliform and lactic acid bacteria counts, while significantly increased the thymus weight. | [77] |
| White Pekin ducklings | Dietary supplementation with 200 mg/kg, 400 mg/kg, 800 mg/kg of CUR | CUR treatment significantly prevented the BW and ADG decrease, while decreased the IL-1β, TNF-α and MDA content, and increased the GSH-Px activity in the jejunal mucosa compared with the OTA ducks. Additionally, CUR increased jejunal mucosa occludin and tight junction protein 1 expression, and decreased those of ρ-associated protein kinase 1. Notably, CUR inhibited the increased expression of apoptosis-related genes, and downregulated mitochondrial transcription factors A, B1 and B2 caused by OTA without any effects on RNA polymerase mitochondrial. | [78] |
| Experimental Animal | Supplementation Dose | Major Findings | References |
|---|---|---|---|
| Duroc × Large White × Landrace piglets | Basal diet supplemented with CUR (200 mg/kg diet) | CUR significantly reduced the liver index as well as the plasma and liver concentrations of AST and LDH in LPS injected weaning piglets. Furthermore, CUR attenuated the LPS induced increase in hepatic SREBP-1c and SCD-1 mRNA. | [79] |
| Duroc × Landrace × Large White | Diet supplemented with CUR (200 mg/kg diet) | CUR significantly decreased the MDA and PC levels in longissimus dorsi muscle improved meat quality and alleviated oxidative stress by activating Nrf2 pathway. Moreover, CUR reduced fat deposition by inhibiting PPAR-γ in IUGR pigs. | [83] |
| Duroc × Landrace × Yorkshire | Diet supplemented with CUR (200 mg/kg, 300 mg/kg, 400 mg/kg) | CUR decreased feed/gain ratio and crypt depth, improved villus height and crypt depth ratio, reduced plasma D-lactate and DAO activity, increased sIgA expression, increased the number of goblet cells (GCs) and reduced the number of intraepithelial lymphocytes. IL-1β, TLR4 and TNF-α expression were also decreased in CUR pigs, but IL-10 mRNA was increased. | [84] |
| Duroc × (Landrace × Yorkshire | Diet supplemented with CUR 400 mg/kg | Dietary CUR supplementation increased feed intake, body-weight gain, antioxidant enzymes activities, and the hepatic Nrf2 and Hmox1 expression in weaned piglets with IUGR. | [45] |
| Duroc boars | CUR in freezing extender (0.125, 0.25, 0.50, 0.75 and 1.0 mmol/L, respectively) | Addition of CUR at 0.25 or 0.50 mmol/L CUR yielded the higher percentage of progressive motility (33.3% and 36.1%, respectively). A significantly higher percentage of acrosome integrity was found in groups administrated with CUR than in the other groups. | [85] |
| Experimental Animal | Supplementation Dose | Major Findings | References |
|---|---|---|---|
| Lacaune sheep | Diet addition 30 mg free CUR/kg concentrate, 3 mg Nano-PCL/kg concentrate, and 3 mg Nano-Eudragit/kg concentrate | The number of total leukocytes and serum globulin levels were lower in 3 mg Nano-Eudragit/kg concentrate than in the control group, antioxidant capacity against peroxyl radicals (ACAP) and catalase enzymes was elevated in 3 mg Nano-Eudragit/kg concentrate, with consequently reduced lipid peroxidation and LPO, and increased ACAP in milk. | [87] |
| Lacaune lambs | Diet addition ethyl polymethacrylate (Eudragit L-100) nanocapsules loaded with CUR (N-CUR) | N-CUR significantly decreased neutrophil and neutrophil counts, increased serum AST concentrations in lambs. Furthermore, N-CUR obviously decreased the serum blood glucose and triglyceride concentrations, and raised the serum SOD in lamb. | [90] |
| Hu sheep | Diet addition CUR 450 mg/kg; 900 mg/kg | CUR significantly increased serum NEFA and GPX, as well as IgA and IgM. Furthermore, dietary CUR supplement increased testicular organ index, serum testosterone level, and testicular star mRNA expression. Moreover, dietary CUR supplement linearly inhibited testicular apoptosis with increased testicular bcl-2 mRNA expression and decreased caspase-3 mRNA expression. | [86] |
| Nili-Ravi buffalo, Angora goats and Holstein bulls | CUR in freezing extender (0.5–10 mM) | At pre-freezing and post-thawing, compared to 0.5 and 1.0 mM CUR and control, 1.5 and 2.0 mM CUR increased total antioxidant contents and decreased lipid peroxidation levels. At post-thawing, rapid velocity and progressive motility were higher with 1.5 mM compared to other doses of CUR. Cryopreservation diluents with antioxidants at three different doses, led to lower percentages of acrosome and total sperm abnormalities, compared to the control. SOD activity was also found to be higher in the presence of CUR at different dose levels and carnitine (5 mM), compared to the other groups. | [85,91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Yan, J.; Xu, X.; Chen, Y.; Chen, X.; Li, F.; Xing, H. Current Development and Future Application Prospects of Plants-Derived Polyphenol Bioactive Substance Curcumin as a Novel Feed Additive in Livestock and Poultry. Int. J. Mol. Sci. 2022, 23, 11905. https://doi.org/10.3390/ijms231911905
Pan S, Yan J, Xu X, Chen Y, Chen X, Li F, Xing H. Current Development and Future Application Prospects of Plants-Derived Polyphenol Bioactive Substance Curcumin as a Novel Feed Additive in Livestock and Poultry. International Journal of Molecular Sciences. 2022; 23(19):11905. https://doi.org/10.3390/ijms231911905
Chicago/Turabian StylePan, Shifeng, Jie Yan, Xingyu Xu, Yongfang Chen, Xinyu Chen, Fei Li, and Hua Xing. 2022. "Current Development and Future Application Prospects of Plants-Derived Polyphenol Bioactive Substance Curcumin as a Novel Feed Additive in Livestock and Poultry" International Journal of Molecular Sciences 23, no. 19: 11905. https://doi.org/10.3390/ijms231911905






