PINK1 Phosphorylates Drp1S616 to Improve Mitochondrial Fission and Inhibit the Progression of Hypertension-Induced HFpEF
Abstract
:1. Introduction
2. Results
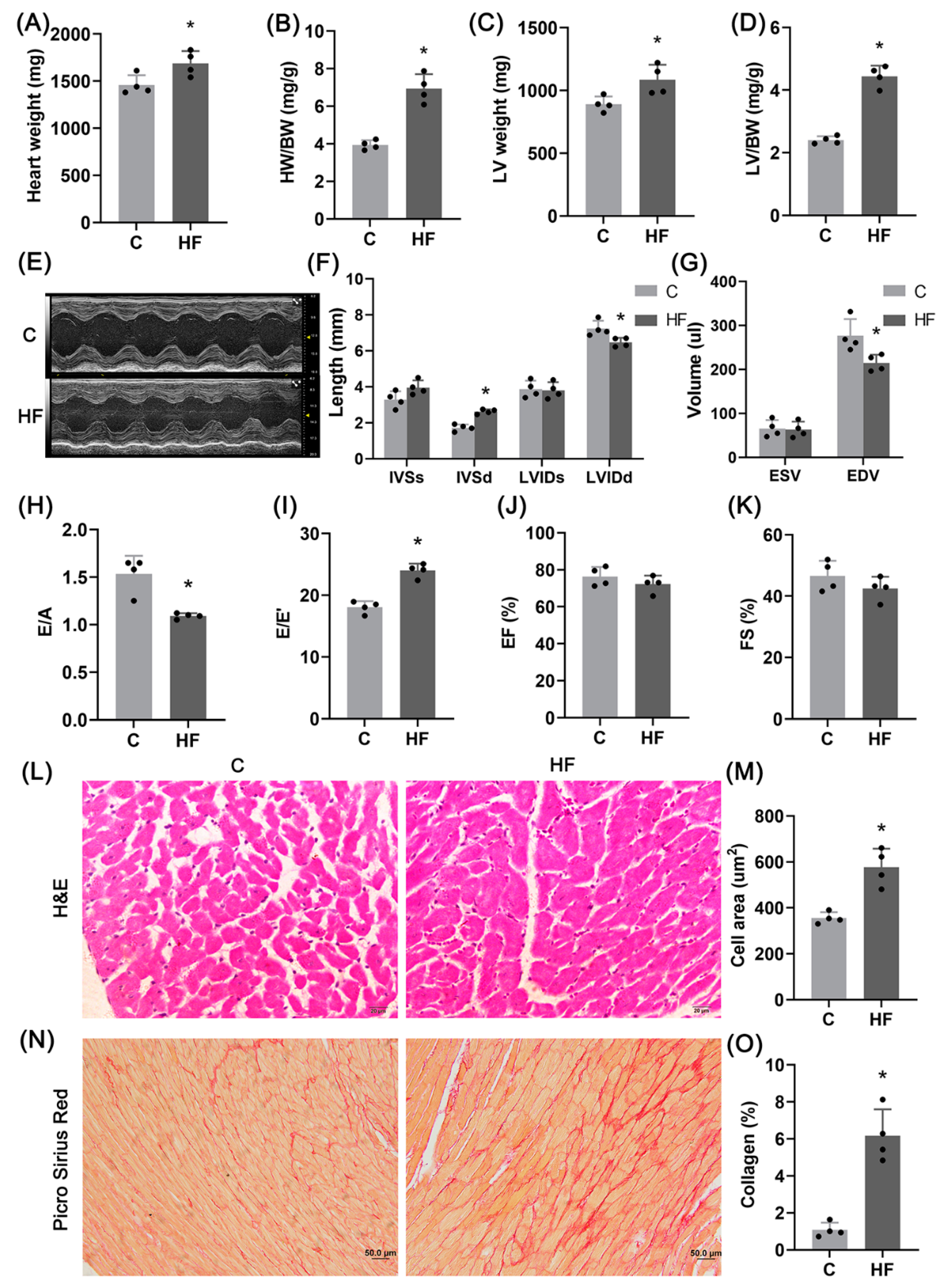
2.1. Hypertension-Induced HFpEF Model
2.2. Mitochondrial Dysfunctions
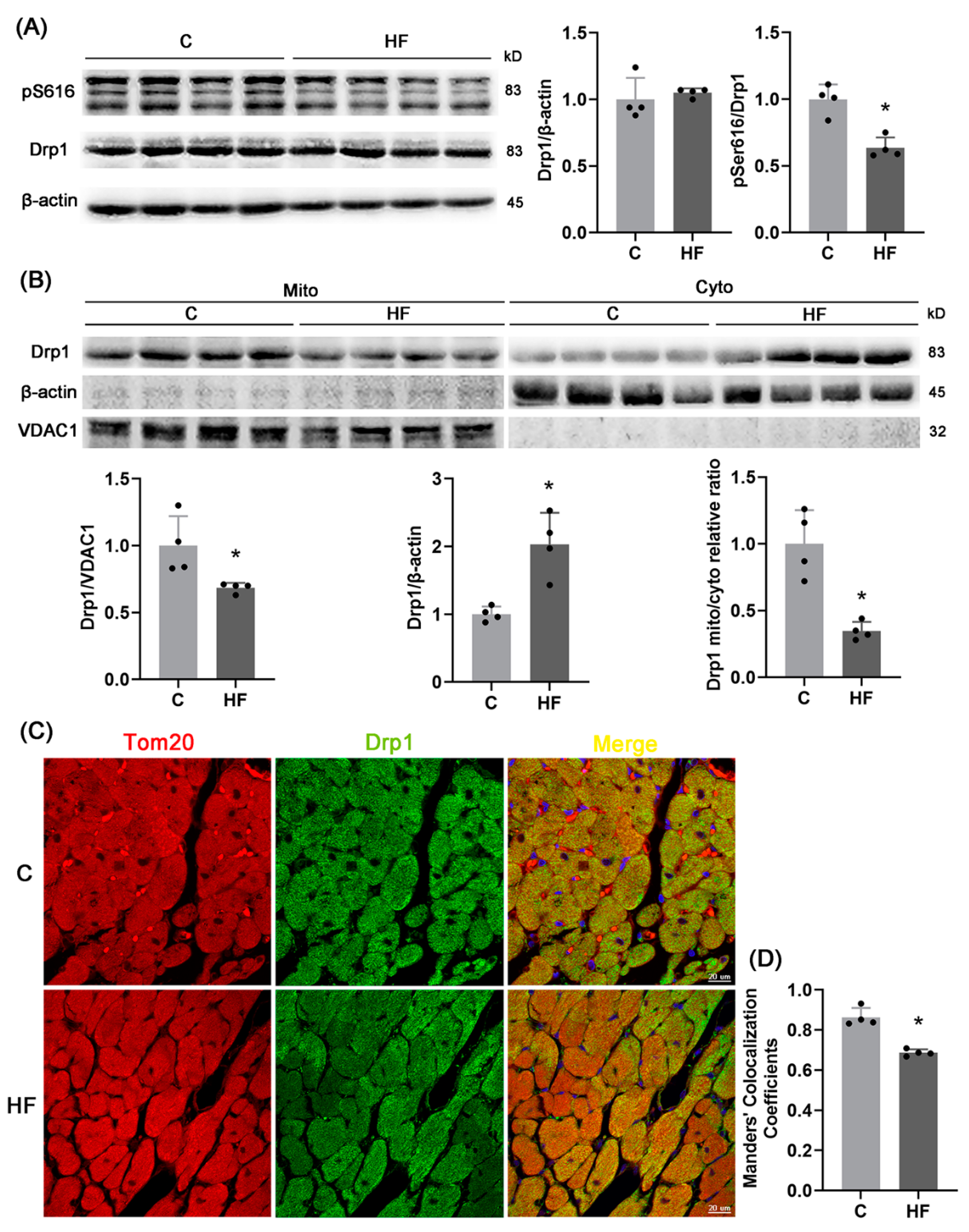
2.3. pDrp1S616-Mediated Mitochondrial Fission
2.4. PINK1-Regulated Mitochondrial Fission
2.5. pDrp1S616-Mediated Mitochondrial Fission Regulates Mitochondrial Function
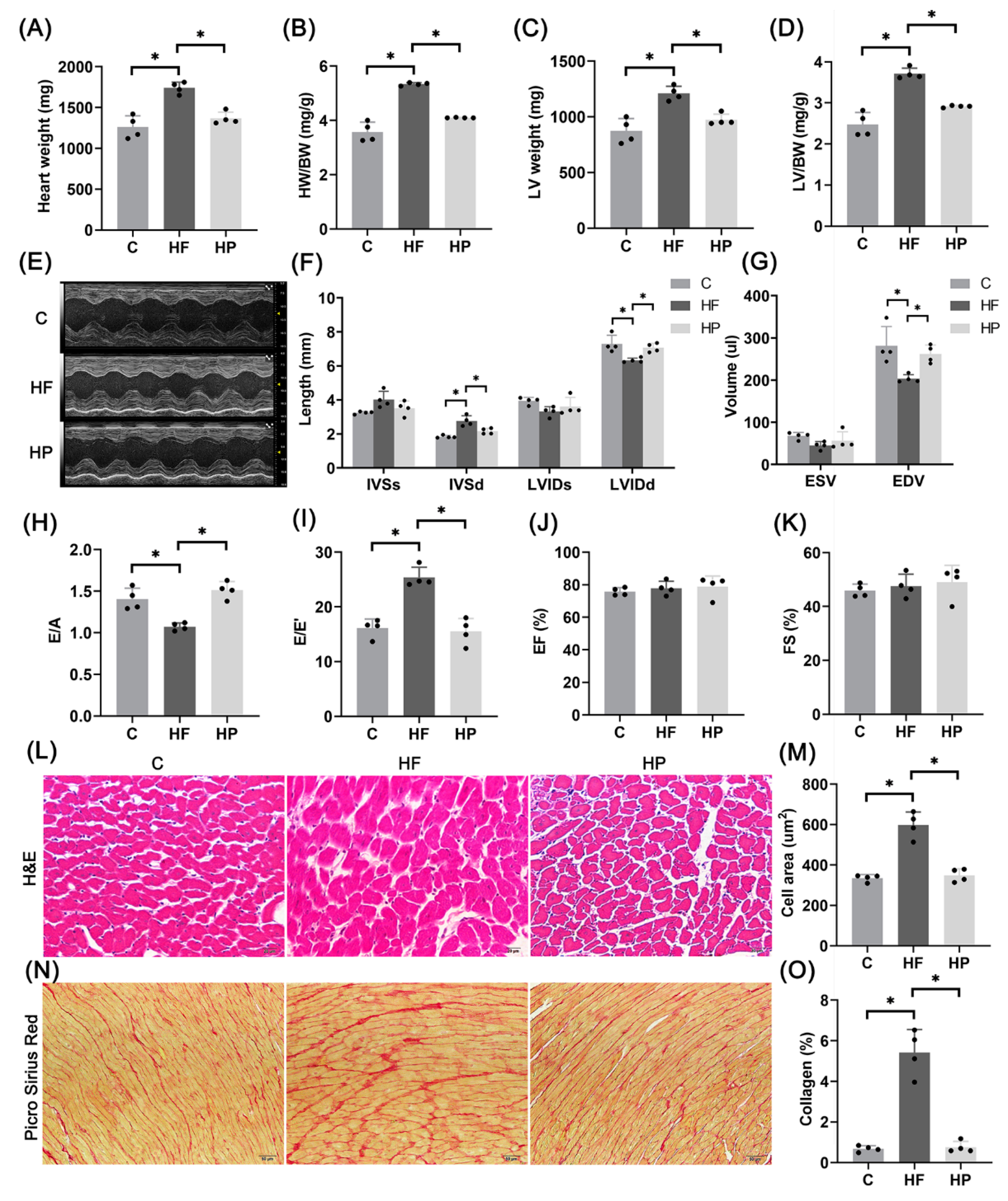
2.6. PINK1 Improves Hypertension-Induced HFpEF Phenotypes
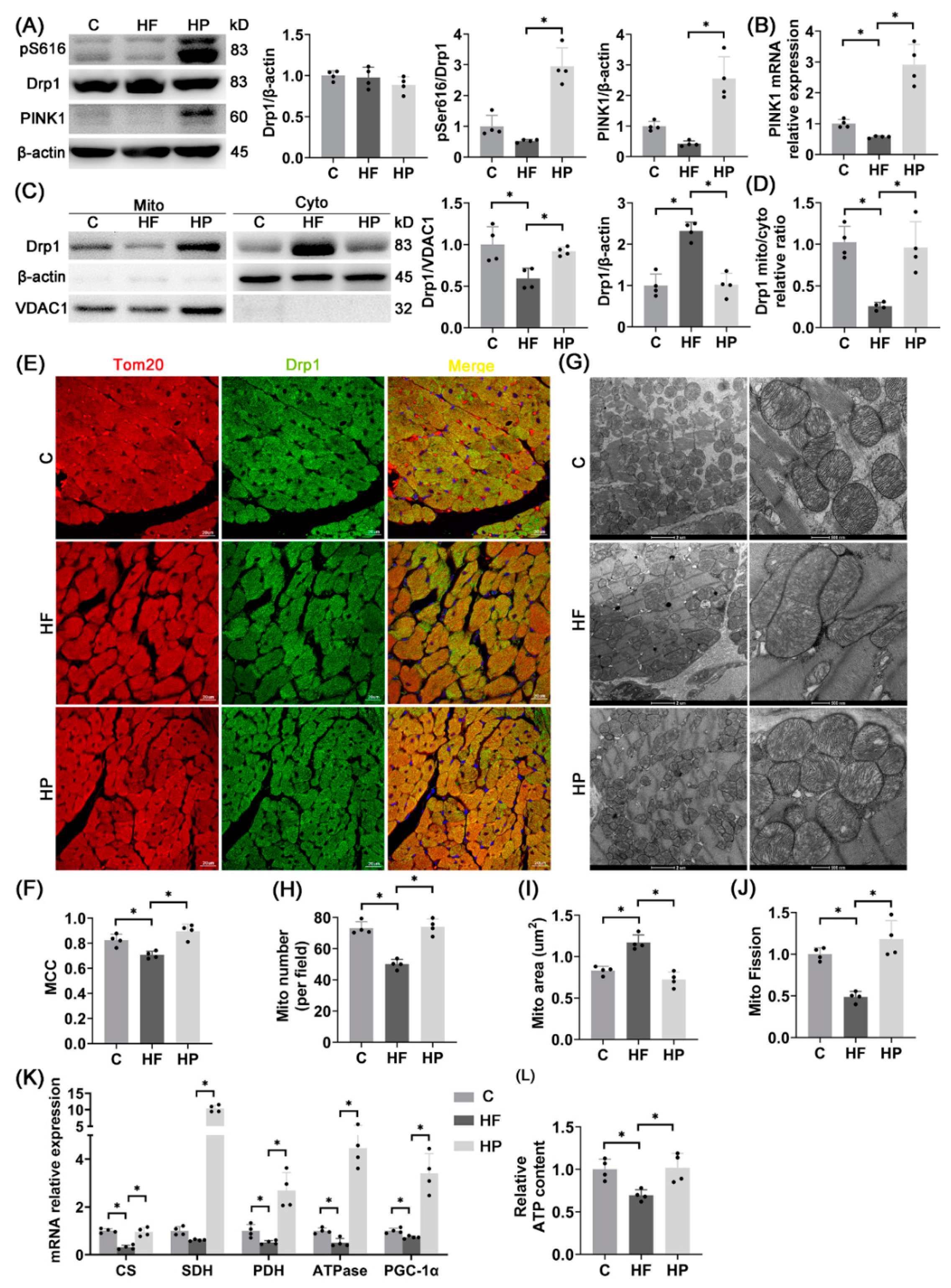
2.7. PINK1 Stimulates pDrp1S616-Mediated Mitochondrial Fission for Improvement of Mitochondrial Function
3. Discussion
Critiques of the Study
4. Materials and Methods
4.1. Animal Experiments
4.2. PINK1 Was Specifically Overexpressed by AAV In Vivo
4.3. Echocardiography
4.4. Transmission Electron Microscope
4.5. H&E and Picro Sirius Red Staining
4.6. Culture of H9C2 Cardiomyocyte Line and Lentivirus Transfection
4.7. Real-Time PCR
4.8. Separation of Mitochondrial Protein and Cytoplasmic Protein
4.9. Western Blot
4.10. Immunofluorescence
4.11. Morphological Analysis of Mitochondria
4.12. Mitochondrial Membrane Potential
4.13. ATP Content Detection
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Non-Standard Abbreviations and Acronyms
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| Drp1 | dynamic related protein 1 |
| PINK1 | PTEN induced putative kinase 1 |
| OPA1 | optical atrophy 1 |
| MFF | mitochondrial fission factor |
| Fis1 | fission protein 1 |
| S616 | Ser 616 |
| AAV | adeno-associated virus |
| LVIDs | Left ventricular end systolic internal diameter |
| LVIDd | left ventricular end diastolic internal diameter |
| IVSs | interventricular septal end systolic thickness |
| IVSd | interventricular septal end diastolic thickness |
| ESV | left ventricular end systolic volume |
| EDV | left ventricular end diastolic volume |
| EF | ejection fraction |
| FS | fraction of shorten |
| E/A | the ratio of early diastolic maximum velocity of mitral valve to systolic maximum velocity of atrium |
| E/E′ | the ratio of the maximum velocity of blood flow in the early diastolic phase of mitral valve to the motion velocity of mitral annulus |
| VDAC1 | voltage dependent anion channel protein 1 |
| Tom20 | the mitochondrial preprotein translocases of the outer membrane 20 |
| TMRE | tetramethylrhodamine ethyl ester |
| CS | citrate synthase |
| SDH | succinate dehydrogenase |
| PDH | pyruvate dehydrogenase |
| ATPase | ATP synthase |
| SIRT3 | sirtuin 3 |
| PGC-1α | peroxisome promoter activated receptor gamma coactivator-1 α |
| NRF-1 | nuclear respiratory factor-1 |
| Mfn1/2 | mitofusin 1/2 |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Wong, L.L.; Liew, O.W.; Richards, A.M. Heart Failure with Reduced Ejection Fraction (HFrEF) and Preserved Ejection Fraction (HFpEF): The Diagnostic Value of Circulating MicroRNAs. Cells 2019, 8, 1651. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Sillje, H.H.W.; Schouten, E.M.; Dokter, M.M.; Voors, A.A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [Green Version]
- Schiattarella, G.G.; Altamirano, F.; Kim, S.Y.; Tong, D.; Ferdous, A.; Piristine, H.; Dasgupta, S.; Wang, X.; French, K.M.; Villalobos, E.; et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat. Commun. 2021, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F. Heart failure with preserved ejection fraction: Insights into diagnosis and pathophysiology. Cardiovasc. Res. 2021, 117, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef]
- Sun, X.; Alford, J.; Qiu, H. Structural and Functional Remodeling of Mitochondria in Cardiac Diseases. Int. J. Mol. Sci. 2021, 22, 4167. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by beta-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Whitley, B.N.; Engelhart, E.A.; Hoppins, S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion 2019, 49, 269–283. [Google Scholar] [CrossRef]
- Boutry, M.; Kim, P.K. ORP1L mediated PI(4)P signaling at ER-lysosome-mitochondrion three-way contact contributes to mitochondrial division. Nat. Commun. 2021, 12, 5354. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Banuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Kalkhoran, S.B.; Kriston-Vizi, J.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Rosdah, A.A.; Lees, J.G.; Costa, J.; Ling, N.X.Y.; Holien, J.K.; Samangouei, P.; et al. Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovasc. Res. 2022, 118, 282–294. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [Green Version]
- Jimah, J.R.; Hinshaw, J.E. Structural Insights into the Mechanism of Dynamin Superfamily Proteins. Trends Cell. Biol. 2019, 29, 257–273. [Google Scholar] [CrossRef]
- Jin, J.Y.; Wei, X.X.; Zhi, X.L.; Wang, X.H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef]
- Tanaka, K. The PINK1-Parkin axis: An Overview. Neurosci. Res. 2020, 159, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, P.; Huang, R.; Wang, C.; Sun, L.; Lan, B.; He, Y.; Zhao, H.; Gao, Y. PINK1: The guard of mitochondria. Life Sci. 2020, 259, 118247. [Google Scholar] [CrossRef]
- Lin, J.; Chen, K.; Chen, W.; Yao, Y.; Ni, S.; Ye, M.; Zhuang, G.; Hu, M.; Gao, J.; Gao, C.; et al. Paradoxical Mitophagy Regulation by PINK1 and TUFm. Mol. Cell 2020, 80, 607–620.e12. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, Y.; Lu, J.; Zhang, W.; Zhu, Y.; Zhang, S.; Ma, G.; Jiang, P.; Zhang, W. PINK1-mediated mitophagy protects against hepatic ischemia/reperfusion injury by restraining NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020, 160, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hamano, K.; Sadoshima, J. Molecular mechanisms and clinical implications of multiple forms of mitophagy in the heart. Cardiovasc. Res. 2021, 117, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Tan, J.; Wang, R.; Wan, H.; He, Y.; Yan, X.; Guo, J.; Gao, Q.; Li, J.; Shang, S.; et al. PINK1 phosphorylates Drp1(S616) to regulate mitophagy-independent mitochondrial dynamics. EMBO Rep. 2020, 21, e48686. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Yao, W.; Li, L.; Niu, P.; Huo, Y.; Tan, W. Morphometric, Hemodynamic, and Multi-Omics Analyses in Heart Failure Rats with Preserved Ejection Fraction. Int. J. Mol. Sci. 2020, 21, 3362. [Google Scholar] [CrossRef] [PubMed]
- Bing, F.; Wang, X.; Shen, W.; Li, L.; Niu, P.; Chen, Y.; Zhang, W.; Tan, W.; Huo, Y. Inhalation of Ultrafine Zinc Particles Impaired Cardiovascular Functions in Hypertension-Induced Heart Failure Rats With Preserved Ejection Fraction. Front. Bioeng. Biotechnol. 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Ann. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Lu, Q.; Wang, Q.; Ding, Y.; Ma, Z.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.H. Binding of FUN14 Domain Containing 1 With Inositol 1,4,5-Trisphosphate Receptor in Mitochondria-Associated Endoplasmic Reticulum Membranes Maintains Mitochondrial Dynamics and Function in Hearts in Vivo. Circulation 2017, 136, 2248–2266. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dang, X.; Franco, A.; Dorn, G.W., 2nd. Reciprocal Regulation of Mitofusin 2-Mediated Mitophagy and Mitochondrial Fusion by Different PINK1 Phosphorylation Events. Front. Cell Dev. Biol. 2022, 10, 868465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhuo, Y.; Hu, Y.; Zhang, Z.; Ye, J.; Liu, L.; Luo, L.; Zhao, C.; Zhou, Q.; et al. SIRT1 alleviates high-magnitude compression-induced senescence in nucleus pulposus cells via PINK1-dependent mitophagy. Aging 2020, 12, 16126–16141. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shou, J.; Huo, Y. PINK1 Phosphorylates Drp1S616 to Improve Mitochondrial Fission and Inhibit the Progression of Hypertension-Induced HFpEF. Int. J. Mol. Sci. 2022, 23, 11934. https://doi.org/10.3390/ijms231911934
Shou J, Huo Y. PINK1 Phosphorylates Drp1S616 to Improve Mitochondrial Fission and Inhibit the Progression of Hypertension-Induced HFpEF. International Journal of Molecular Sciences. 2022; 23(19):11934. https://doi.org/10.3390/ijms231911934
Chicago/Turabian StyleShou, Jian, and Yunlong Huo. 2022. "PINK1 Phosphorylates Drp1S616 to Improve Mitochondrial Fission and Inhibit the Progression of Hypertension-Induced HFpEF" International Journal of Molecular Sciences 23, no. 19: 11934. https://doi.org/10.3390/ijms231911934
APA StyleShou, J., & Huo, Y. (2022). PINK1 Phosphorylates Drp1S616 to Improve Mitochondrial Fission and Inhibit the Progression of Hypertension-Induced HFpEF. International Journal of Molecular Sciences, 23(19), 11934. https://doi.org/10.3390/ijms231911934






