Anti-c-MET Fab-Grb2-Gab1 Fusion Protein-Mediated Interference of c-MET Signaling Pathway Induces Methuosis in Tumor Cells
Abstract
:1. Introduction
2. Results
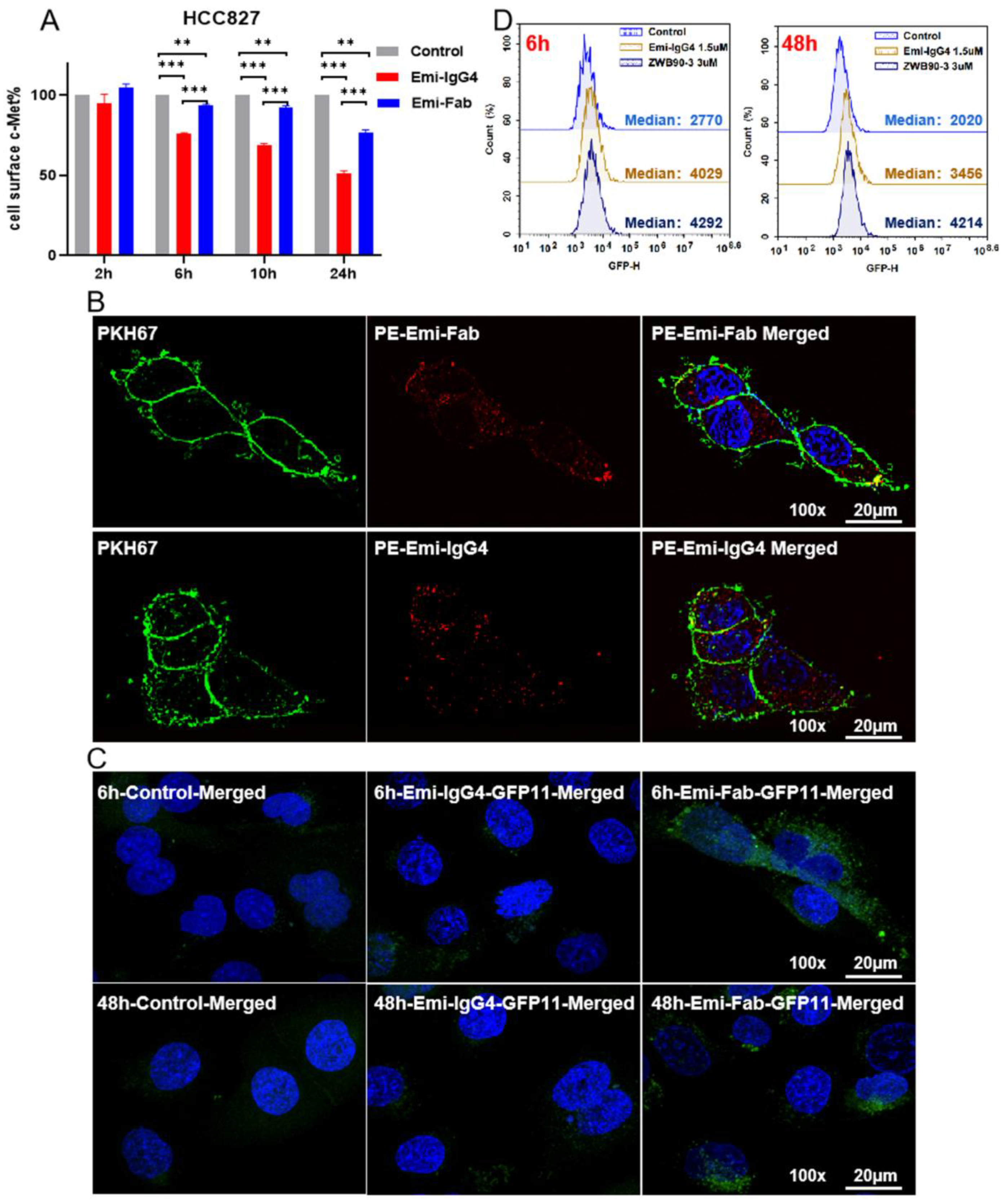
2.1. Emi-Based Fab Acts as an Intracellular Delivery Carrier
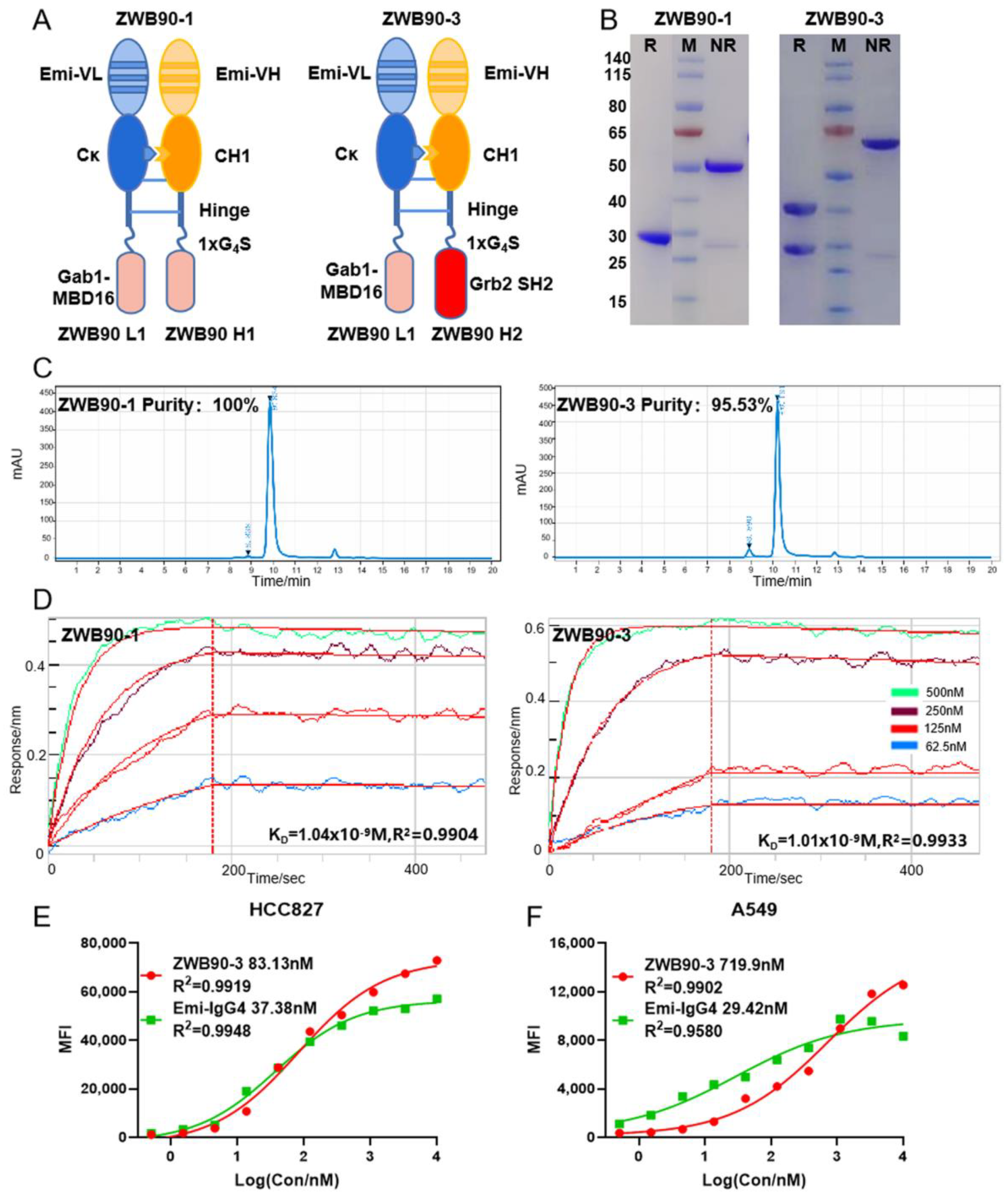
2.2. Construction, Expression, and Identification of the Recombinant Fusion Proteins
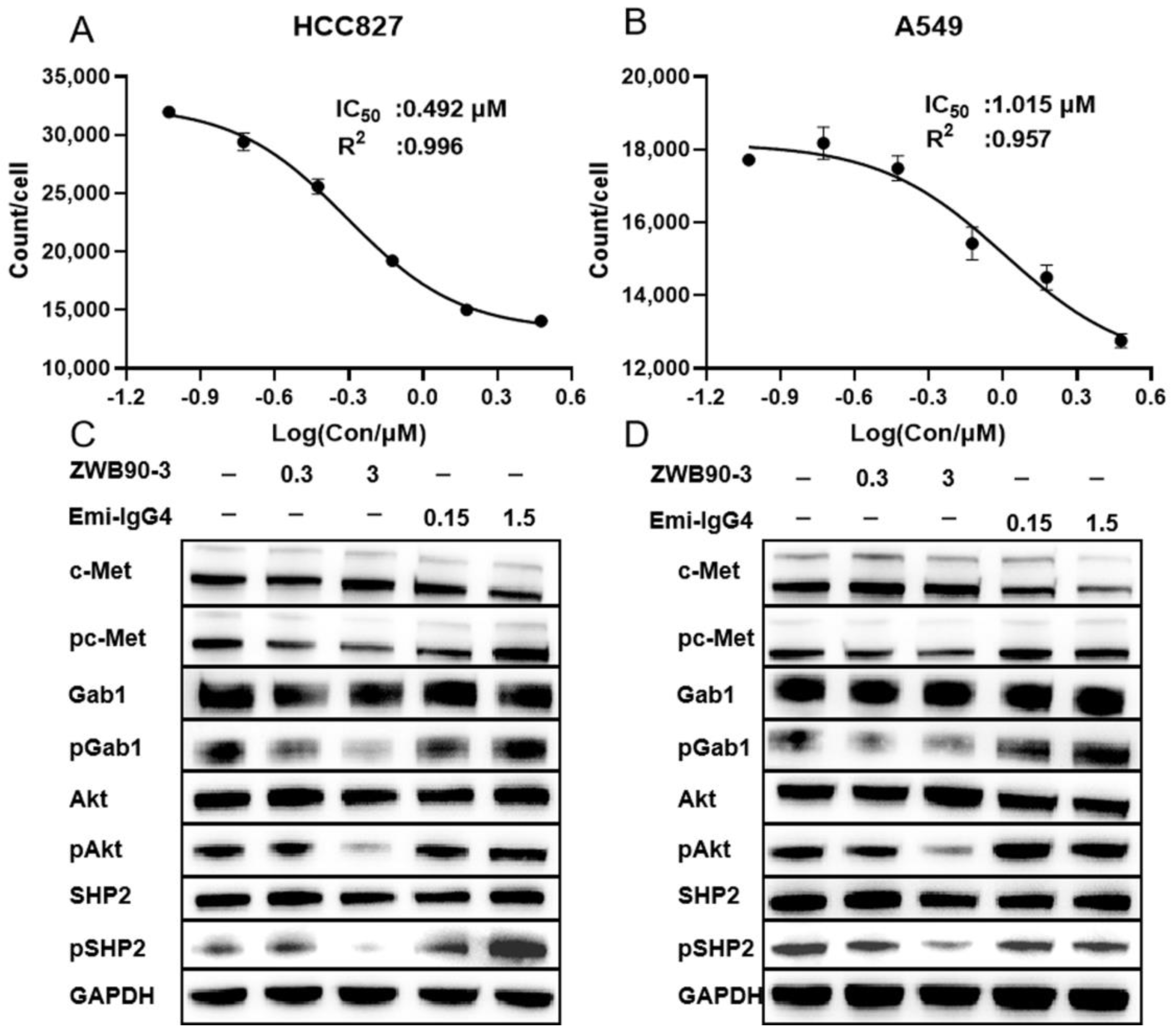
2.3. ZWB90-3 Inhibited Tumor Cell Proliferation and Blocked the c-MET Signaling Pathway
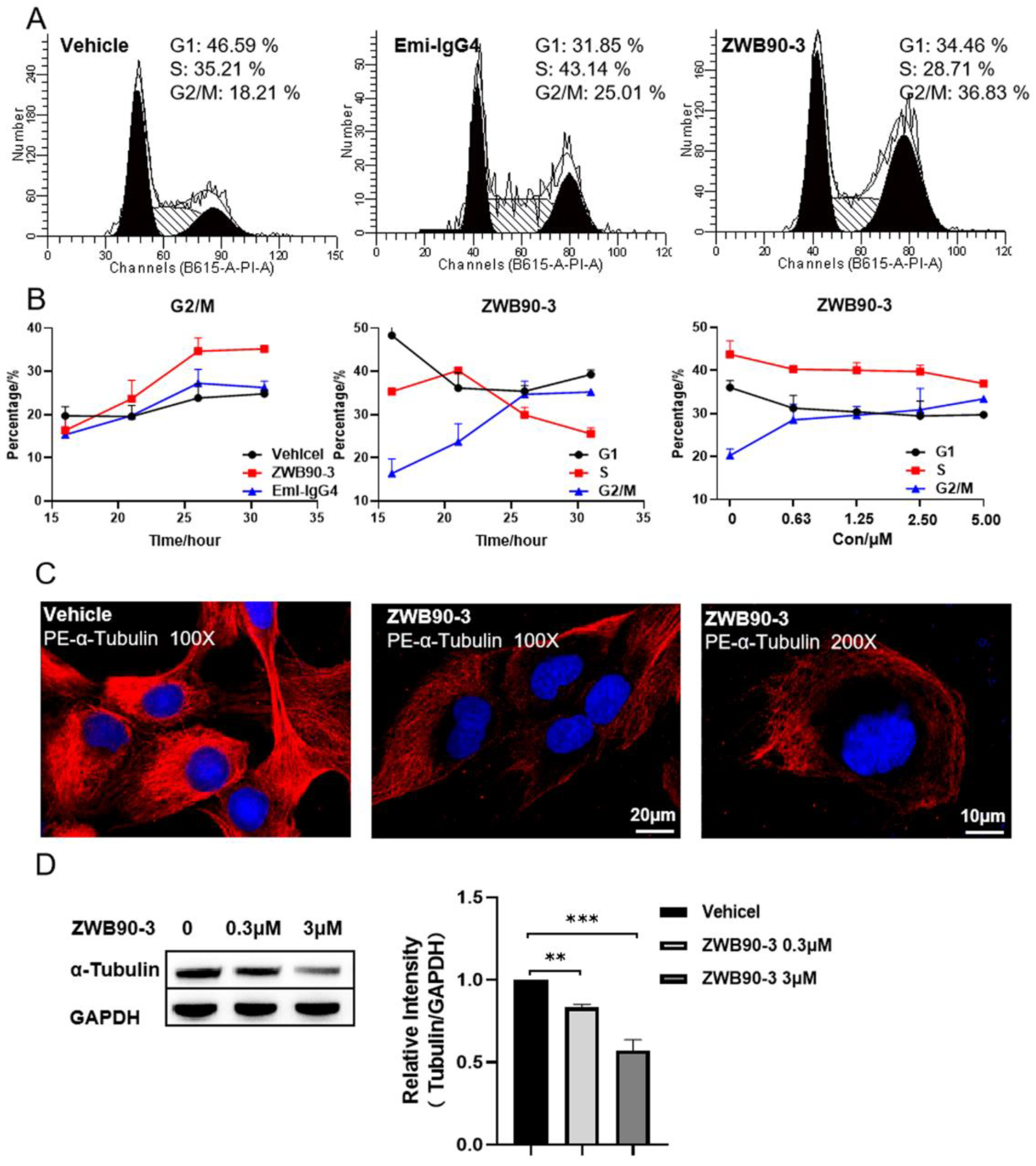
2.4. ZWB90-3 Induced Cell Cycle Arrest at the G2/M-Phase
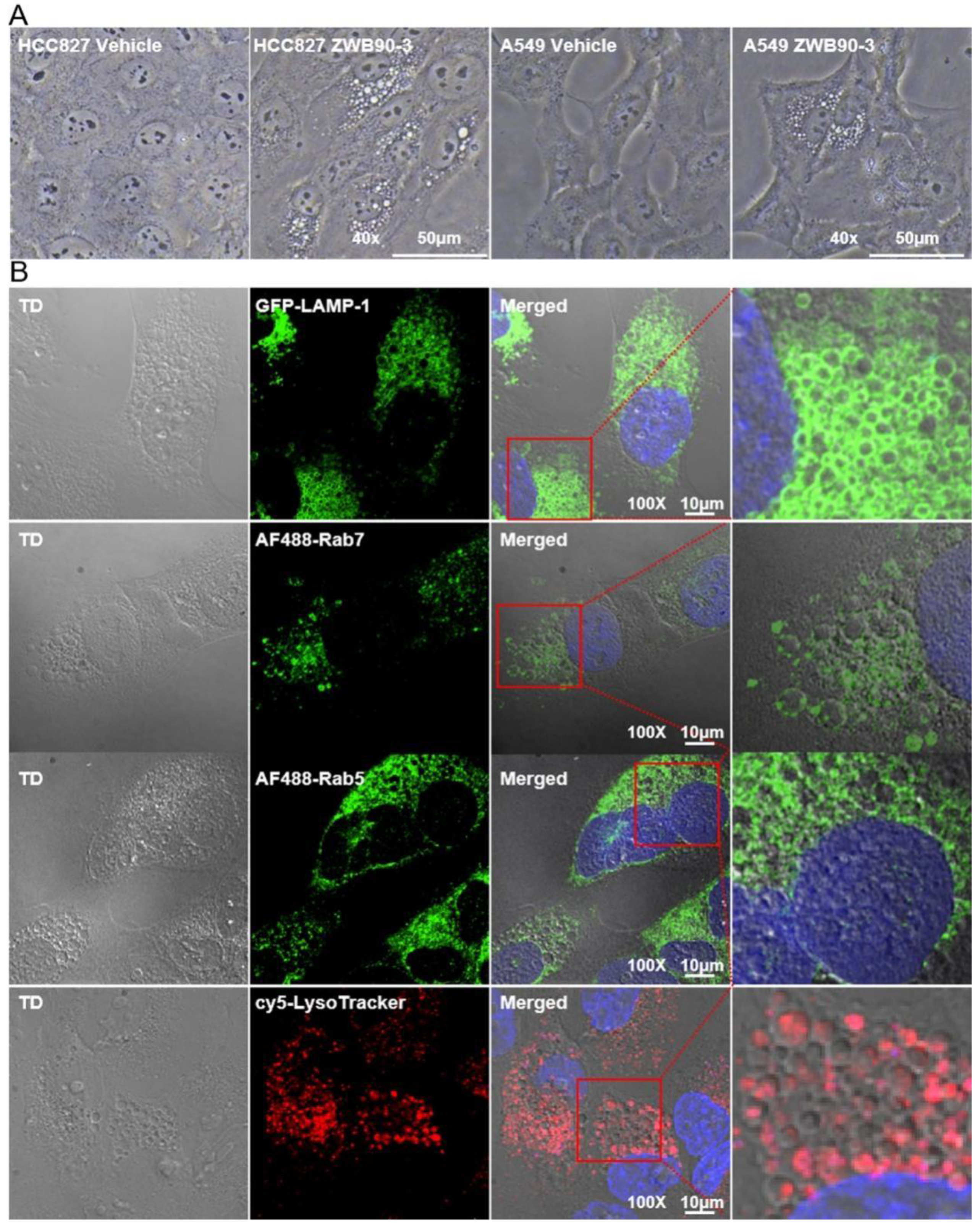
2.5. ZWB90-3 Induced Methuosis of Tumor Cells
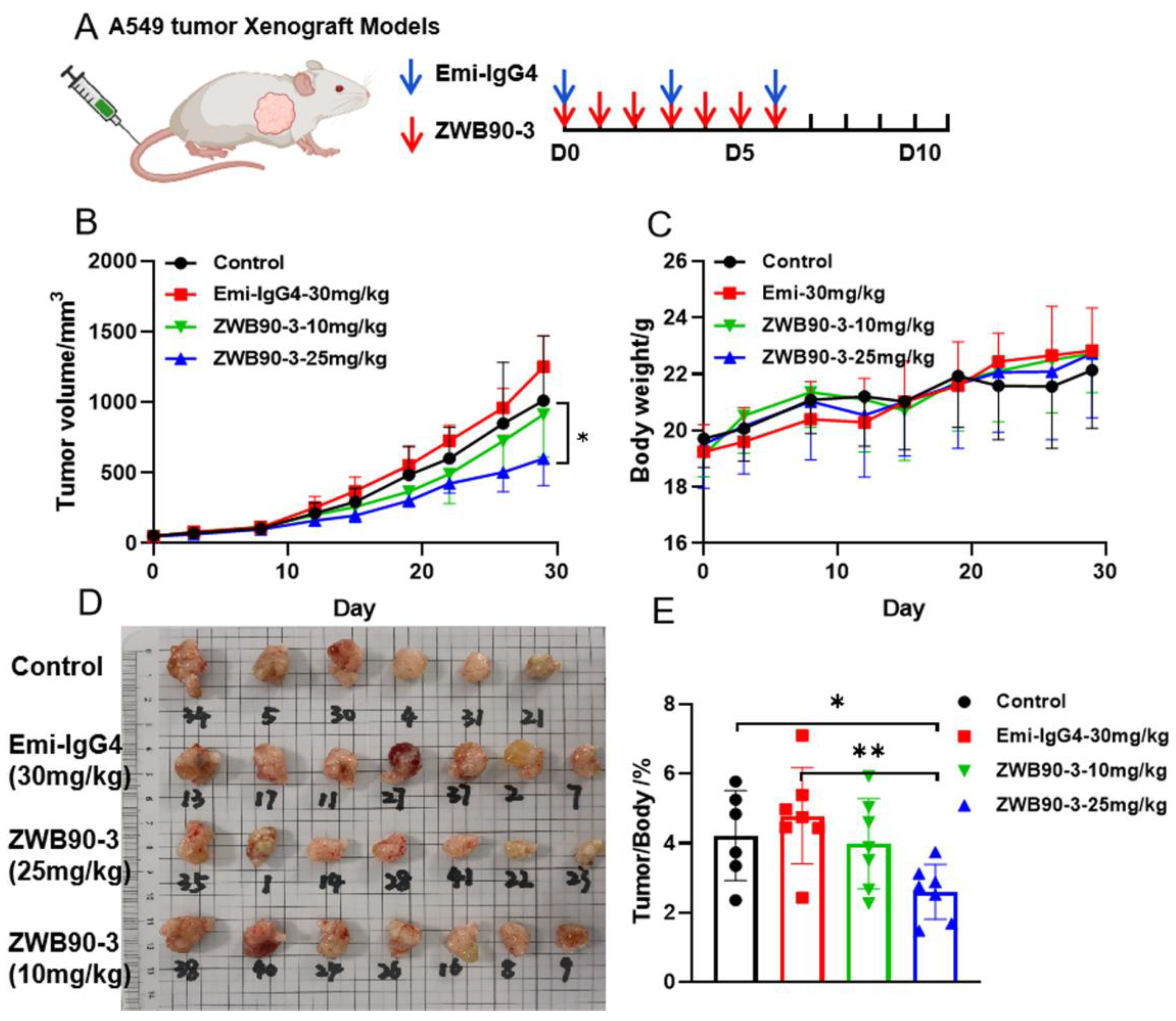
2.6. ZWB90-3 Inhibited Tumor Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Lentiviral Transfection
4.2. Protein Expression and Purification
4.3. Flow Cytometry Analysis
4.4. Cell Proliferation Assay
4.5. Confocal Immunofluorescence (IF) Microscopy
4.6. Western Blotting (WB)
4.7. Xenograft Mouse Models
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khera, N.; Rajput, S. Therapeutic Potential of Small Molecule Inhibitors. J. Cell. Biochem. 2017, 118, 959–961. [Google Scholar] [CrossRef]
- Hojjat-Farsangi, M. Small-molecule inhibitors of the receptor tyrosine kinases: Promising tools for targeted cancer therapies. Int. J. Mol. Sci. 2014, 15, 13768–13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, W. Creation of Functional Molecules Based on Biomolecular Interactions; Development toward Chemical Biology. Yakugaku Zasshi 2017, 137, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R. Challenges to macromolecular drug delivery. Biochem. Soc. Trans. 2007, 35 Pt 1, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.M.; Amouzadeh, H.R.; Engwall, M.J. Nonclinical strategy considerations for safety pharmacology: Evaluation of biopharmaceuticals. Expert Opin. Drug Saf. 2013, 12, 91–102. [Google Scholar] [CrossRef]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Lv, J.; Gao, X.; Wang, X.; Wang, H.; Chen, H.; He, X.; Li, L.; Cheng, Y. Rational Design of a Polymer with Robust Efficacy for Intracellular Protein and Peptide Delivery. Nano Lett. 2017, 17, 1678–1684. [Google Scholar] [CrossRef]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 2017, 87, 50–63. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Tomizawa, K. Cell-Penetrating Peptide as a Means of Directing the Differentiation of Induced-Pluripotent Stem Cells. Int. J. Mol. Sci. 2015, 16, 26667–26676. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Langel, U. Recent CPP-based applications in medicine. Expert Opin. Drug Deliv. 2019, 16, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Pellois, J.P. Peptide translocation through the plasma membrane of human cells: Can oxidative stress be exploited to gain better intracellular access? Commun. Integr. Biol. 2016, 9, e1205771. [Google Scholar] [CrossRef] [PubMed]
- Anton, J.; Sudibio, S.; Handoko, H.; Permata, T.B.M.; Kodrat, H.; Nuryadi, E.; Sofyan, H.; Susanto, E.; Mulyadi, R.; Aman, R.A.; et al. Prognosis in Glioblastoma Multiforme: A Systematic Review and Meta-Analyses. Asian Pac. J. Cancer Prev. 2021, 22, 3075–3080. [Google Scholar] [CrossRef]
- Huang, X.; Li, E.; Shen, H.; Wang, X.; Tang, T.; Zhang, X.; Xu, J.; Tang, Z.; Guo, C.; Bai, X.; et al. Targeting the HGF/MET Axis in Cancer Therapy: Challenges in Resistance and Opportunities for Improvement. Front. Cell Dev. Biol. 2020, 8, 152. [Google Scholar] [CrossRef]
- Pietronave, S.; Forte, G.; Locarno, D.; Merlin, S.; Zamperone, A.; Nicotra, G.; Isidoro, C.; Nardo, P.D.; Prat, M. Agonist monoclonal antibodies against HGF receptor protect cardiac muscle cells from apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1155–H1165. [Google Scholar] [CrossRef] [Green Version]
- Merchant, M.; Ma, X.; Maun, H.R.; Zheng, Z.; Peng, J.; Romero, M.; Huang, A.; Yang, N.Y.; Nishimura, M.; Greve, J.; et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl. Acad. Sci. USA 2013, 110, E2987–E2996. [Google Scholar] [CrossRef] [Green Version]
- Sakai, D.; Chung, H.C.; Oh, D.Y.; Park, S.H.; Kadowaki, S.; Kim, Y.H.; Tsuji, A.; Komatsu, Y.; Kang, Y.K.; Uenaka, K.; et al. A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother. Pharmacol. 2017, 80, 1197–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scagliotti, G.; Moro-Sibilot, D.; Kollmeier, J.; Favaretto, A.; Cho, E.K.; Grosch, H.; Kimmich, M.; Girard, N.; Tsai, C.M.; Hsia, T.C.; et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients. J. Thorac. Oncol. 2020, 15, 80–90. [Google Scholar] [CrossRef]
- Spigel, D.R.; Edelman, M.J.; O’Byrne, K.; Paz-Ares, L.; Mocci, S.; Phan, S.; Shames, D.S.; Smith, D.; Yu, W.; Paton, V.E.; et al. Results from the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J. Clin. Oncol. 2017, 35, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, S.; Lipfert, L.; Chevalier, K.; Bushey, B.S.; Henley, B.; Lenhart, R.; Sendecki, J.; Beqiri, M.; Millar, H.J.; Packman, K.; et al. Amivantamab (JNJ-61186372), an Fc Enhanced EGFR/cMet Bispecific Antibody, Induces Receptor Downmodulation and Antitumor Activity by Monocyte/Macrophage Trogocytosis. Mol. Cancer Ther. 2020, 19, 2044–2056. [Google Scholar] [CrossRef]
- Schaeper, U.; Gehring, N.H.; Fuchs, K.P.; Sachs, M.; Kempkes, B.; Birchmeier, W. Coupling of Gab1 to c-Met, Grb2, and Shp2 Mediates Biological Responses. J. Cell Biol. 2000, 149, 1419–1432. [Google Scholar] [CrossRef] [Green Version]
- Sorkin, A.; McClure, M.; Huang, F.; Carter, R. Interaction of EGF receptor and Grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 2000, 10, 1395–1398. [Google Scholar] [CrossRef] [Green Version]
- Guo, A.; Villén, J.; Kornhauser, J.; Lee, K.A.; Stokes, M.P.; Rikova, K.; Possemato, A.; Nardone, J.; Innocenti, G.; Wetzel, R.; et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl. Acad. Sci. USA 2008, 105, 692–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Shinohara, N.; Moriya, K.; Sazawa, A.; Kobayashi, Y.; Ogiso, Y.; Takiguchi, M.; Yasuda, J.; Koyanagi, T.; Kuzumaki, N.; et al. Significance of the Grb2 and son of sevenless (Sos) proteins in human bladder cancer cell lines. IUBMB Life 2000, 49, 317–320. [Google Scholar] [PubMed]
- Nguyen, L.; Holgado-Madruga, M.; Maroun, C.; Fixman, E.D.; Kamikura, D.; Fournier, T.; Charest, A.; Tremblay, M.L.; Wong, A.J.; Park, M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J. Biol. Chem. 1997, 272, 20811–20819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Furukawa, T.; Arano, Y.; Fujibayashi, Y.; Saga, T. Fusion protein based on Grb2-SH2 domain for cancer therapy. Biochem. Biophys. Res. Commun. 2010, 399, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Cai, Z.; Zhang, L.; Zhang, J.; He, X.; Du, X.; Wang, Q.; Lu, J. A recombined fusion protein PTD-Grb2-SH2 inhibits the proliferation of breast cancer cells in vitro. Int. J. Oncol. 2013, 42, 1061–1069. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, W.; Wortinger, M.A.; Yan, S.B.; Cornwell, P.; Peek, V.L.; Stephens, J.R.; Tetreault, J.W.; Xia, J.; Manro, J.R.; et al. LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth. Clin. Cancer Res. 2014, 20, 6059–6070. [Google Scholar] [CrossRef] [Green Version]
- Cabantous, S.; Terwilliger, T.C.; Waldo, G.S. Protein tagging and detection with engineered self-assembling fragments of green fluorescent protein. Nat. Biotechnol. 2005, 23, 102–107. [Google Scholar] [CrossRef]
- Lonn, P.; Kacsinta, A.D.; Cui, X.S.; Hamil, A.S.; Kaulich, M.; Gogoi, K.; Dowdy, S.F. Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics. Sci. Rep. 2016, 6, 32301. [Google Scholar] [CrossRef]
- Bonisch, M.; Sellmann, C.; Maresch, D.; Halbig, C.; Becker, S.; Toleikis, L.; Hock, B.; Ruker, F. Novel CH1:CL interfaces that enhance correct light chain pairing in heterodimeric bispecific antibodies. Protein Eng. Des. Sel. 2017, 30, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.Y.; Chai, Y.; Zhang, N.L.; Zhao, D.M.; Yang, C. Microtubule Stabilization Promotes Microcirculation Reconstruction After Spinal Cord Injury. J. Mol. Neurosci. 2021, 71, 583–595. [Google Scholar] [CrossRef]
- Maltese, W.A.; Overmeyer, J.H. Methuosis: Non-apoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Zhang, Y.; Ding, T.; Ji, N.; Zhao, H. The Dual Role of Macropinocytosis in Cancers: Promoting Growth and Inducing Methuosis to Participate in Anticancer Therapies as Targets. Front. Oncol. 2020, 10, 570108. [Google Scholar] [CrossRef]
- Bhanot, H.; Young, A.M.; Overmeyer, J.H.; Maltese, W.A. Induction of non-apoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol. Cancer Res. 2010, 8, 1358–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S.; Stayton, P.S.; Press, O.; Murthy, N.; Lackey, C.A.; Cheung, C.; Black, F.; Campbell, J.; Fausto, N.; Kyriakides, T.R.; et al. Design of “Smart” polymers that can direct intracellular drug delivery. Polym. Adv. Technol. 2002, 13, 992–999. [Google Scholar] [CrossRef]
- Prat, M.; Crepaldi, T.; Pennacchietti, S.; Bussolino, F.; Comoglio, P.M. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J. Cell Sci. 1998, 111 Pt 2, 237–247. [Google Scholar] [CrossRef]
- Foss, S.; Watkinson, R.; Sandlie, I.; James, L.C.; Andersen, J.T. TRIM21: A cytosolic Fc receptor with broad antibody isotype specificity. Immunol. Rev. 2015, 268, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Gay, B.; Suarez, S.; Caravatti, G.; Furet, P.; Meyer, T.; Schoepfer, J. Selective GRB2 SH2 inhibitors as anti-Ras therapy. Int. J. Cancer 1999, 83, 235–241. [Google Scholar] [CrossRef]
- Lin, C.C.; Wieteska, L.; Suen, K.M.; Kalverda, A.P.; Ahmed, Z.; Ladbury, J.E. Grb2 binding induces phosphorylation-independent activation of Shp2. Commun. Biol. 2021, 4, 437. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.C.; Terracciano, M.; Mancarella, C.; Sciandra, M.; Guerzoni, C.; Pasello, M.; Grilli, A.; Zini, M.; Picci, P.; Colombo, M.P.; et al. CD99 triggering induces methuosis of Ewing sarcoma cells through IGF-1R/RAS/Rac1 signaling. Oncotarget 2016, 7, 79925–79942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overmeyer, J.H.; Young, A.M.; Bhanot, H.; Maltese, W.A. A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol. Cancer 2011, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Kamiyama, D.; Sekine, S.; Barsi-Rhyne, B.; Hu, J.; Chen, B.; Gilbert, L.A.; Ishikawa, H.; Leonetti, M.D.; Marshall, W.F.; Weissman, J.S.; et al. Versatile protein tagging in cells with split fluorescent protein. Nat. Commun. 2016, 7, 11046. [Google Scholar] [CrossRef] [Green Version]
- Dyson, M.R. Fundamentals of Expression in Mammalian Cells. Adv. Exp. Med. Biol. 2016, 896, 217–224. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, X.; Xu, Q.; Dong, B.; Xu, G.; Qian, N.; Yang, C.; Li, H.; Chen, L.; Gao, X.; Song, H. Anti-c-MET Fab-Grb2-Gab1 Fusion Protein-Mediated Interference of c-MET Signaling Pathway Induces Methuosis in Tumor Cells. Int. J. Mol. Sci. 2022, 23, 12018. https://doi.org/10.3390/ijms231912018
Dou X, Xu Q, Dong B, Xu G, Qian N, Yang C, Li H, Chen L, Gao X, Song H. Anti-c-MET Fab-Grb2-Gab1 Fusion Protein-Mediated Interference of c-MET Signaling Pathway Induces Methuosis in Tumor Cells. International Journal of Molecular Sciences. 2022; 23(19):12018. https://doi.org/10.3390/ijms231912018
Chicago/Turabian StyleDou, Xiaoqian, Qinzhi Xu, Bo Dong, Guili Xu, Niliang Qian, Cuima Yang, Hongjie Li, Liting Chen, Xin Gao, and Haifeng Song. 2022. "Anti-c-MET Fab-Grb2-Gab1 Fusion Protein-Mediated Interference of c-MET Signaling Pathway Induces Methuosis in Tumor Cells" International Journal of Molecular Sciences 23, no. 19: 12018. https://doi.org/10.3390/ijms231912018





