Effects of iNOS in Hepatic Warm Ischaemia and Reperfusion Models in Mice and Rats: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
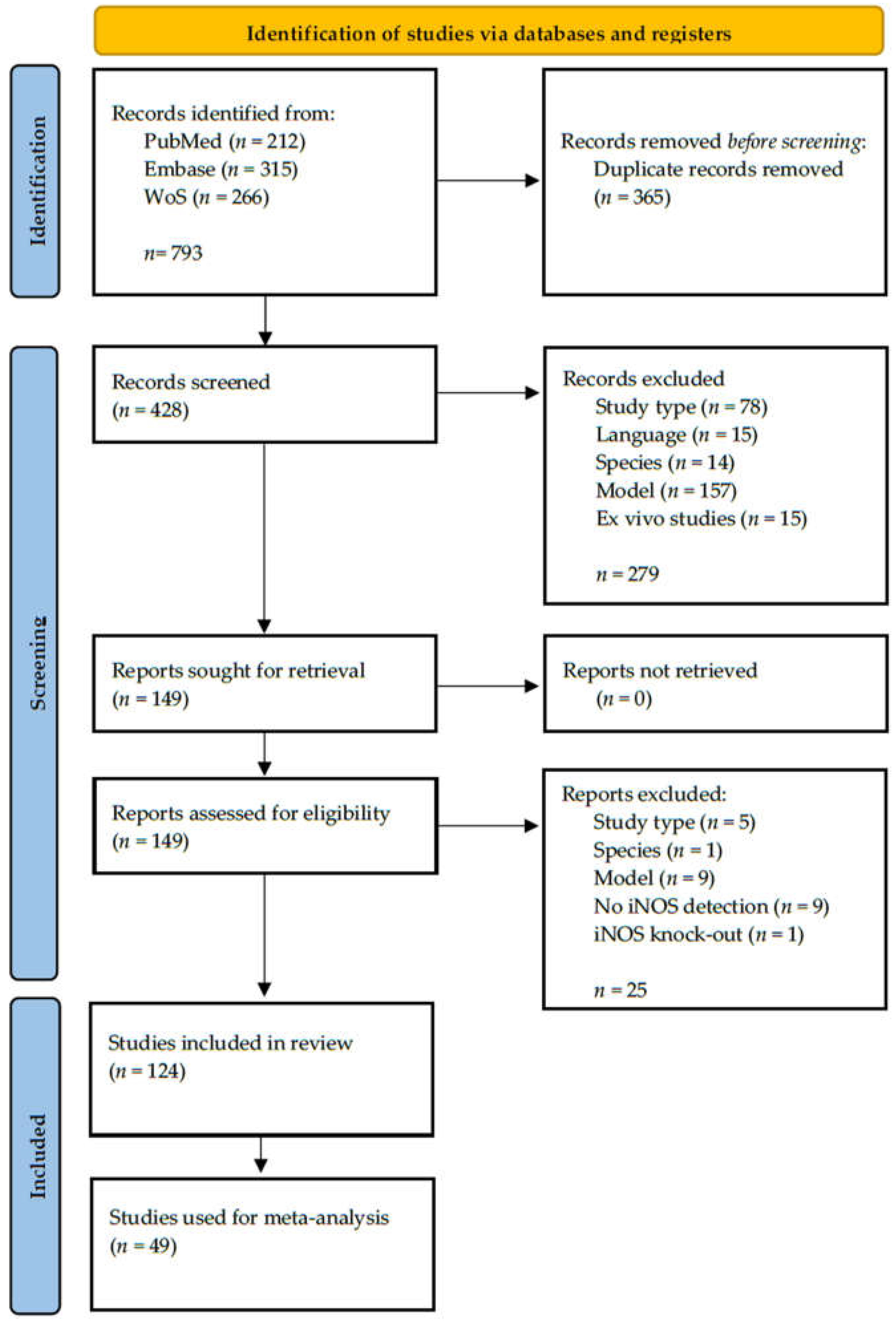
2.1. Results of the Search
2.2. General Characteristics
2.3. Details for Rat Models
| Reference | Year | Strain | Reperfusion Time (h) | Gender (%) | BW (g) | iNOS Detection Method | NO Detection | eNOS Detection | Survival Rate |
|---|---|---|---|---|---|---|---|---|---|
| 30 min ischemia time | |||||||||
| Abd-Elbaset [32] | 2017 | Wistar | 0.5 | ♂ 100% | 300–350 | IHC | - | + | - |
| Fouad [33] | 2011 | SD | 72 | ♂ 100% | 190–210 | IHC | + | - | - |
| Liu [34] | 2000 | Fischer | 4 | ♂ 100% | 275–300 | RNA | + | - | - |
| Liu [35] | 1998 | Fischer | 4 | ♂ 100% | 245–290 | SP | - | + | - |
| Rhee [36] | 2002 | SD | 1/6 | ♂ 100% | 100–150 | indirect | + | - | + |
| Wang [37] | 2017 | SD | 1 | ♂ 100% | 200–250 | Protein | - | - | - |
| Wang [38] | 2003 | Wistar | 6/1/3/5/7/336 | ♂ 100% | 240–260 | RNA/WB | - | + | - |
| 45 min ischemia time | |||||||||
| El-Emam [39] | 2020 | SD | 24 | ♂ 100% | 250–280 | ELISA | - | - | - |
| Hur [40] | 1999 | SD | 0.5/1/3/5/12/24 | ♂ 100% | 350–400 | RNA | - | - | - |
| Koti [21] | 2005 | SD | 2 | ♂ 100% | 250–300 | WB | + | + | - |
| Mostafa-Hedeab [41] | 2019 | Wistar | N/A | ♂ 100% | 140–250 | RNA | - | + | - |
| Serracino-Iglott [42] | 2003 | Wistar | 1 | ♂ 100% | 250–300 | WB/IHC | - | + | - |
| Yang [43] | 2011 | SD | 2 | ♂ 100% | 250–300 | IHC | + | + | - |
| 60 min ischemia time | |||||||||
| Curek [44] | 2010 | Wistar | 1 | ♂ 100% | 350–450 | IHC | - | - | - |
| Eum [45] | 2004 | SD | 5 | ♂ 100% | 270–300 | RNA | - | + | - |
| Eum [46] | 2004 | SD | 5/24 | ♂ 100% | 260–300 | RNA | - | - | - |
| Fernández [47] | 2009 | SD | 0.33 | ♂ 100% | 180–200 | RNA | - | - | - |
| Ferrigno [48] | 2020 | Wistar | 1 | ♂ 100% | N/A | WB | - | - | - |
| Ferrigno [49] | 2020 | Wistar | 1/2 | ♂ 100% | N/A | WB | - | + | - |
| Hataji [50] | 2010 | SD | 24 | ♂ 100% | N/A | IHC | + | + | - |
| Hsu [51] | 2002 | SD | 2 | ♂/♀ 50% | 200–275 | IHC/activity | - | - | - |
| Kang [52] | 2011 | SD | 1/5 | ♂ 100% | 270–300 | RNA/WB | - | - | - |
| Kim [53] | 2012 | SD | 5 | ♂ 100% | 150–170 | WB/RNA | - | - | - |
| Kim [54] | 2010 | SD | 5 | ♂ 100% | 270–300 | RNA/WB | - | - | - |
| Kim [55] | 2004 | SD | 5 | ♂ 100% | 260–320 | RNA | - | + | - |
| Kurabayashi [56] | 2005 | SD | 5 | ♂ 100% | 230–290 | IHC | - | - | - |
| Lee [57] | 2008 | SD | 5 | ♂ 100% | 270–300 | RNA | - | + | - |
| Man [58] | 2005 | SD | 0.33/1/1.5/6/24 | ♂ 100% | 220–280 | RNA | - | - | - |
| Park [59] | 2007 | SD | 5 | ♂ 100% | 270–300 | RNA | - | + | - |
| Ramalho [60] | 2014 | Wistar | 6 | ♂ 100% | 150–200 | WB | + | + | - |
| Ren [61] | 2019 | Wistar | 1/3/6/12 | ♂ 100% | 220–280 | RNA | - | - | - |
| Sonin [62] | 1999 | SD | 6 | ♂ 100% | 250–330 | RNA | - | - | - |
| Tao [63] | 2014 | Wistar | 6 | ♂ 100% | 180–220 | ELISA | - | - | + |
| Trocha [64] | 2014 | Wistar | 4 | ♂ 100% | N/A | ELISA | - | - | - |
| Unal [65] | 2017 | Wistar | 1 | ♂ 100% | 350–450 | IHC | + | - | - |
| Yang [66] | 2007 | SD | 1/3/5 | ♂ 100% | 230–250 | mRNA | + | + | - |
| Yun [67] | 2012 | SD | 5 | ♂ 100% | 270–300 | RNA/WB | - | - | - |
| Yun [68] | 2010 | SD | 1/2/4/6/8/12/24 | ♂ 100% | 270–300 | RNA/WB | - | - | - |
| 90 min ischemia time | |||||||||
| Bektas [69] | 2016 | Wistar | 2 | ♂ 100% | 250–300 | IHC | - | + | - |
| Grezzana-Filho [70] | 2020 | Wistar | 24 | ♂ 100% | 250–310 | WB | - | + | - |
| Kim [71] | 2004 | SD | 6 | ♂ 100% | 260–300 | RNA | - | + | - |
| Kuncewitch [72] | 2013 | SD | 24 | ♂ 100% | 250–275 | WB | - | - | + |
| Lin [73] | 2004 | Wistar | 1.5 | ♂ 100% | 300–350 | IHC | + | + | - |
| Longo [74] | 2016 | Wistar | 2 | ♂ 100% | 200–250 | WB | - | + | - |
| Takamatsu [75] | 2006 | SD | 3/6/12/24 | ♂ 100% | 230–300 | RNA | + | - | - |
| Yao [76] | 2009 | SD | 1/3/6/24/168 | ♂ 100% | 220–240 | Protein | + | - | + |
| Yun [77] | 2012 | SD | 3/24 | ♂ 100% | 270–300 | WB | - | - | - |
| Studies not included in meta-analysis | |||||||||
| 20 min ischemia time | |||||||||
| Wang [78] | 1998 | Fischar | 0.5 | ♂ 100% | 240–320 | N/A | + | - | - |
| 35 min ischemia time | |||||||||
| Kireev [79] | 2012 | Wistar | 36 | ♂ 100% | N/A | RNA | - | + | - |
| Kireev [80] | 2013 | fa/fa Zucker | 36 | ♂ 100% | 496 | RNA | - | + | - |
| 40 min ischemia time | |||||||||
| Duan [81] | 2017 | SD | 2 | ♂ 100% | 190–210 | WB | + | + | - |
| Trocha [82] | 2010 | Wistar | 1 | ♂ 100% | 240–303 | ELISA | - | + | - |
| 100 min ischemia time | |||||||||
| Hara [83] | 2005 | N/A | 12 | N/A | N/A | WB | - | - | - |
| 120 min ischemia time | |||||||||
| Ishizaki [84] | 2008 | SD | 1/3/6/9/12/24 | ♂ 100% | 240–270 | RNA/WB | + | - | - |
| Unknown ischemia time | |||||||||
| Hsieh [85] | 2015 | SD | 3.5/24 | ♂ 100% | 250–300 | RNA | - | - | - |
| Reference | Year | Strain | Reperfusion Time (h) | Gender | BW (g) | iNOS Detection Method | NO Detection | eNOS Detection | Survival Rate |
|---|---|---|---|---|---|---|---|---|---|
| 30 min ischemia time | |||||||||
| Acquaviva [86] | 2009 | Wistar | 3 | ♂ 100% | 200–220 | WB | - | + | - |
| Chen [87] | 2014 | SD | 2 | ♂ 100% | 250–300 | - | + | - | - |
| Lanteri [88] | 2007 | Wistar | 0.5/3 | ♂ 100% | 200–220 | WB | - | + | - |
| Morisuee [89] | 2003 | Wistar | 3/4/6 | ♂ 100% | 350–450 | IHC | + | - | + |
| Nii [90] | 2014 | Wistar | 2 | ♂ 100% | 250–300 | RNA | - | - | - |
| Uchiami [91] | 2002 | Wistar | 0.5/1/2 | ♂ 100% | 280–320 | RNA | - | - | - |
| 45 min ischemia time | |||||||||
| Atef [92] | 2017 | Wistar | 1 | ♂ 100% | 200–250 | RNA | + | + | - |
| El-Shintany [93] | 2015 | albino | 2 | ♂ 100% | 180–200 | IHC | + | - | - |
| Ibrahim [94] | 2020 | albino | 24 | ♂ 100% | 200–300 | ELISA | - | + | - |
| Ibrahim [95] | 2014 | albino | 1 | ♂ 100% | 200–230 | IHC | + | - | - |
| Sankary [96] | 1999 | SD | 0.25/0.5/1/2/3/144 | ♂ 100% | 200–250 | - | - | - | + |
| Sehitoglu [97] | 2019 | Wistar | 24 | ♂ 100% | 250–300 | IHC | - | - | - |
| Yaylak [98] | 2008 | Wistar | 0.75 | ♂ 100% | 150–220 | IHC | - | - | - |
| 60 min ischemia time | |||||||||
| Miyake [28] | 2013 | Wistar | 5/3 | ♂ 100% | 250–300 | WB/RNA | + | - | + |
| Rodríguez-Reynoso [99] | 2001 | SD | 1/2 | ♂ 100% | 250–300 | RNA | + | - | + |
| Sankary [96] | 1999 | SD | 0.25/0.5/1/2/3/144 | ♂ 100% | 200–250 | - | - | - | + |
| Yang [100] | 2007 | SD | 5 | ♂ 100% | 230–250 | mRNA | + | + | - |
| Studies not included in meta-analysis | |||||||||
| 5/15 min ischemia time | |||||||||
| Miyake [28] | 2013 | Wistar | 5,3 | ♂ 100% | 250–300 | WB/RNA | + | + | - |
| 20 min ischemia time | |||||||||
| Harada [101] | 2003 | SD | 2/6/24 | ♂ 100% | 225–250 | RNA | - | - | - |
| Suetsugu [102] | 2005 | SD | 72 | ♂ 100% | 240–255 | WB | - | - | - |
| 40 min ischemia time | |||||||||
| Abdel-Gaber [103] | 2015 | Albino | 1 | ♂ 100% | 200–230 | IHC | + | + | - |
| 90 min ischemia time | |||||||||
| Koeppel [26] | 2007 | SD | 3/6/12/24 | ♂ 100% | 280–350 | RNA | - | + | - |
| Rodriguez-Reynos [104] | 2018 | SD | 1/5/168 | ♂ 100% | 250–300 | RNA | + | - | + |
| Xue [105] | 2010 | SD | 24/48/72/168 | ♂ 100% | 200–250 | RNA | + | + | + |
| Model | Reference | Year | Strain | Reperfusion Time (h) | Gender | BW (g) | iNOS Detection Method | NO Detection | eNOS Detection | Survival Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| 30% HIRI 60 min ischemia time | Wang [106] | 2012 | SD | 6 | ♂ 100% | 250–300 | WB | - | - | - |
| 40% HIRI 45 min ischemia time | Björnsson [107] | 2015 | SD | 4 | ♂ 100% | 313–444 | RNA/IHC | + | - | - |
| 40% HIRI 60 min ischemia time | Björnsson [108] | 2014 | SD | 1/4 | ♂ 100% | 258–444 | RNA | + | - | - |
| 32% HIRI + 68% PH 30 min ischemia time | Liang [109] | 2009 | SD | 3/8/48/168 | ♀ 100% | 200–220 | IHC | - | - | + |
| 50%HIRI + 50%PH 45 min ischemia time | Iwasaki [110] | 2019 | Wistar | 1/3/24/168 | ♂ 100% | 200–300 | RNA/WB | + | + | - |
| 70% HIRI + 30%PH 45 min ischemia time | Shen [111] | 2007 | SD | 3/12/24/168 | ♂ 100% | 220–300 | SP | + | + | + |
| 70% HIRI + 30%PH 45 min ischemia time | Shen [112] | 2007 | SD | 3/12/24 | ♂ 100% | 250–300 | SP | + | + | + |
| 70% HIRI + 30%PH 60 min ischemia time | El-Gohary [113] | 2017 | Wistar | 1/5 | ♂ 100% | 200–250 | WB | - | + | - |
| 70% HIRI + 30%PH 60 min ischemia time | Zhang [114] | 2015 | SD | 6/168 | ♂ 100% | 250–300 | WB | + | - | - |
| 70% HIRI + 70%PH 30 min ischemia time | Duval [115] | 2010 | SD | 0/0.5/1/3/6/9/12/15/18/21/24/30/48/72 | ♂ 100% | 200–225 | RNA | - | - | - |
| 100% HIRI + 70%PH 15 min ischemia time | Kawai [116] | 2010 | Wistar | 1 | ♂ 100% | 250–300 | RNA/IHC | + | + | - |
| 120, 150 min ischemia time + SCS | Sankary [96] | 1999 | SD | ≤144 | ♂ 100% | 200–250 | IHC | - | - | - |
2.4. Details for Mouse Models
| Reference | Year | Strain | Reper-fusion Time (h) | Gender | Age (Weeks) | iNOS Detection Method | NO Detec. | eNOS Detec. | Survival Rate |
|---|---|---|---|---|---|---|---|---|---|
| 45 min ischemia time | |||||||||
| Bae [117] | 2014 | C57Bl/6 | 1/6/24 | ♂ 100% | 8–10 | RNA | - | - | - |
| Datta [118] | 2014 | C57Bl/6 (eNOS −/− and WT) | 2 | ♂ 100% | 8–12 | WB | - | + | - |
| Hines [119] | 2001 | C57Bl/6 (iNOS −/− and WT) | 1/3/6 | ♂ 100% | N/A | RNA | - | - | - |
| Hines [120] | 2002 | C57Bl/6 (iNOS −/−, eNOS −/− and WT) | 1/3 | ♂ 100% | N/A | RNA | - | + | - |
| Kawachi [121] | 2000 | C57Bl/6 (NOS −/−, eNOS −/− and WT) | 5 | ♂ 100% | N/A | RNA | - | + | - |
| Lee [122] | 2001 | C57Bl/6 (iNOS −/−, eNOS −/− and WT) | 6 | ♂/♀ 50% | N/A | RNA | - | - | - |
| 60 min ischemia time | |||||||||
| Chen [123] | 2017 | C57Bl/6 (NLRC5 −/− and WT) | N/A | ♂ 100% | 8–10 | ELISA | - | - | - |
| Gao [124] | 2016 | Cav-1tm1Mls/J (Cav-1 −/−and WT) | 1/6/12 | ♂ 100% | 8–12 | WB/ICH | - | - | - |
| Guo [125] | 2011 | BALB/c | 2/4/24 | ♂ 100% | N/A | SP | + | + | - |
| Guo [126] | 2011 | BALB/c | 2/4/12 | ♂ 100% | N/A | WB/SP | - | + | - |
| Jeyabalan [127] | 2008 | C57Bl/6 | 3/6 | ♂ 100% | 8–12 | RNA | + | + | - |
| Kim [128] | 2015 | C57Bl/6 | 6 | ♂ 100% | 6–8 | RNA | - | - | - |
| Klune [129] | 2012 | C57BL/6 (IRF2 −/−, IRF2 +/− and WT) | 6 | ♂ 100% | 8–12 | RNA | - | - | - |
| Lee [122] | 2001 | C57Bl/6 (iNOS −/−, eNOS −/− and WT) | 6 | ♂/♀ 50% | N/A | RNA | - | - | - |
| Luedde [130] | 2005 | Ikk2 −/−, Nemo −/− | 6/24 | ♂ 100% | 8–10 | IHC | - | - | - |
| Moon [131] | 2008 | C57Bl/6 | 2 | ♂ 100% | N/A | WB | - | - | - |
| Mukhopadhyay [132] | 2011 | C57Bl/6 | 2/6/24 | ♂ 100% | N/A | RNA | - | - | - |
| Qiao [133] | 2020 | C57Bl/6 (iNOS −/− and WT) | 6/24 | ♂ 100% | 8–10 | WB | - | - | - |
| Qiao [134] | 2019 | C57Bl/6 (iNOS −/− and WT) | 6/24 | ♂ 100% | ~8 | RNA/WB | - | - | - |
| Sanches [135] | 2014 | Swiss | 6 | ♂ 100% | N/A | WB | + | + | - |
| Shaker [136] | 2016 | C57Bl/6 | 3/12 | ♂ 100% | 8–10 | RNA | - | - | - |
| Shi [137] | 2012 | C57Bl/6 | 2/6/168 | ♂ 100% | 8–9 | WB | - | - | + |
| Tsung [138] | 2006 | C57Bl/6 (IRF−1 −/− and WT) | 1/3/6 | ♂ 100% | 8–12 | RNA | - | - | - |
| Tsurui [139] | 2005 | C57Bl/6 | 2/6 | ♂ 100% | 8–12 | RNA | - | + | - |
| Zhao [140] | 2017 | BALB/c (H−2d) | 0/6 | ♂ 100% | 6–8 | RNA | - | - | - |
| 90 min ischemia time | |||||||||
| Ajamieh [141] | 2015 | foz/foz and WT | 24 | ♂ 100% | 8 | RNA | - | + | - |
| Duarte [142] | 2012 | C57Bl/6 (TIMP −/− and WT) | 6/48/168 | ♂ 100% | N/A | RNA | - | - | + |
| Freitas [143] | 2010 | C57Bl/6 | 6 | ♂ 100% | 8–12 | RNA | - | - | - |
| Hamada [144] | 2009 | C57Bl/6 (iNOS −/− and WT) and C57Bl/6 (MMP−9 −/− and WT) | 3/6/24 | ♂ 100% | 8–10 | WB | + | - | - |
| Lee [145] | 2016 | C57BL/6 | 6 | ♂ 100% | 8 | WB | - | - | - |
| Okaya [146] | 2004 | C57Bl/6 (PPARa −/− and WT) | 8 | ♂ 100% | 8–12 | WB | + | + | - |
| Zhou [147] | 2020 | C57Bl/6 | 6 | ♂ 100% | 8 | ELISA/RNA | - | - | - |
| Studies not included in meta-analysis | |||||||||
| 30 min ischemia time | |||||||||
| Tao [148] | 2016 | C57Bl/6 | 6 | ♂ 100% | 12 | RNA/WB | - | - | - |
| Lee [122] | 2001 | C57Bl/6 (iNOS −/−, eNOS −/− and WT) | 6 | ♂/♀ | N/A | RNA | - | - | - |
| 40 min ischemia time | |||||||||
| Patouraux [149] | 2014 | C57Bl/6 (Opn −/− and WT) | 4 | ♂ 100% | 10–12 | RNA | - | - | - |
| 75 min ischemia time | |||||||||
| Kobayashi [150] | 2008 | BALB/c | 24 | N/A | 6–8 | WB/IHC | - | - | - |
| Lee [122] | 2001 | C57Bl/6 (iNOS −/−, eNOS −/− and WT) | 6 | ♂/♀ 50% | N/A | RNA | - | - | - |
| Model | Reference | Year | Strain | Gender | Age (Weeks) | Reper-fusion Time (h) | iNOS Detection Method | NO Detec. | eNOS Detec. | Survival Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| 100% HIRI 3, 20 min ischemia time | Jessup [151] | 2005 | Nude and C57bl/6 (IL-10 −/−, IL-6 −/− and WT) | ♂ 100% | 8 | 4 | RNA | + | - | + |
| 70% HIRI + 30% PH | Godwin [152] | 2014 | C57bl/6 | ♂ 100% | N/A | 24 | RNA | - | - | - |
2.5. Risk of Bias
2.6. Effects of Intervention
2.6.1. Analysis of Rat Models with 70% HIRI and 30 min Warm Ischaemia Time
2.6.2. Analysis of Rat Models with 70% HIRI and 60 min Warm Ischaemia Time
2.6.3. Analysis of Rat Models with 70% HIRI and 90 min Warm Ischaemia Time
2.6.4. Analysis of Rat Models with 100% HIRI and 45 min Warm Ischaemia Time
2.6.5. Analysis of Mouse Models with 70% HIRI and 60 min Warm Ischaemia Time
2.6.6. Analysis Comparing NO Values between SHAM and HIRI Groups in Rats
2.6.7. Analysis of eNOS Values Compared between the SHAM and HIRI Groups in Rats
3. Discussion
3.1. Summary of Main Results
3.2. Rat and Mouse Models within This Systematic Review
3.3. Warm Ischaemia and Reperfusion Models
3.4. Overall Completeness and Applicability of Evidence
3.4.1. Sex Distribution
3.4.2. iNOS Generation and Function
3.4.3. iNOS in HIRI versus SHAM
3.4.4. Increased iNOS May Induce Hepatic Damage Via Significant Production of ROS
3.5. Agreements and Disagreements with Other Studies or Reviews
3.5.1. Nitric Oxide in HIRI Models versus SHAM
3.5.2. eNOS in HIRI Models versus SHAM
3.5.3. iNOS and Survival
3.6. Potential Biases in the Review Process
4. Materials and Methods
4.1. Search Strategy
- Web of Science Core Collection Indexes;
- Science Citation Index Expanded (SCI-Expanded) (1900-);
- Social Sciences Citation Index (SSCI) (1900-);
- Arts & Humanities Citation Index (A&HCI) (1975-);
- Conference Proceedings Citation Index—Science (CPCI-S) (1990-);
- Conference Proceedings Citation Index—Social Sciences & Humanities (CPCI-SSH) (1990-);
- Book Citation Index—Science (BKCI-S) (2005-);
- Book Citation Index—Social Sciences & Humanities (BKCI-SSH) (2005-);
- Current Chemical Reactions (CCR-Expanded) (1985-);
- Index Chemicus (IC) (1993-);
- BIOSIS Previews (1926-): Journals, patents and conference proceedings in biomedicine;
- MEDLINE (1950-);
- Russian Science Citation Index (2005-);
- SciELO Citation Index (1997-);
- Journal Citation Reports.
4.2. Searching Other Resources
4.3. Criteria for Considering Studies for This Review
4.3.1. Types of Studies
4.3.2. Types of Species
4.3.3. Types of Intervention
4.3.4. Types of Comparison
4.4. Outcomes
- We compared iNOS values of SHAM to HIRI groups using subgroups of different reperfusion times:
- Rat 70% HIRI at 30 min warm ischaemia time, data grouped by reperfusion time;
- Rat 70% HIRI at 60 min warm ischaemia time, data grouped by reperfusion time;
- Rat 100% HIRI at 45 min warm ischaemia time, data grouped by reperfusion time;
- Mouse 70% HIRI at 60 min warm ischaemia time, data grouped by reperfusion time.
- Effects of parameters related to iNOS:
- NO parameters of SHAM groups versus HIRI groups in rat 70% HIRI data grouped by warm ischaemia times;
- eNOS parameters of SHAM groups versus HIRI groups.
4.5. Differences between Protocol and Review
4.6. Data Collection and Analysis
4.7. Handling of Missing Data
- Species/strain and liver model;
- Warm ischaemia and reperfusion time for the HIRI and SHAM groups;
- Sex distribution of mice and rats for each study;
- Weight or age of experimental animals for each study;
- iNOS detection method;
- NO parameter and eNOS parameters assessed parallel to iNOS for the SHAM and HIRI groups.
4.8. Risk of Bias and Quality Assessment
4.9. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirnezami, R.; Mirnezami, A.H.; Chandrakumaran, K.; Abu Hilal, M.; Pearce, N.W.; Primrose, J.N.; Sutcliffe, R.P. Short- and long-term outcomes after laparoscopic and open hepatic resection: Systematic review and meta-analysis. HPB 2011, 13, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelat, V.G.; Cipriani, F.; Basseres, T.; Armstrong, T.H.; Takhar, A.S.; Pearce, N.W.; AbuHilal, M. Pure laparoscopic liver resection for large malignant tumors: Does size matter? Ann. Surg. Oncol. 2015, 22, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Shelat, V.G.; Serin, K.; Samim, M.; Besselink, M.G.; Al Saati, H.; Gioia, P.D.; Pearce, N.W.; Abu Hilal, M. Outcomes of repeat laparoscopic liver resection compared to the primary resection. World J. Surg. 2014, 38, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Chacon, E.; Eman, P.; Dugan, A.; Davenport, D.; Marti, F.; Ancheta, A.; Gupta, M.; Shah, M.; Gedaly, R. Effect of operative duration on infectious complications and mortality following hepatectomy. HPB 2019, 21, 1727–1733. [Google Scholar] [CrossRef]
- Farges, O.; Goutte, N.; Bendersky, N.; Falissard, B. Incidence and risks of liver resection: An all-inclusive French nationwide study. Ann. Surg. 2012, 256, 697–704. [Google Scholar] [CrossRef]
- Filmann, N.; Walter, D.; Schadde, E.; Bruns, C.; Keck, T.; Lang, H.; Oldhafer, K.; Schlitt, H.J.; Schön, M.R.; Herrmann, E.; et al. Mortality after liver surgery in Germany. Br. J. Surg. 2019, 106, 1523–1529. [Google Scholar] [CrossRef]
- Kenjo, A.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Shimada, M.; Baba, H.; Tomita, N.; Kimura, W.; Sugihara, K.; Mori, M. Risk stratification of 7732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J. Am. Coll. Surg. 2014, 218, 412–422. [Google Scholar] [CrossRef]
- Pringle, J.H.V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann. Surg. 1908, 48, 541–549. [Google Scholar] [CrossRef]
- Belghiti, J.; Noun, R.; Malafosse, R.; Jagot, P.; Sauvanet, A.; Pierangeli, F.; Marty, J.; Farges, O. Continuous versus intermittent portal triad clamping for liver resection: A controlled study. Ann. Surg. 1999, 229, 369–375. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Yoshimoto, J.; Miwa, K.; Sugo, H.; Kawasaki, S. Safety of prolonged intermittent pringle maneuver during hepatic resection. Arch. Surg. 2006, 141, 649–653. [Google Scholar] [CrossRef]
- van der Bilt, J.D.; Livestro, D.P.; Borren, A.; van Hillegersberg, R.; Borel Rinkes, I.H. European survey on the application of vascular clamping in liver surgery. Dig. Surg. 2007, 24, 423–435. [Google Scholar] [CrossRef]
- Lee, K.F.; Cheung, Y.S.; Wong, J.; Chong, C.C.; Wong, J.S.; Lai, P.B. Randomized clinical trial of open hepatectomy with or without intermittent Pringle manoeuvre. Br. J. Surg. 2012, 99, 1203–1209. [Google Scholar] [CrossRef]
- Capussotti, L.; Muratore, A.; Ferrero, A.; Massucco, P.; Ribero, D.; Polastri, R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br. J. Surg. 2006, 93, 685–689. [Google Scholar] [CrossRef]
- Man, K.; Fan, S.T.; Ng, I.O.; Lo, C.M.; Liu, C.L.; Wong, J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann. Surg. 1997, 226, 704–711. [Google Scholar] [CrossRef]
- Zaki, H.F.; Abdelsalam, R.M. Vinpocetine protects liver against ischemia-reperfusion injury. Can. J. Physiol. Pharm. 2013, 91, 1064–1070. [Google Scholar] [CrossRef]
- Massip-Salcedo, M.; Roselló-Catafau, J.; Prieto, J.; Avíla, M.A.; Peralta, C. The response of the hepatocyte to ischemia. Liver Int. 2007, 27, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharm. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharm. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Canbakan, B.; Akin, H.; Tahan, G.; Tarcin, O.; Eren, F.; Atug, O.; Tahan, V.; Imeryuz, N.; Yapicier, O.; Avsar, E.; et al. The effects of pegylated interferon alpha 2b on bile-duct ligation induced liver fibrosis in rats. Ann. Hepatol. 2009, 8, 234–240. [Google Scholar] [CrossRef]
- Erwin, P.A.; Lin, A.J.; Golan, D.E.; Michel, T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2005, 280, 19888–19894. [Google Scholar] [CrossRef]
- Koti, R.S.; Tsui, J.; Lobos, E.; Yang, W.; Seifalian, A.M.; Davidson, B.R. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005, 19, 1155–1157. [Google Scholar] [CrossRef]
- McNaughton, L.; Puttagunta, L.; Martinez-Cuesta, M.A.; Kneteman, N.; Mayers, I.; Moqbel, R.; Hamid, Q.; Radomski, M.W. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc. Natl. Acad. Sci. USA 2002, 99, 17161–17166. [Google Scholar] [CrossRef] [Green Version]
- Amersi, F.; Shen, X.D.; Moore, C.; Melinek, J.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Coito, A.J. Fibronectin-alpha 4 beta 1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am. J. Pathol. 2003, 162, 1229–1239. [Google Scholar] [CrossRef]
- Cescon, M.; Grazi, G.L.; Grassi, A.; Ravaioli, M.; Vetrone, G.; Ercolani, G.; Varotti, G.; D’Errico, A.; Ballardini, G.; Pinna, A.D. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006, 12, 628–635. [Google Scholar] [CrossRef]
- Kimura, H.; Katsuramaki, T.; Isobe, M.; Nagayama, M.; Meguro, M.; Kukita, K.; Nui, A.; Hirata, K. Role of inducible nitric oxide synthase in pig liver transplantation. J. Surg. Res. 2003, 111, 28–37. [Google Scholar] [CrossRef]
- Koeppel, T.A.; Mihaljevic, N.; Kraenzlin, B.; Loehr, M.; Jesenofsky, R.; Post, S.; Palma, P. Enhanced iNOS gene expression in the steatotic rat liver after normothermic ischemia. Eur. Surg. Res. 2007, 39, 303–311. [Google Scholar] [CrossRef]
- Meguro, M.; Katsuramaki, T.; Nagayama, M.; Kimura, H.; Isobe, M.; Kimura, Y.; Matsuno, T.; Nui, A.; Hirata, K. A novel inhibitor of inducible nitric oxide synthase (ONO-1714) prevents critical warm ischemia-reperfusion injury in the pig liver. Transplantation 2002, 73, 1439–1446. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Yokoyama, Y.; Kokuryo, T.; Mizutani, T.; Imamura, A.; Nagino, M. Endothelial nitric oxide synthase plays a main role in producing nitric oxide in the superacute phase of hepatic ischemia prior to the upregulation of inducible nitric oxide synthase. J. Surg. Res. 2013, 183, 742–751. [Google Scholar] [CrossRef]
- Colasanti, M.; Suzuki, H. The dual personality of NO. Trends Pharm. Sci. 2000, 21, 249–252. [Google Scholar] [CrossRef]
- Jiang, W.W.; Kong, L.B.; Li, G.Q.; Wang, X.H. Expression of iNOS in early injury in a rat model of small-for-size liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 146–151. [Google Scholar]
- Zhang, Y.Q.; Ding, N.; Zeng, Y.F.; Xiang, Y.Y.; Yang, M.W.; Hong, F.F.; Yang, S.L. New progress in roles of nitric oxide during hepatic ischemia reperfusion injury. World J. Gastroenterol. 2017, 23, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elbaset, M.; Arafa, E.S.A.; El Sherbiny, G.A.; Abdel-Bakky, M.S.; Elgendy, A.N.A.M. Thymoquinone mitigate ischemia-reperfusion-induced liver injury in rats: A pivotal role of nitric oxide signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Jresat, I. Therapeutic potential of cannabidiol against ischemia/reperfusion liver injury in rats. Eur. J. Pharmacol. 2011, 670, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, B.; Spokas, E.; Lai, P.S.; Wong, P.Y. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-repefusion. Shock 2000, 13, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, K.; Nagele, R.; Wong, P.Y. Inhibition of nitric oxide synthase attenuates peroxynitrite generation, but augments neutrophil accumulation in hepatic ischemia-reperfusion in rats. J. Pharm. Exp. 1998, 284, 1139–1146. [Google Scholar]
- Rhee, J.E.; Jung, S.E.; Shin, S.D.; Suh, G.J.; Noh, D.Y.; Youn, Y.K.; Oh, S.K.; Choe, K.J. The effects of antioxidants and nitric oxide modulators on hepatic ischemic-reperfusion injury in rats. J. Korean Med. Sci. 2002, 17, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yan, B.J. A polysaccharide (PNPA) from Pleurotus nebrodensis ameliorates hepatic ischemic/reperfusion (I/R) injury in rats. Int. J. Biol. Macromol. 2017, 105, 447–451. [Google Scholar] [CrossRef]
- Wang, L.M.; Tian, X.F.; Song, Q.Y.; Gao, Z.M.; Luo, F.W.; Yang, C.M. Expression and role of inducible nitric oxide synthase in ischemia-reperfusion liver in rats. Hepatobiliary Pancreat. Dis. Int. 2003, 2, 252–258. [Google Scholar]
- El-Emam, S.Z.; Soubh, A.A.; Al-Mokaddem, A.K.; Abo El-Ella, D.M. Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1849–1858. [Google Scholar] [CrossRef]
- Hur, G.M.; Ryu, Y.S.; Yun, H.Y.; Jeon, B.H.; Kim, Y.M.; Seok, J.H.; Lee, J.H. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-κB. Biochem. Biophys. Res. Commun. 1999, 261, 917–922. [Google Scholar] [CrossRef]
- Mostafa-Hedeab, G.; Hanyelhady; El-Nahass, E.S.; Sabry, D. Tadalafil mitigate experimental liver ischemia-reperfusion injury in rats via anti-oxidant, anti-inflammatory and anti-apoptotic action. Int. J. Pharm. Res. 2019, 11, 47–54. [Google Scholar]
- Serracino-Inglott, F.; Virlos, I.T.; Habib, N.A.; Williamson, R.C.; Mathie, R.T. Differential nitric oxide synthase expression during hepatic ischemia-reperfusion. Am. J. Surg. 2003, 185, 589–595. [Google Scholar] [CrossRef]
- Yang, L.Q.; Tao, K.M.; Liu, Y.T.; Cheung, C.W.; Irwin, M.G.; Wong, G.T.; Lv, H.; Song, J.G.; Wu, F.X.; Yu, W.F. Remifentanil preconditioning reduces hepatic ischemia-reperfusion injury in rats via inducible nitric oxide synthase expression. Anesthesiology 2011, 114, 1036–1047. [Google Scholar] [CrossRef]
- Curek, G.D.; Cort, A.; Yucel, G.; Demir, N.; Ozturk, S.; Elpek, G.O.; Savas, B.; Aslan, M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology 2010, 267, 147–153. [Google Scholar] [CrossRef]
- Eum, H.A.; Lee, S.M. Effect of Trolox on altered vasoregulatory gene expression in hepatic ischemia/reperfusion. Arch. Pharm. Res. 2004, 27, 225–231. [Google Scholar] [CrossRef]
- Eum, H.A.; Lee, S.M. Effects of Trolox on the activity and gene expression of cytochrome P450 in hepatic ischemia/reperfusion. Br. J. Pharm. 2004, 142, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, V.; Tapia, G.; Varela, P.; Cornejo, P.; Videla, L.A. Upregulation of liver inducible nitric oxide synthase following thyroid hormone preconditioning: Suppression by N-acetylcysteine. Biol. Res. 2009, 42, 487–495. [Google Scholar] [CrossRef]
- Ferrigno, A.P.; Pasqua, L.G.D.G.; Berardo, C.; Richelmi, P.; Cadamuro, M.; Fabris, L.; Perlini, S.; Adorini, L.; Vairetti, M. Obeticholic acid reduces biliary and hepatic matrix metalloproteinases activity in rat hepatic ischemia/reperfusion injury. PLoS ONE 2020, 15, e0238543. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Palladini, G.; Berardo, C.; Verta, R.; Richelmi, P.; Perlini, S.; Collotta, D.; Collino, M.; Vairetti, M. Transient expression of reck under hepatic ischemia/reperfusion conditions is associated with mapk signaling pathways. Biomolecules 2020, 10, 747. [Google Scholar] [CrossRef]
- Hataji, K.; Watanabe, T.; Oowada, S.; Nagaya, M.; Kamibayashi, M.; Murakami, E.; Kawakami, H.; Ishiuchi, A.; Kumai, T.; Nakano, H.; et al. Effects of a calcium-channel blocker (CV159) on hepatic ischemia/reperfusion injury in rats: Evaluation with selective NO/pO2 electrodes and an electron paramagnetic resonance spin-trapping method. Biol. Pharm. Bull. 2010, 33, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.M.; Wang, J.S.; Liu, C.H.; Chen, L.W. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock 2002, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Koh, E.J.; Lee, S.M. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J. Pineal. Res. 2011, 50, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.M. Effect of baicalin on toll-like receptor 4-mediated ischemia/reperfusion inflammatory responses in alcoholic fatty liver condition. Toxicol. Appl. Pharmacol. 2012, 258, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Moon, Y.J.; Lee, S.M. Protective effects of baicalin against ischemia/reperfusion injury in rat liver. J. Nat. Prod. 2010, 73, 2003–2008. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.M. Role of Kupffer cells in the vasoregulatory gene expression during hepatic ischemia/reperfusion. Arch. Pharmacal. Res. 2004, 27, 111–117. [Google Scholar] [CrossRef]
- Kurabayashi, M.; Takeyoshi, I.; Yoshinari, D.; Koibuchi, Y.; Ohki, T.; Matsumoto, K.; Morishita, Y. NO donor ameliorates ischemia-reperfusion injury of the rat liver with iNOS attenuation. J. Investig. Surg. 2005, 18, 193–200. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.H.; Lee, S.M. Effect of pyrrolidine dithiocarbamate on hepatic vascular stress gene expression during ischemia and reperfusion. Eur. J. Pharm. 2008, 595, 100–107. [Google Scholar] [CrossRef]
- Man, K.; Ng, K.T.; Lee, T.K.; Lo, C.M.; Sun, C.K.; Li, X.L.; Zhao, Y.; Ho, J.W.; Fan, S.T. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am. J. Transpl. 2005, 5, 40–49. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, S.M.; Lee, S.M. Effect of melatonin on altered expression of vasoregulatory genes during hepatic ischemia/reperfusion. Arch. Pharmacal. Res. 2007, 30, 1619–1624. [Google Scholar] [CrossRef]
- Ramalho, L.N.Z.; Pasta, A.A.C.; Terra, V.A.; Augusto, M.J.; Sanches, S.C.; Souza-Neto, F.P.; Cecchini, R.; Gulin, F.; Ramalho, F.S. Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food Chem. Toxicol. 2014, 74, 270–278. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, L.H.; Deng, F.S.; Li, J.S.; Jiang, L. Protective effect and mechanism of alpha-lipoic acid on partial hepatic ischemia-reperfusion injury in adult male rats. Physiol. Res. 2019, 68, 739–745. [Google Scholar] [CrossRef]
- Sonin, N.V.; Garcia-Pagan, J.C.; Nakanishi, K.; Zhang, J.X.; Clemens, M.G. Patterns of vasoregulatory gene expression in the liver response to ischemia/reperfusion and endotoxemia. Shock 1999, 11, 175–179. [Google Scholar] [CrossRef]
- Tao, X.; Wan, X.; Xu, Y.; Xu, L.; Qi, Y.; Yin, L.; Han, X.; Lin, Y.; Peng, J. Dioscin attenuates hepatic ischemia-reperfusion injury in rats through inhibition of oxidative-nitrative stress, inflammation and apoptosis. Transplantation 2014, 98, 604–611. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Lad, A.; Chlebda-Sieragowska, E.; Szuba, A.; Piesniewska, M.; Fereniec-Golebiewska, L.; Kwiatkowska, J.; Szelag, A.; Sozanski, T. Age-related changes in ADMA-DDAH-NO pathway in rat liver subjected to partial ischemia followed by global reperfusion. Exp. Gerontol. 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Unal, B.; Ozcan, F.; Tuzcu, H.; Kirac, E.; Elpek, G.O.; Aslan, M. Inhibition of neutral sphingomyelinase decreases elevated levels of nitrative and oxidative stress markers in liver ischemia-reperfusion injury. Redox. Rep. 2017, 22, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.L.; Lou, Y.J. Sodium nitroprusside decreased leukotriene C4 generation by inhibiting leukotriene C4 synthase expression and activity in hepatic ischemia-reperfusion injured rats. Biochem. Pharmacol. 2007, 73, 724–735. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Yun, N.; Eum, H.A.; Lee, S.M. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid. Redox. Signal 2010, 13, 1503–1512. [Google Scholar] [CrossRef]
- Bektas, S.; Karakaya, K.; Can, M.; Bahadir, B.; Guven, B.; Erdogan, N.; Ozdamar, S.O. The effects of tadalafil and pentoxifylline on apoptosis and nitric oxide synthase in liver ischemia/reperfusion injury. Kaohsiung J. Med. Sci. 2016, 32, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Grezzana-Filho, T.J.M.L.; Santos, J.L.L.; Gabiatti, G.; Boffi, C.; Santos, E.B.; Cerski, C.T.S.; Chedid, M.F.; Corso, C.O. Induction of selective liver hypothermia prevents significant ischemia/reperfusion injury in wistar rats after 24 hours. Acta Cir. Bras. 2020, 35, 1–12. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M. Expression of hepatic vascular stress genes following ischemia/reperfusion and subsequent endotoxemia. Arch. Pharm. Res. 2004, 27, 769–775. [Google Scholar] [CrossRef]
- Kuncewitch, M.; Yang, W.L.; Molmenti, E.; Nicastro, J.; Coppa, G.F.; Wang, P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock 2013, 39, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.I.; Wang, D.; Leu, F.J.; Chen, C.F.; Chen, H.I. Ischemia and reperfusion of liver induces eNOS and iNOS expression: Effects of a NO donor and NOS inhibitor. Chin. J. Physiol. 2004, 47, 121–127. [Google Scholar]
- Longo, L.; Sinigaglia-Fratta, L.X.; Weber, G.R.; Janz-Moreira, A.; Kretzmann, N.A.; Grezzana-Filho Tde, J.; Possa-Marroni, N.; Corso, C.O.; Schmidt-Cerski, C.T.; Reverbel-da-Silveira, T.; et al. Hypothermia is better than ischemic preconditioning for preventing early hepatic ischemia/reperfusion in rats. Ann. Hepatol. 2016, 15, 110–120. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Shimada, K.; Yamaguchi, K.; Kuroki, S.; Chijiiwa, K.; Tanaka, M. Inhibition of inducible nitric oxide synthase prevents hepatic, but not pulmonary, injury following ischemia-reperfusion of rat liver. Dig. Dis. Sci. 2006, 51, 571–579. [Google Scholar] [CrossRef]
- Yao, X.M.; Chen, H.; Li, Y. Protective effect of bicyclol on liver injury induced by hepatic warm ischemia/reperfusion in rats. Hepatol. Res. 2009, 39, 833–842. [Google Scholar] [CrossRef]
- Yun, N.; Kim, S.H.; Lee, S.M. Differential consequences of protein kinase C activation during early and late hepatic ischemic preconditioning. J. Physiol. Sci. 2012, 62, 199–209. [Google Scholar] [CrossRef]
- Wang, Y.; Lawson, J.A.; Jaeschke, H. Differential effect of 2-aminoethyl-isothiourea, an inhibitor of the inducible nitric oxide synthase, on microvascular blood flow and organ injury in models of hepatic ischemia-reperfusion and endotoxemia. Shock 1998, 10, 20–25. [Google Scholar] [CrossRef]
- Kireev, R.A.; Cuesta, S.; Ibarrola, C.; Bela, T.; Gonzalez, E.M.; Vara, E.; Tresguerres, J.A.F. Age-related differences in hepatic ischemia/reperfusion: Gene activation, liver injury, and protective effect of melatonin. J. Surg. Res. 2012, 178, 922–934. [Google Scholar] [CrossRef]
- Kireev, R.; Bitoun, S.; Cuesta, S.; Tejerina, A.; Ibarrola, C.; Moreno, E.; Vara, E.; Tresguerres, J.A. Melatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosis. Eur. J. Pharm. 2013, 701, 185–193. [Google Scholar] [CrossRef]
- Duan, Y.F.; An, Y.; Zhu, F.; Jiang, Y. Remote ischemic preconditioning protects liver ischemia-reperfusion injury by regulating eNOS-NO pathway and liver microRNA expressions in fatty liver rats. Hepatobiliary Pancreat Dis. Int. 2017, 16, 387–394. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Lad, A.; Szuba, A.; Chlebda, E.; Piesniewska, M.; Sozanski, T.; Szelag, A. Effect of simvastatin on nitric oxide synthases (eNOS, iNOS) and arginine and its derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat liver. Pharm. Rep. 2010, 62, 343–351. [Google Scholar] [CrossRef]
- Hara, Y.; Teramoto, K.; Kumashiro, Y.; Sato, E.; Nakamura, N.; Takatsu, S.; Kawamura, T.; Arii, S. Beneficial effect of tetrahydrobiopterin on the survival of rats exposed to hepatic ischemia-reperfusion injury. Transpl. Proc. 2005, 37, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, M.; Kaibori, M.; Uchida, Y.; Tanaka, H.; Ozaki, T.; Saito, T.; Matsui, K.; Kamiyama, Y.; Nishizawa, M.; Okumura, T. Protective effect of FR183998, a Na+/H+ exchanger inhibitor, and its inhibition of inducible nitric oxide synthase induction in hepatic ischemia-reperfusion injury in rats. Am. J. Transplant. 2008, 8, 496. [Google Scholar]
- Hsieh, C.C.; Hsu, S.M.; Hwang, L.S.; Chiu, J.H.; Lu, W.C.; Wu, Y.L.; Hsieh, S.C. Protective effects of the extract from longan flower against hepatic ischemia/reperfusion injury in rats. J. Funct. Foods 2015, 15, 570–579. [Google Scholar] [CrossRef]
- Acquaviva, R.; Lanteri, R.; Li Destri, G.; Caltabiano, R.; Vanella, L.; Lanzafame, S.; Di Cataldo, A.; Li Volti, G.; Di Giacomo, C. Beneficial effects of rutin and L-arginine coadministration in a rat model of liver ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G664–G670. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Liao, F.T.; Yang, Y.C.; Wang, J.J. Inhibition of inducible nitric oxide synthesis ameliorates liver ischemia and reperfusion injury induced transient increase in arterial stiffness. Transpl. Proc. 2014, 46, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, R.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Li Destri, G.; Santangelo, M.; Vanella, L.; Di Cataldo, A. Rutin in rat liver ischemia/reperfusion injury: Effect on DDAH/NOS pathway. Microsurgery 2007, 27, 245–251. [Google Scholar] [CrossRef]
- Morisue, A.; Wakabayashi, G.; Shimazu, M.; Tanabe, M.; Mukai, M.; Matsumoto, K.; Kawachi, S.; Yoshida, M.; Yamamoto, S.; Kitajima, M. The role of nitric oxide after a short period of liver ischemia-reperfusion. J. Surg. Res. 2003, 109, 101–109. [Google Scholar] [CrossRef]
- Nii, A.; Utsunomiya, T.; Shimada, M.; Ikegami, T.; Ishibashi, H.; Imura, S.; Morine, Y.; Ikemoto, T.; Sasaki, H.; Kawashima, A. Hydrolyzed whey peptide-based diet ameliorates hepatic ischemia-reperfusion injury in the rat nonalcoholic fatty liver. Surg. Today 2014, 44, 2354–2360. [Google Scholar] [CrossRef]
- Uchinami, H.; Yamamoto, Y.; Kume, M.; Yonezawa, K.; Ishikawa, Y.; Taura, K.; Nakajima, A.; Hata, K.; Yamaoka, Y. Effect of heat shock preconditioning on NF-kappaB/I-kappaB pathway during I/R injury of the rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G962–G971. [Google Scholar] [CrossRef]
- Atef, Y.; El-Fayoumi, H.M.; Abdel-Mottaleb, Y.; Mahmoud, M.F. Effect of cardamonin on hepatic ischemia reperfusion induced in rats: Role of nitric oxide. Eur. J. Pharmacol. 2017, 815, 446–453. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Desoky, K. Cromoglycate, not ketotifen, ameliorated the injured effect of warm ischemia/reperfusion in rat liver: Role of mast cell degranulation, oxidative stress, proinflammatory cytokine, and inducible nitric oxide synthase. Drug Des. Dev. Ther. 2015, 9, 5237–5246. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.G.; El-Emam, S.Z.; Mohamed, E.A.; Abd Ellah, M.F. Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int. Immunopharmacol. 2020, 80, 106131. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdel-Gaber, S.A.; Amin, E.F.; Ibrahim, S.A.; Mohammed, R.K.; Abdelrahman, A.M. Molecular mechanisms contributing to the protective effect of levosimendan in liver ischemia-reperfusion injury. Eur. J. Pharm. 2014, 741, 64–73. [Google Scholar] [CrossRef]
- Sankary, H.N.; Yin, D.P.; Chong, A.S.F.; Ma, L.L.; Blinder, L.; Shen, J.K.; Foster, P.; Liu, L.P.; Li, C.; Williams, J.W. The portosystemic shunt protects liver against ischemic reperfusion injury. Transplantation 1999, 68, 958–963. [Google Scholar] [CrossRef]
- Sehitoglu, M.H.; Karaboga, I.; Kiraz, A.; Kiraz, H.A. The hepatoprotective effect of Aloe vera on ischemia-reperfusion injury in rats. N. Clin. Istanb. 2019, 6, 203–209. [Google Scholar]
- Yaylak, F.; Canbaz, H.; Caglikulekci, M.; Dirlik, M.; Tamer, L.; Ogetman, Z.; Polat, Y.; Kanik, A.; Aydin, S. Liver tissue inducible nitric oxide synthase (iNOS) expression and lipid peroxidation in experimental hepatic ischemia reperfusion injury stimulated with lipopolysaccharide: The role of aminoguanidine. J. Surg. Res. 2008, 148, 214–223. [Google Scholar] [CrossRef]
- Rodríguez-Reynoso, S.; Leal, C.; Portilla, E.; Olivares, N.; Muñiz, J. Effect of exogenous melatonin on hepatic energetic status during ischemia/reperfusion: Possible role of tumor necrosis factor-α and nitric oxide. J. Surg. Res. 2001, 100, 141–149. [Google Scholar] [CrossRef]
- Yang, S.L.; Chen, L.J.; Kong, Y.; Xu, D.; Lou, Y.J. Sodium nitroprusside regulates mRNA expressions of LTC4 synthesis enzymes in hepatic ischemia/reperfusion injury rats via NF-kappaB signaling pathway. Pharmacology 2007, 80, 11–20. [Google Scholar] [CrossRef]
- Harada, N.; Iimuro, Y.; Nitta, T.; Yoshida, M.; Uchinami, H.; Nishio, T.; Hatano, E.; Yamamoto, N.; Yamamoto, Y.; Yamaoka, Y. Inactivation of the small GTPase Rac1 protects the liver from ischemia/reperfusion injury in the rat. Surgery 2003, 134, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, H.; Iimuro, Y.; Uehara, T.; Nishio, T.; Harada, N.; Yoshida, M.; Hatano, E.; Son, G.; Fujimoto, J.; Yamaoka, Y. Nuclear factor kappa B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut 2005, 54, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Gaber, S.A.; Ibrahim, M.A.; Amin, E.F.; Ibrahim, S.A.; Mohammed, R.K.; Abdelrahman, A.M. Effect of selective versus non-selective cyclooxygenase inhibitors on ischemia-reperfusion-induced hepatic injury in rats. Life Sci. 2015, 134, 42–48. [Google Scholar] [CrossRef]
- Rodriguez-Reynos, S.; Leal-Cortes, C.; Portilla-de Buen, E.; Lopez-De la Torre, S.P. Ischemic Preconditioning Preserves Liver Energy Charge and Function on Hepatic Ischemia/Reperfusion Injury in Rats. Arch. Med. Res. 2018, 49, 373–380. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, J.J.; Xu, L.M.; Zhang, C.; Xia, Q. Protective effects of HGF-MSP chimer (metron factor-1) on liver ischemia-reperfusion injury in rat model. J. Dig. Dis. 2010, 11, 299–305. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, G.T.; Man, K.; Irwin, M.G. Pretreatment with intrathecal or intravenous morphine attenuates hepatic ischaemia-reperfusion injury in normal and cirrhotic rat liver. Br. J. Anaesth. 2012, 109, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Björnsson, B.; Bojmar, L.; Olsson, H.; Sundqvist, T.; Sandström, P. Nitrite, a novel method to decrease ischemia/reperfusion injury in the rat liver. World J. Gastroenterol. 2015, 21, 1775–1783. [Google Scholar] [CrossRef]
- Björnsson, B.; Winbladh, A.; Bojmar, L.; Sundqvist, T.; Gullstrand, P.; Sandström, P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J. Gastroenterol. 2014, 20, 9506–9512. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Nickkholgh, A.; Hoffmann, K.; Kern, M.; Schneider, H.; Sobirey, M.; Zorn, M.; Buchler, M.W.; Schemmer, P. Melatonin protects from hepatic reperfusion injury through inhibition of IKK and JNK pathways and modification of cell proliferation. J. Pineal Res. 2009, 46, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, J.; Afify, M.; Bleilevens, C.; Klinge, U.; Weiskirchen, R.; Steitz, J.; Vogt, M.; Yagi, S.; Nagai, K.; Uemoto, S.; et al. The Impact of a Nitric Oxide Synthase Inhibitor (L-NAME) on Ischemia(-)Reperfusion Injury of Cholestatic Livers by Pringle Maneuver and Liver Resection after Bile Duct Ligation in Rats. Int. J. Mol. Sci. 2019, 20, 2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.Q.; Zhang, Y.; Xiang, J.J.; Xiong, C.L. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J. Gastroenterol. 2007, 13, 1953–1961. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.Q.; Zhang, Y.; Xiong, C.L. The protective effects of 17beta-estradiol on hepatic ischemia-reperfusion injury in rat model, associated with regulation of heat-shock protein expression. J. Surg. Res. 2007, 140, 67–76. [Google Scholar] [CrossRef]
- El-Gohary, O.A. Obestatin improves hepatic injury induced by ischemia/reperfusion in rats: Role of nitric oxide. Gen. Physiol. Biophys. 2017, 36, 109–115. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Y.; Xu, K.Q.; Huang, W.Q. Pretreatment of parecoxib attenuates hepatic ischemia/reperfusion injury in rats. BMC Anesth. 2015, 15, 165. [Google Scholar] [CrossRef] [Green Version]
- Duval, H.; Mbatchi, S.F.; Grandadam, S.; Legendre, C.; Loyer, P.; Ribault, C.; Piquet-Pellorce, C.; Guguen-Guillouzo, C.; Boudjema, K.; Corlu, A. Reperfusion stress induced during intermittent selective clamping accelerates rat liver regeneration through JNK pathway. J. Hepatol. 2010, 52, 560–569. [Google Scholar] [CrossRef]
- Kawai, K.; Yokoyama, Y.; Kokuryo, T.; Watanabe, K.; Kitagawa, T.; Nagino, M. Inchinkoto, an herbal medicine, exerts beneficial effects in the rat liver under stress with hepatic ischemia-reperfusion and subsequent hepatectomy. Ann. Surg. 2010, 251, 692–700. [Google Scholar] [CrossRef]
- Bae, U.J.; Yang, J.D.; Ka, S.O.; Koo, J.H.; Woo, S.J.; Lee, Y.R.; Yu, H.C.; Cho, B.H.; Zhao, H.Y.; Ryu, J.H.; et al. SPA0355 attenuates ischemia/reperfusion-induced liver injury in mice. Exp. Mol. Med. 2014, 46, e109. [Google Scholar] [CrossRef]
- Datta, G.; Luong, T.V.; Fuller, B.J.; Davidson, B.R. Endothelial nitric oxide synthase and heme oxygenase-1 act independently in liver ischemic preconditioning. J. Surg. Res. 2014, 186, 417–428. [Google Scholar] [CrossRef]
- Hines, I.N.; Harada, H.; Bharwani, S.; Pavlick, K.P.; Hoffman, J.M.; Grisham, M.B. Enhanced post-ischemic liver injury in iNOS-deficient mice: A cautionary note. Biochem. Biophys. Res. Commun. 2001, 284, 972–976. [Google Scholar] [CrossRef]
- Hines, I.N.; Kawachi, S.; Harada, H.; Pavlick, K.P.; Hoffman, J.M.; Bharwani, S.; Wolf, R.E.; Grisham, M.B. Role of nitric oxide in liver ischemia and reperfusion injury. Mol. Cell Biochem. 2002, 234, 229–237. [Google Scholar] [CrossRef]
- Kawachi, S.; Hines, I.N.; Laroux, F.S.; Hoffman, J.; Bharwani, S.; Gray, L.; Leffer, D.; Grisham, M.B. Nitric oxide synthase and postischemic liver injury. Biochem. Biophys. Res. Commun. 2000, 276, 851–854. [Google Scholar] [CrossRef]
- Lee, V.G.; Johnson, M.L.; Baust, J.; Laubach, V.E.; Watkins, S.C.; Billiar, T.R. The roles of iNOS in liver ischemia-reperfusion injury. Shock 2001, 16, 355–360. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, T.; Ma, C.G. Dexmedetomidine (DEX) protects against hepatic ischemia/reperfusion (I/R) injury by suppressing inflammation and oxidative stress in NLRC5 deficient mice. Biochem. Biophys. Res. Commun. 2017, 493, 1143–1150. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Peng, T.; Yang, D.; Wang, Q.; Lv, Z.; Shen, J. Caveolin-1 protects against hepatic ischemia/reperfusion injury through ameliorating peroxynitrite-mediated cell death. Free Radic. Biol. Med. 2016, 95, 209–215. [Google Scholar] [CrossRef]
- Guo, J.Y.; Yang, T.; Sun, X.G.; Zhou, N.Y.; Li, F.S.; Long, D.; Lin, T.; Li, P.Y.; Feng, L. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J. Biomed. Sci. 2011, 18, 79. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yang, T.; Lu, J.; Li, S.; Wan, L.; Long, D.; Li, Q.; Feng, L.; Li, Y. Rb1 postconditioning attenuates liver warm ischemia-reperfusion injury through ROS-NO-HIF pathway. Life. Sci. 2011, 88, 598–605. [Google Scholar] [CrossRef]
- Jeyabalan, G.; Klune, J.R.; Nakao, A.; Martik, N.; Wu, G.; Tsung, A.; Geller, D.A. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric. Oxide 2008, 19, 29–35. [Google Scholar] [CrossRef]
- Kim, H.J.; Joe, Y.; Yu, J.K.; Chen, Y.; Jeong, S.O.; Mani, N.; Cho, G.J.; Pae, H.O.; Ryter, S.W.; Chung, H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim. Biophys. Acta 2015, 1852, 1550–1559. [Google Scholar] [CrossRef] [Green Version]
- Klune, J.R.; Dhupar, R.; Kimura, S.; Ueki, S.; Cardinal, J.; Nakao, A.; Nace, G.; Evankovich, J.; Murase, N.; Tsung, A.; et al. Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G666–G673. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Assmus, U.; Wüstefeld, T.; Meyer zu Vilsendorf, A.; Roskams, T.; Schmidt-Supprian, M.; Rajewsky, K.; Brenner, D.A.; Manns, M.P.; Pasparakis, M.; et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J. Clin. Investig. 2005, 115, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.H.; Hood, B.L.; Mukhopadhyay, P.; Rajesh, M.; Abdelmegeed, M.A.; Kwon, Y.I.; Conrads, T.P.; Veenstra, T.D.; Song, B.J.; Pacher, P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology 2008, 135, 1344–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Rajesh, M.; Horvath, B.; Batkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Xiao, F.; Li, W.; Yu, M.; Du, P.; Fang, Z.; Sun, J. Hepatocellular HO-1 mediated iNOS-induced hepatoprotection against liver ischemia reperfusion injury. Biochem. Biophys. Res. Commun. 2020, 521, 1095–1100. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, X.; Zhao, G.; Liu, Z.; Yu, M.; Fang, Z.; Li, X. Hepatocellular iNOS protects liver from ischemia/reperfusion injury through HSF1-dependent activation of HSP70. Biochem. Biophys. Res. Commun. 2019, 512, 882–888. [Google Scholar] [CrossRef]
- Sanches, S.C.; Ramalho, L.N.; Mendes-Braz, M.; Terra, V.A.; Cecchini, R.; Augusto, M.J.; Ramalho, F.S. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
- Shaker, M.E.; Trawick, B.N.; Mehal, W.Z. The novel TLR9 antagonist COV08-0064 protects from ischemia/reperfusion injury in non-steatotic and steatotic mice livers. Biochem. Pharm. 2016, 112, 90–101. [Google Scholar] [CrossRef]
- Shi, Y.R.H.; Ramshesh, V.K.; Schwartz, J.; Liu, Q.; Krishnasamy, Y.; Zhang, X.; Lemasters, J.J.; Smith, C.D.; Zhong, Z. Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. J. Hepatol. 2012, 56, 137–145. [Google Scholar] [CrossRef]
- Tsung, A.; Stang, M.T.; Ikeda, A.; Critchlow, N.D.; Izuishi, K.; Nakao, A.; Chan, M.H.; Jeyabalan, G.; Yim, J.H.; Geller, D.A. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1261–G1268. [Google Scholar] [CrossRef] [Green Version]
- Tsurui, Y.; Sho, M.; Kuzumoto, Y.; Hamada, K.; Akashi, S.; Kashizuka, H.; Ikeda, N.; Nomi, T.; Mizuno, T.; Kanehiro, H.; et al. Dual role of vascular endothelial growth factor in hepatic ischemia-reperfusion injury. Transplantation 2005, 79, 1110–1115. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Fu, C.; Wang, L.; Zhu, L.; Yan, Y.T.; Xiang, Y.; Zheng, F.; Gong, F.L.; Chen, S.; Chen, G. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ajamieh, H.; Farrell, G.C.; McCuskey, R.S.; Yu, J.; Chu, E.; Wong, H.J.; Lam, W.; Teoh, N.C. Acute atorvastatin is hepatoprotective against ischaemia-reperfusion injury in mice by modulating eNOS and microparticle formation. Liver Int. 2015, 35, 2174–2186. [Google Scholar] [CrossRef]
- Duarte, S.; Hamada, T.; Kuriyama, N.; Busuttil, R.W.; Coito, A.J. TIMP-1 deficiency leads to lethal partial hepatic ischemia and reperfusion injury. Hepatology 2012, 56, 1074–1085. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.C.; Uchida, Y.; Zhao, D.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Blockade of Janus kinase-2 signaling ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2010, 16, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Hamada, T.; Duarte, S.; Tsuchihashi, S.; Busuttil, R.W.; Coito, A.J. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury. Am. J. Pathol. 2009, 174, 2265–2277. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.M.; Jang, H.J.; Kim, S.S.; Kim, H.J.; Lee, S.Y.; Oh, M.Y.; Kwan, H.C.; Jang, D.S.; Eom, D.W. Protective Effect of Eupatilin Pretreatment Against Hepatic Ischemia-Reperfusion Injury in Mice. Transpl. Proc. 2016, 48, 1226–1233. [Google Scholar] [CrossRef]
- Okaya, T.; Lentsch, A.B. Peroxisome proliferator-activated receptor-α regulates postischemic liver injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G606–G612. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.M.; Sun, J.; Zhong, W.Z.; Pan, X.X.; Liu, C.M.; Cheng, F.; Wang, P.; Rao, Z.Q. Dexmedetomidine preconditioning alleviated murine liver ischemia and reperfusion injury by promoting macrophage M2 activation via PPAR gamma/STAT3 signaling. Int. Immunopharmacol. 2020, 82, 106363. [Google Scholar] [CrossRef]
- Tao, J.; Shen, X.H.; Ai, Y.H.; Han, X.J. Tea polyphenols protect against ischemia/reperfusion-induced liver injury in mice through anti-oxidative and anti-apoptotic properties. Exp. Ther. Med. 2016, 12, 3433–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patouraux, S.; Rousseau, D.; Rubio, A.; Bonnafous, S.; Lavallard, V.J.; Lauron, J.; Saint-Paul, M.C.; Bailly-Maitre, B.; Tran, A.; Crenesse, D.; et al. Osteopontin deficiency aggravates hepatic injury induced by ischemia-reperfusion in mice. Cell Death Dis. 2014, 5, e1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.P.; Watanabe, T.; Oowada, S.; Hirayama, A.; Nagase, S.; Kamibayashi, M.; Otsubo, T. Effect of CV159-Ca(2+)/calmodulin blockade on redox status hepatic ischemia-reperfusion injury in mice evaluated by a newly developed in vivo EPR imaging technique. J. Surg. Res. 2008, 147, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jessup, J.M.; Samara, R.; Battle, P.; Laguinge, L.M. Carcinoembryonic antigen promotes tumor cell survival in liver through an IL-10-dependent pathway. Clin. Exp. Metastasis 2005, 21, 709–717. [Google Scholar] [CrossRef]
- Godwin, A.; Yang, W.L.; Sharma, A.; Khader, A.; Wang, Z.; Zhang, F.; Nicastro, J.; Coppa, G.F.; Wang, P. Blocking cold-inducible RNA-binding protein (CIRP) protects liver from ischemia/reperfusion injury. Shock 2014, 43, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Braz, M.; Elias-Miró, M.; Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Ramalho, F.S.; Peralta, C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J. Biomed. Biotechnol. 2012, 2012, 298657. [Google Scholar] [CrossRef] [Green Version]
- Man, K.; Fan, S.T.; Ng, I.O.; Lo, C.M.; Liu, C.L.; Yu, W.C.; Wong, J. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch. Surg. 1999, 134, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Peralta, C.; Bartrons, R.; Riera, L.; Manzano, A.; Xaus, C.; Gelpí, E.; Roselló-Catafau, J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G163–G171. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, X.; Li, H.; Guo, L.; Zhang, B.; Gong, Z.; Zhang, J.; Ye, Q. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J. Surg. Oncol. 2016, 114, 112–118. [Google Scholar] [CrossRef]
- Nakatake, R.; Tsuda, T.; Matsuura, T.; Miki, H.; Hishikawa, H.; Matsushima, H.; Ishizaki, M.; Matsui, K.; Kaibori, M.; Nishizawa, M.; et al. Genipin Inhibits the Induction of Inducible Nitric Oxide Synthase Through the Inhibition of NF-κB Activation in Rat Hepatocytes. Drug. Metab. Lett. 2017, 10, 254–263. [Google Scholar] [CrossRef]
- Butcher, R.L.; Collins, W.E.; Fugo, N.W. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974, 94, 1704–1708. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Which gender is better positioned in the process of liver surgery? Male or female? Surg. Today 2007, 37, 823–830. [Google Scholar] [CrossRef]
- Harada, H.; Pavlick, K.P.; Hines, I.N.; Hoffman, J.M.; Bharwani, S.; Gray, L.; Wolf, R.E.; Grisham, M.B. Selected contribution: Effects of gender on reduced-size liver ischemia and reperfusion injury. J. Appl. Physiol. 2001, 91, 2816–2822. [Google Scholar] [CrossRef]
- Eckhoff, D.E.; Bilbao, G.; Frenette, L.; Thompson, J.A.; Contreras, J.L. 17-Beta-estradiol protects the liver against warm ischemia/reperfusion injury and is associated with increased serum nitric oxide and decreased tumor necrosis factor-alpha. Surgery 2002, 132, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnal, J.F.; Clamens, S.; Pechet, C.; Negre-Salvayre, A.; Allera, C.; Girolami, J.P.; Salvayre, R.; Bayard, F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc. Natl. Acad. Sci. USA 1996, 93, 4108–4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burra, P. Liver abnormalities and endocrine diseases. Best Pr. Res. Clin. Gastroenterol. 2013, 27, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Zeni, N.; Zanetto, A.; Adam, R.; Karam, V.; Belli, L.S.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Cherqui, D.; et al. Influence of donor and recipient gender on liver transplantation outcomes in Europe. Liver Int. 2020, 40, 1961–1971. [Google Scholar] [CrossRef]
- Tang, J.; Cao, Y.; Rose, R.L.; Brimfield, A.A.; Dai, D.; Goldstein, J.A.; Hodgson, E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab. Dispos. 2001, 29, 1201–1204. [Google Scholar]
- Löfgren, S.; Hagbjörk, A.L.; Ekman, S.; Fransson-Steen, R.; Terelius, Y. Metabolism of human cytochrome P450 marker substrates in mouse: A strain and gender comparison. Xenobiotica 2004, 34, 811–834. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Mansukhani, N.A.; Stubbs, V.C.; Helenowski, I.B.; Woodruff, T.K.; Kibbe, M.R. Sex bias exists in basic science and translational surgical research. Surgery 2014, 156, 508–516. [Google Scholar] [CrossRef]
- Kleinert, H.; Schwarz, P.M.; Förstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Kröncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Kim, P.K.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Datta, G.; Fuller, B.J.; Davidson, B.R. Molecular mechanisms of liver ischemia reperfusion injury: Insights from transgenic knockout models. World J. Gastroenterol. 2013, 19, 1683–1698. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kubota, K.; Ogura, T.; Esumi, H.; Inoue, K.; Takayama, T.; Makuuchi, M. Increased nitric oxide production in the liver in the perioperative period of partial hepatectomy with Pringle’s maneuver. J. Hepatol. 1998, 28, 212–220. [Google Scholar] [CrossRef]
- Abu-Amara, M.; Yang, S.Y.; Seifalian, A.; Davidson, B.; Fuller, B. The nitric oxide pathway—Evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012, 32, 531–543. [Google Scholar] [CrossRef]
- Shah, V.; Chen, A.F.; Cao, S.; Hendrickson, H.; Weiler, D.; Smith, L.; Yao, J.; Katusic, Z.S. Gene transfer of recombinant endothelial nitric oxide synthase to liver in vivo and in vitro. Am. J Physiol. Gastrointest. Liver Physiol. 2000, 279, G1023–G1030. [Google Scholar] [CrossRef]
- Yamashita, T.; Kawashima, S.; Ohashi, Y.; Ozaki, M.; Rikitake, Y.; Inoue, N.; Hirata, K.; Akita, H.; Yokoyama, M. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension 2000, 36, 97–102. [Google Scholar] [CrossRef]
- Behar-Cohen, F.F.; Heydolph, S.; Faure, V.; Droy-Lefaix, M.T.; Courtois, Y.; Goureau, O. Peroxynitrite cytotoxicity on bovine retinal pigmented epithelial cells in culture. Biochem. Biophys. Res. Commun. 1996, 226, 842–849. [Google Scholar] [CrossRef]
- Fan, C.; Zwacka, R.M.; Engelhardt, J.F. Therapeutic approaches for ischemia/reperfusion injury in the liver. J. Mol. Med. 1999, 77, 577–592. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-based inhibitors of nitric oxide synthase: Therapeutic potential and challenges. Mediat. Inflamm. 2012, 2012, 318087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Nonami, T.; Kurokawa, T.; Takeuchi, Y.; Harada, A.; Nakao, A.; Takagi, H. Role of endogenous nitric oxide in ischemia-reperfusion injury in rat liver. J. Surg. Res. 1995, 59, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R.; Ayav, A.; Navarra, G.; Sommerville, C.; Pai, M.; Damrah, O.; Khorsandi, S.; Habib, N.A. Laparoscopic liver resection assisted by the laparoscopic Habib Sealer. Surgery 2008, 144, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Efanov, M.; Kazakov, I.; Alikhanov, R.; Vankovich, A.; Koroleva, A.; Kovalenko, D.; Salimgereeva, D.; Tsvirkun, V.; Khatkov, I. A randomized prospective study of the immediate outcomes of the use of a hydro-jet dissector and an ultrasonic surgical aspirator for laparoscopic liver resection. HPB 2021, 23, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Rau, H.G.; Wichmann, M.W.; Schinkel, S.; Buttler, E.; Pickelmann, S.; Schauer, R.; Schildberg, F.W. Surgical techniques in hepatic resections: Ultrasonic aspirator versus Jet-Cutter. A prospective randomized clinical trial. Zent. Chir. 2001, 126, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, R.; Wahba, R.; Bangard, C.; Prenzel, K.; Hölscher, A.H.; Stippel, D. Radiomorphology of the Habib sealer-induced resection plane during long-time followup: A longitudinal single center experience after 64 radiofrequency-assisted liver resections. HPB Surg. 2010, 2010, 403097. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Chen, K.; Wei, Y.; Li, B.; Liu, F. Laparoscopic liver resection with “ultrasonic scalpel mimic CUSA” technique. Surg. Endosc. 2022, 1–8. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS. Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]













| Method | N° of Used Method | N° Used for Meta-Analysis | % of Used Methods |
|---|---|---|---|
| ELISA | 7 | 3 | 2.2 |
| IHC | 24 | 8 | 6.0 |
| RNA | 61 | 18 | 13.4 |
| Spectrometry | 5 | 3 | 2.2 |
| WB | 37 | 8 | 6.0 |
| Total | 134 | 40 | 29.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatake, R.; Schulz, M.; Kalvelage, C.; Benstoem, C.; Tolba, R.H. Effects of iNOS in Hepatic Warm Ischaemia and Reperfusion Models in Mice and Rats: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 11916. https://doi.org/10.3390/ijms231911916
Nakatake R, Schulz M, Kalvelage C, Benstoem C, Tolba RH. Effects of iNOS in Hepatic Warm Ischaemia and Reperfusion Models in Mice and Rats: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(19):11916. https://doi.org/10.3390/ijms231911916
Chicago/Turabian StyleNakatake, Richi, Mareike Schulz, Christina Kalvelage, Carina Benstoem, and René H. Tolba. 2022. "Effects of iNOS in Hepatic Warm Ischaemia and Reperfusion Models in Mice and Rats: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 23, no. 19: 11916. https://doi.org/10.3390/ijms231911916





