Silver Nanoparticles Produced by Laser Ablation and Re-Irradiation Are Effective Preventing Peri-Implantitis Multispecies Biofilm Formation
Abstract
:1. Introduction
2. Results
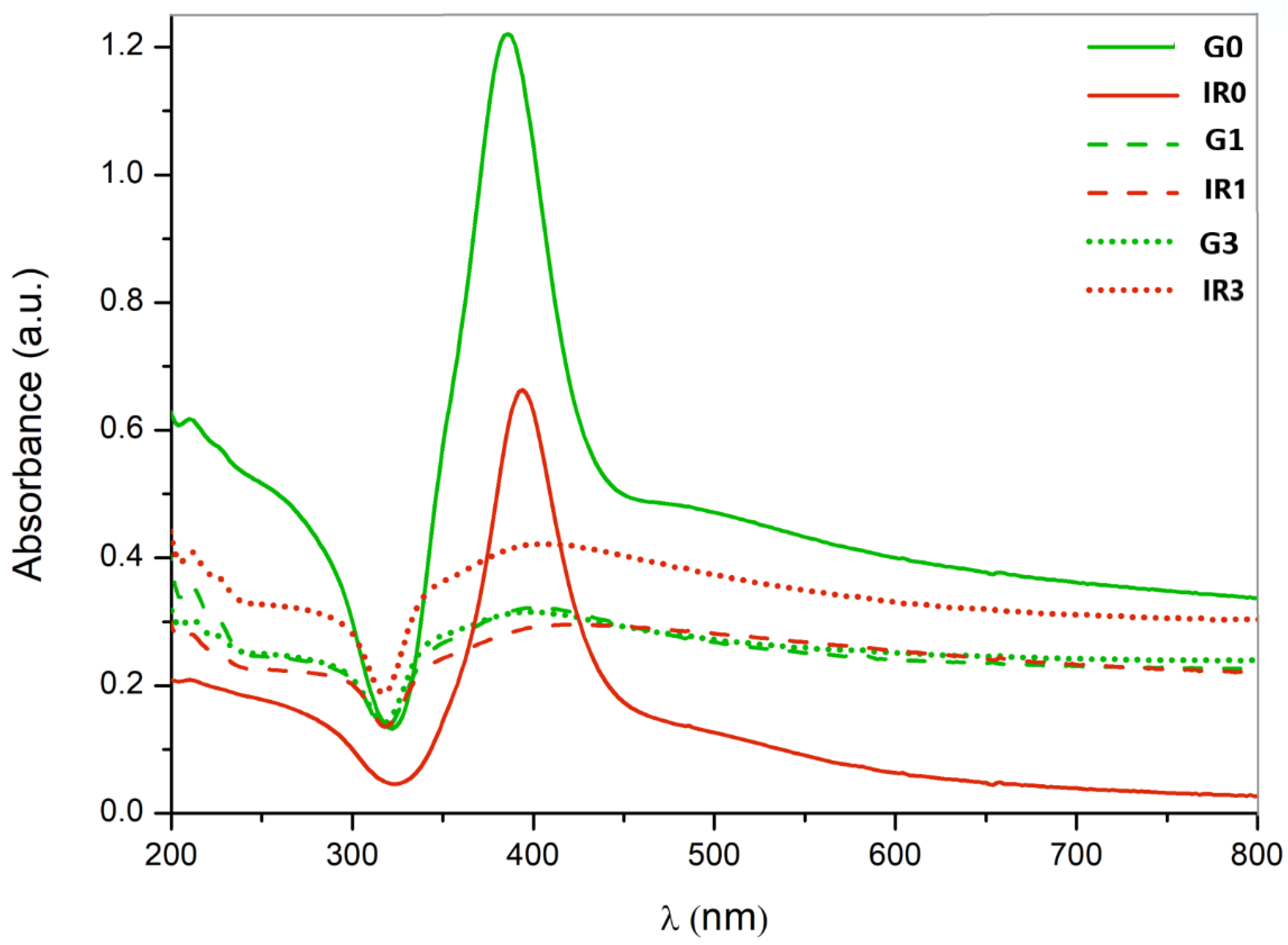
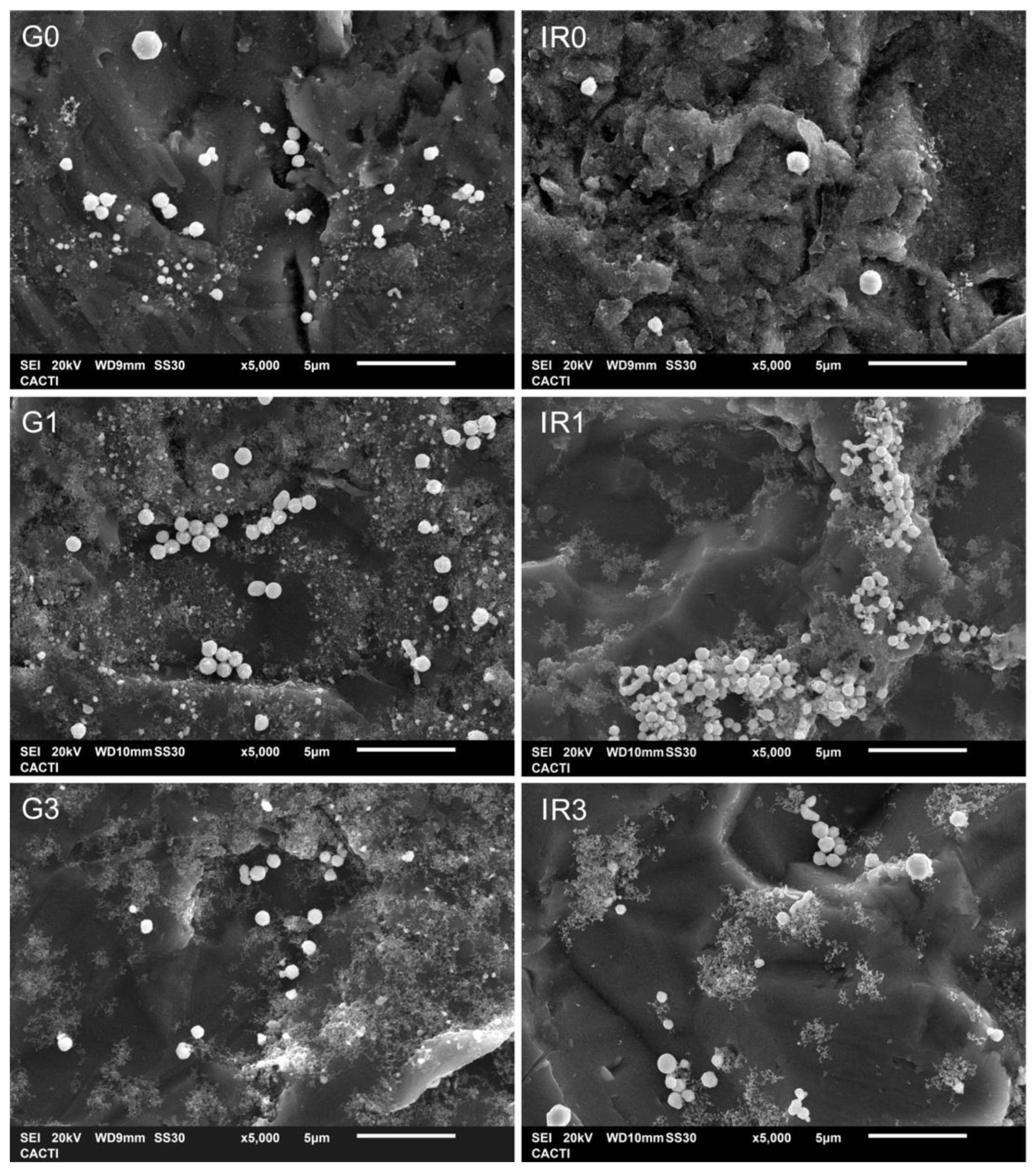
2.1. Physico-Chemical Characterization of the Nanoparticles
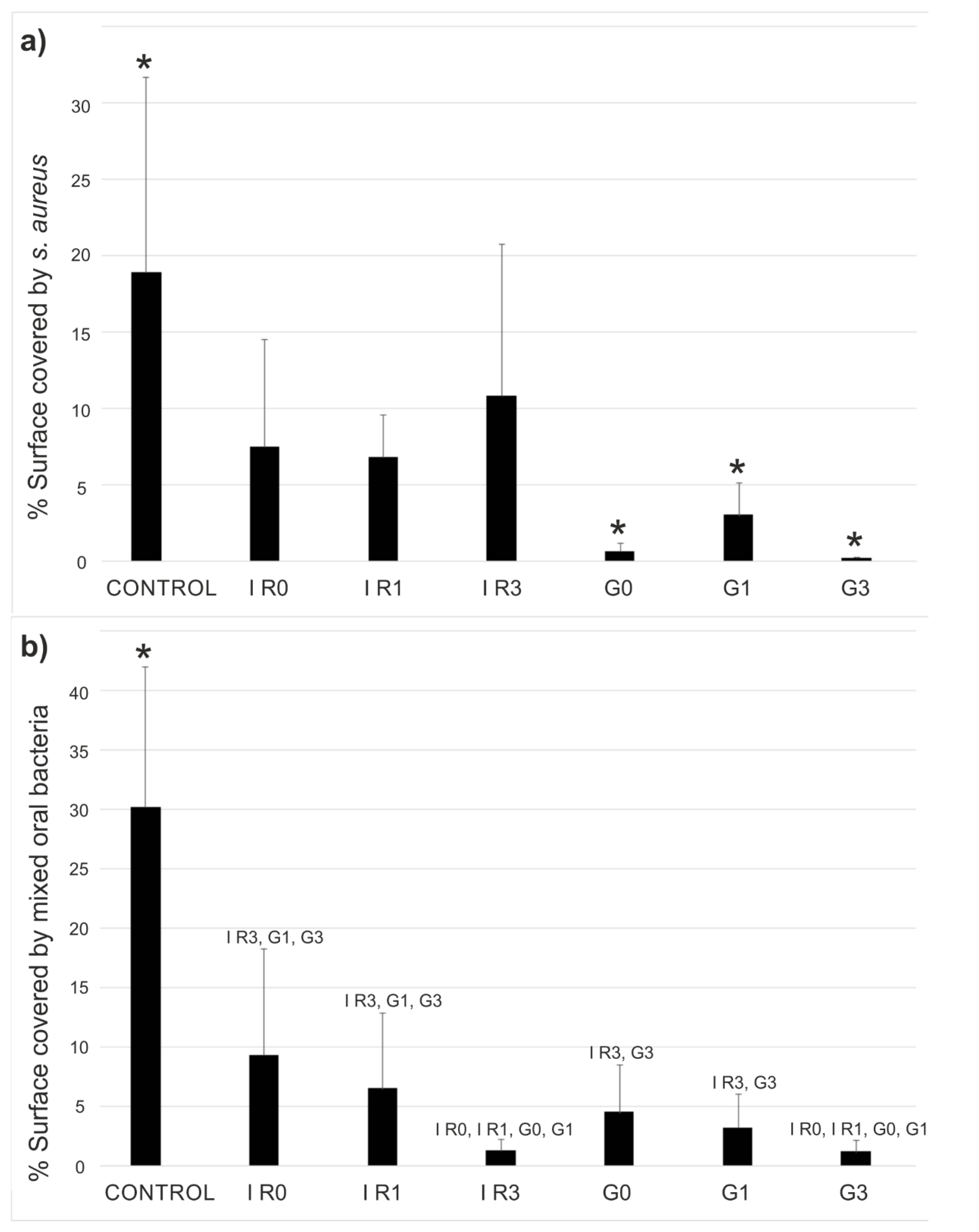
2.2. Bacterial Adhesion Tests
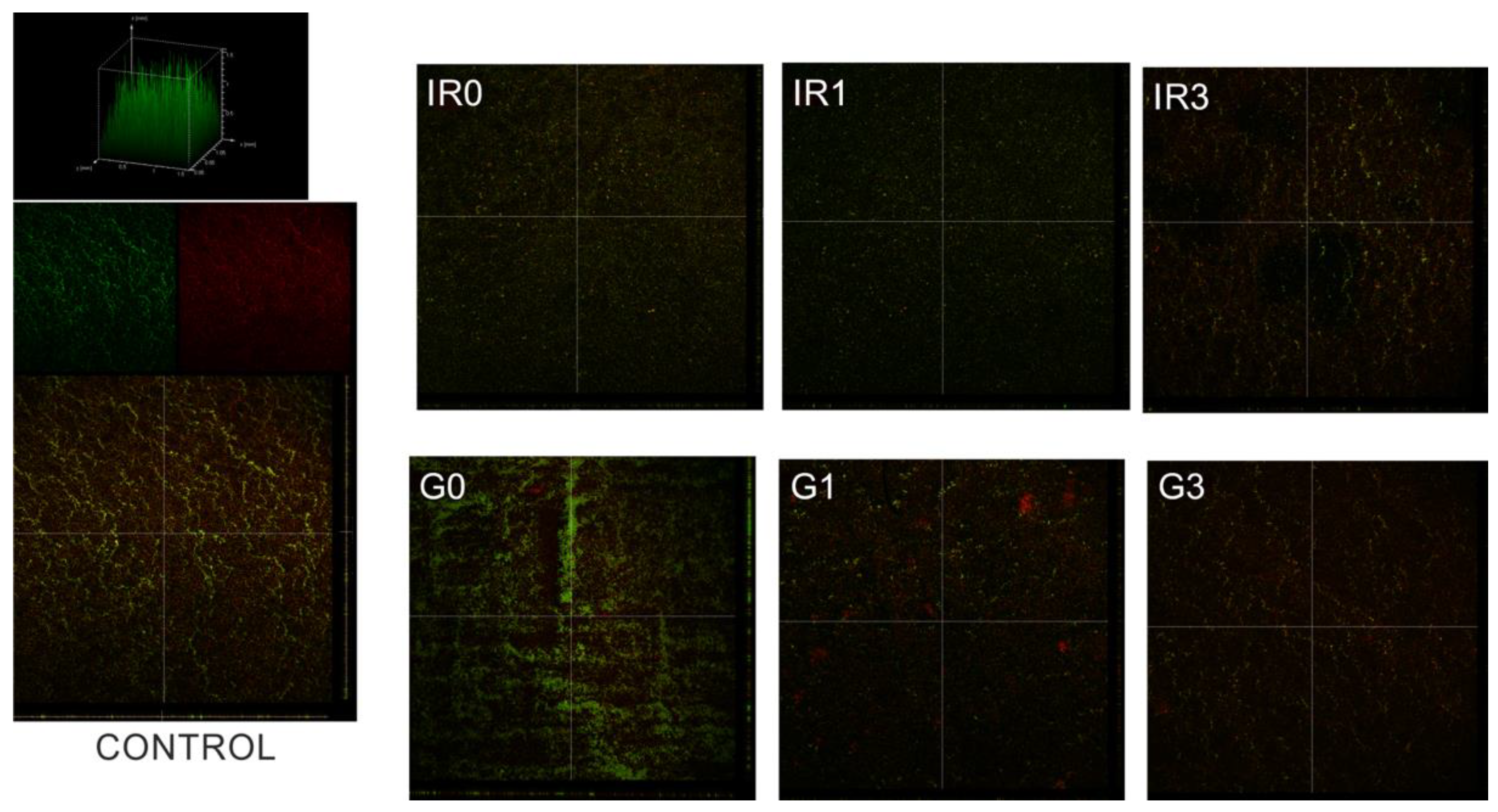
2.3. Biofilm
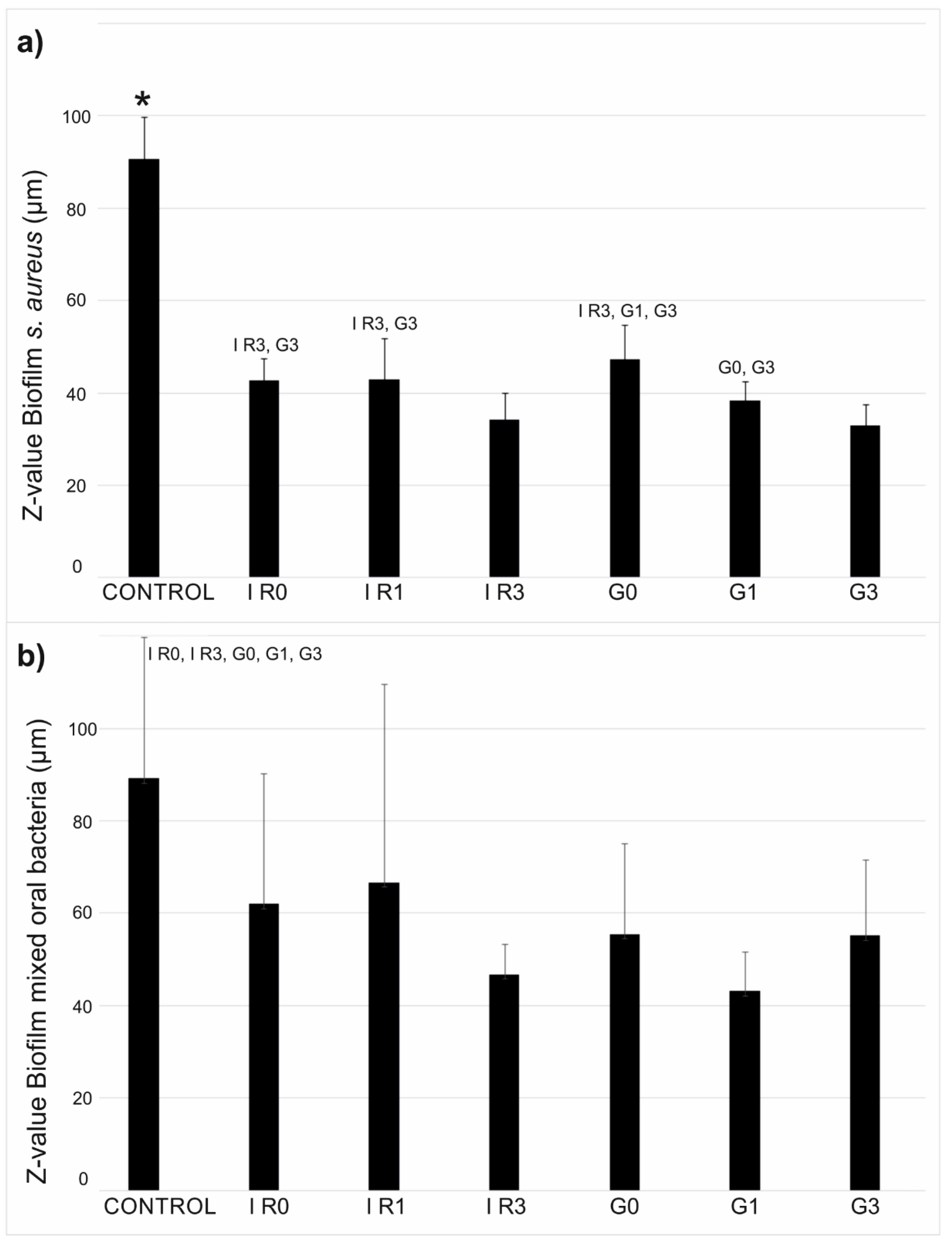
2.3.1. Percentage of Surface Covered by Biofilm
2.3.2. Thickness of Biofilm
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Production
4.2. Sample Preparation and Characterization Techniques
4.3. Bacterial Adhesion and Biofilm Formation Experiments
4.3.1. Bacterial Adhesion
4.3.2. Biofilm Formation
4.3.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinstein, R.A.; Darouiche, R.O. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Nguemeleu, E.T.; Beogo, I.; Sia, D.; Kilpatrick, K.; Séguin, C.; Baillot, A.; Jabbour, M.; Parisien, N.; Robins, S.; Boivin, S.; et al. Economic analysis of healthcare-associated infection prevention and control interven-tions in medical and surgical units: Systematic review using a discounting approach. J. Hosp. Infect. 2020, 106, 134–154. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Sereti, C.; Ioannidis, A.; Mitchell, C.A.; Ball, A.R.; Magiorkinis, E.; Chatzipanagiotou, S.; Hamblin, M.R.; Hadjifrangiskou, M.; Tegos, G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018, 31, e00084-16. [Google Scholar] [CrossRef] [Green Version]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontology 2000 2010, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Mouhyi, J.; Ehrenfest, D.M.D.; Albrektsson, T. The Peri-Implantitis: Implant Surfaces, Microstructure, and Physicochemical Aspects. Clin. Implant Dent. Relat. Res. 2009, 14, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Watanabe, T. Risk Factors for Peri-Implant Diseases; Springer: Cham, Switzerland, 2020; pp. 11–21. [Google Scholar]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Ms, D.A.S.; Guida, L.; Ms, S.S.; Valderrama, P.; Wilson, T.G.; Rodrigues, D.C. Evaluation of oral microbial corrosion on the surface degradation of dental implant materials. J. Periodontol. 2018, 90, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, M.; Lai, Q.J.; Albrecht, R.M.; Mosher, D.F.; Proctor, R.A. Adhesion of Staphylococcus aureus to Surface-Bound Platelets: Role of Fibrinogen/Fibrin and Platelet Integrins. J. Infect. Dis. 1993, 167, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and surface properties influence biofilm proliferation. Adv. Colloid Interface Sci. 2020, 288, 102336. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K.D.V. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. BioMed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B.; et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: Multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol. Assess. 2012, 16. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Tanoira, R.; Pérez-Jorge, C.; Endrino, J.L.; Gómez-Barrena, E.; Horwat, D.; Pierson, J.F.; Esteban, J. Bacterial adhesion on biomedical surfaces covered by micrometric silver Islands. J. Biomed. Mater. Res. Part A 2012, 100A, 1521–1528. [Google Scholar] [CrossRef]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles-the real “silver bullet” in clinical medicine? Med. Chem. Commun. 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Thomas, R.; Soumya, K.; Mathew, J.; Radhakrishnan, E. Inhibitory effect of silver nanoparticle fabricated urinary catheter on colonization efficiency of Coagulase Negative Staphylococci. J. Photochem. Photobiol. B Biol. 2015, 149, 68–77. [Google Scholar] [CrossRef]
- Pérez-Tanoira, R.; Horwat, D.; Kinnari, T.J.; Pérez-Jorge, C.; Gómez-Barrena, E.; Migot, S.; Esteban, J. Bacterial adhesion on biomedical surfaces covered by yttria stabilized zirconia. J. Mater. Sci. Mater. Med. 2016, 27, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutinguiza, M.; Fernández-Arias, M.; del Val, J.; Buxadera-Palomero, J.; Rodríguez, F.L.; Lusquiños, F.; Gil, F.; Pou, J. Synthesis and deposition of silver nanoparticles on cp Ti by laser ablation in open air for antibacterial effect in dental implants. Mater. Lett. 2018, 231, 126–129. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Perito, B.; Giorgetti, E.; Marsili, P.; Muniz-Miranda, M. Antibacterial activity of silver nanoparticles obtained by pulsed laser ablation in pure water and in chloride solution. Beilstein J. Nanotechnol. 2016, 7, 465–473. [Google Scholar] [CrossRef]
- D’Urso, L.; Nicolosi, V.; Compagnini, G.; Puglisi, O. Size distribution of silver nanoclusters induced by ion, electron, laser beams and thermal treatments of an organometallic precursor. Appl. Surf. Sci. 2004, 226, 131–136. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Badr, Y.; El Wahed, M.A.; Mahmoud, M. On 308nm photofragmentation of the silver nanoparticles. Appl. Surf. Sci. 2006, 253, 2502–2507. [Google Scholar] [CrossRef]
- Compagnini, G.; Messina, E.; Puglisi, O.; Nicolosi, V. Laser synthesis of Au/Ag colloidal nano-alloys: Optical properties, structure and composition. Appl. Surf. Sci. 2007, 254, 1007–1011. [Google Scholar] [CrossRef]
- Petica, A.; Gavriliu, S.; Lungu, M.V.; Buruntea, N.; Panzaru, C. Colloidal silver solutions with antimicrobial properties. Mater. Sci. Eng. B 2008, 152, 22–27. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Nadaf, N.H.; Ghosh, S.J.; Pawar, S.H. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012, 72, 92–94. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Z.; Yuwen, L.; Tian, J.; Zhu, X.; Wang, L. Synthesis of silver nanoparticles on reduced graphene oxide under microwave irradiation with starch as an ideal reductant and stabilizer. Appl. Surf. Sci. 2013, 266, 188–193. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef]
- Al-Masoodi, A.H.H.; Talik, N.A.; Goh, B.T.; Sarjidan, M.A.M.; Al-Masoodi, A.H.; Majid, W.H.A. Effect of silver nanoparticles deposited on indium tin oxide by plasma-assisted hot-filament evaporation on phosphorescent organic light-emitting diode performance. Appl. Surf. Sci. 2021, 570, 151280. [Google Scholar] [CrossRef]
- Švecová, M.; Volochanskyi, O.; Král, M.; Dendisová, M.; Matějka, P. Advantages and drawbacks of the use of immobilized “green-synthesized” silver nanoparticles on gold nanolayer for near-field vibrational spectroscopic study of riboflavin. Appl. Surf. Sci. 2021, 557, 149832. [Google Scholar] [CrossRef]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Hermanns, M.I.; Pohl, C.; Baldi, G.; E Unger, R.; Kirkpatrick, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Neddersen, J.; Chumanov, G.; Cotton, T.M. Laser Ablation of Metals: A New Method for Preparing SERS Active Colloids. Appl. Spectrosc. 1993, 47, 1959–1964. [Google Scholar] [CrossRef]
- Simakin, A.; Voronov, V.; Kirichenko, N.; Shafeev, G. Nanoparticles produced by laser ablation of solids in liquid environment. Appl. Phys. A 2004, 79, 1127–1132. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodríguez-González, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser-assisted production of spherical TiO2nanoparticles in water. Nanotechnology 2011, 22, 195606. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2012, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2012, 15, 3022–3026. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef] [Green Version]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laserfabricated surfactant-free gold nanoparti-cles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067. [Google Scholar]
- Barcikowski, S.; Menéndez-Manjón, A.; Chichkov, B.; Brikas, M.; Raciukaitis, G. Generation of nanoparticle colloids by picosecond and femtosecond laser ablations in liquid flow. Appl. Phys. Lett. 2007, 91, 083113. [Google Scholar] [CrossRef]
- Xu, B.; Song, R.G. Fabrication of Ag Nanoparticles Colloids by Pulsed Laser Ablation in Liquid. Adv. Mater. Res. 2010, 123–125, 675–678. [Google Scholar] [CrossRef]
- Mendivil, M.I.; Krishnan, B.; Sanchez, F.A.; Martinez, S.; Aguilar-Martinez, J.A.; Castillo, G.A.; Garcia-Gutierrez, D.I.; Shaji, S. Synthesis of silver nanoparticles and antimony oxide nanocrystals by pulsed laser ablation in liquid media. Appl. Phys. A 2012, 110, 809–816. [Google Scholar] [CrossRef]
- Rao, S.V.; Podagatlapalli, G.K.; Hamad, S. Ultrafast Laser Ablation in Liquids for Nanomaterials and Applications. J. Nanosci. Nanotechnol. 2014, 14, 1364–1388. [Google Scholar] [CrossRef]
- Valverde-Alva, M.; García-Fernández, T.; Villagrán-Muniz, M.; Sánchez-Aké, C.; Castañeda-Guzmán, R.; Esparza-Alegría, E.; Valdés, C.F.S.; Llamazares, J.S.; Herrera, C.M. Synthesis of silver nanoparticles by laser ablation in ethanol: A pulsed photoacoustic study. Appl. Surf. Sci. 2015, 355, 341–349. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Mangini, V.; Valenza, G.; De Pascale, O.; De Stradis, A.; Natile, G.; Arnesano, F.; De Giacomo, A. Silver and gold nanoparticles produced by pulsed laser ablation in liquid to investigate their interaction with Ubiquitin. Appl. Surf. Sci. 2016, 374, 297–304. [Google Scholar] [CrossRef]
- Resano-Garcia, A.; Champmartin, S.; Battie, Y.; Koch, A.; Naciri, A.E.; Ambari, A.; Chaoui, N. Highly-repeatable generation of very small nanoparticles by pulsed-laser ablation in liquids of a high-speed rotating target. Phys. Chem. Chem. Phys. 2016, 18, 32868–32875. [Google Scholar] [CrossRef] [Green Version]
- Kohsakowski, S.; Santagata, A.; Dell’Aglio, M.; De Giacomo, A.; Barcikowski, S.; Wagener, P.; Gökce, B. High productive and continuous nanoparticle fabrication by laser ablation of a wire-target in a liquid jet. Appl. Surf. Sci. 2017, 403, 487–499. [Google Scholar] [CrossRef]
- Scardaci, V.; Condorelli, M.; Barcellona, M.; Salemi, L.; Pulvirenti, M.; Fragalà, M.E.; Compagnini, G. Fast One-Step Synthesis of Anisotropic Silver Nanoparticles. Appl. Sci. 2021, 11, 8949. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Wagener, P.; Winkel, A.; Sajti, C.L.; Barcikowski, S.; Stiesch, M. Therapeutic Window of Ligand-Free Silver Nanoparticles in Agar-Embedded and Colloidal State: In Vitro Bactericidal Effects and Cytotoxicity. Adv. Eng. Mater. 2012, 14, B231–B239. [Google Scholar] [CrossRef]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver Nanoparticles Synthesized by Pulsed Laser Ablation: As a Potent Antibacterial Agent for Human Enteropathogenic Gram-Positive and Gram-Negative Bacterial Strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Liu, Z.; Wang, T. The Molecular Mechanisms of the Antibacterial Effect of Picosecond Laser Generated Silver Nanoparticles and Their Toxicity to Human Cells. PLoS ONE 2016, 11, e0160078. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; del Val, J.; Medina, E.; Rodríguez, D.; Riveiro, A.; Comesaña, R.; Lusquiños, F.; Gil, F.; Pou, J. RE-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl. Surf. Sci. 2018, 473, 548–554. [Google Scholar] [CrossRef]
- Menazea, A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 2019, 168, 108616. [Google Scholar] [CrossRef]
- Nikolov, A.; Stankova, N.; Karashanova, D.; Nedyalkov, N.; Pavlov, E.; Koev, K.T.; Najdenski, H.; Kussovski, V.; Avramov, L.; Ristoscu, C.; et al. Synergistic effect in a two-phase laser procedure for production of silver nanoparticles colloids applicable in ophthalmology. Opt. Laser Technol. 2021, 138, 106850. [Google Scholar] [CrossRef]
- Ruiz, S.; Wang, F.; Liu, L.; Lu, Y.; Duan, B.; Korshoj, L.E.; Kielian, T.; Cui, B. Antibacterial properties of silver nanoparticles synthesized via nanosecond pulsed laser ablation in water. J. Laser Appl. 2022, 34, 012031. [Google Scholar] [CrossRef]
- Ratti, M.; Naddeo, J.J.; Tan, Y.; Griepenburg, J.C.; Tomko, J.; Trout, C.; O’Malley, S.M.; Bubb, D.M.; Klein, E.A. Irradiation with visible light enhances the antibacterial toxicity of silver nanoparticles produced by laser ablation. Appl. Phys. A 2016, 122, 346. [Google Scholar] [CrossRef]
- Baiee, R.; Liu, Z.; Li, L. Understanding the stability and durability of laser-generated Ag nanoparticles and effects on their antibacterial activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 035001. [Google Scholar] [CrossRef]
- Krce, L.; Šprung, M.; Maravić, A.; Umek, P.; Salamon, K.; Krstulović, N.; Aviani, I. Bacteria Exposed to Silver Nanoparticles Synthesized by Laser Ablation in Water: Modelling E. coli Growth and Inactivation. Materials 2020, 13, 653. [Google Scholar] [CrossRef] [Green Version]
- Hajiesmaeilbaigi, F.; Mohammadalipour, A.; Sabbaghzadeh, J.; Hoseinkhani, S.; Fallah, H.R. Preparation of silver nanoparticles by laser ablation and fragmentation in pure water. Laser Phys. Lett. 2006, 3, 252–256. [Google Scholar] [CrossRef]
- Wagener, P.; Barcikowski, S. Laser fragmentation of organic microparticles into colloidal nanoparticles in a free liquid jet. Appl. Phys. A 2010, 101, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wei, J.; Xie, S.; Tao, X.; Zhang, Z.; Ran, P.; Li, X. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 2018, 11, 1410–1422. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-W.; Yoon, S.; Choi, H.W.; Kim, J.; Farson, D.; Cho, S.-H. The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers. Appl. Sci. 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Pyatenko, A.; Wang, H.; Koshizaki, N.; Tsuji, T. Mechanism of pulse laser interaction with colloidal nanoparticles. Laser Photon Rev. 2013, 7, 596–604. [Google Scholar] [CrossRef]
- Dadras, S.; Torkamany, M.J.; Jafarkhani, P. Analysis and optimization of silver nanoparticles laser synthesis with emission spectroscopy of induced plasma. J. Nanosci. Nanotechnol. 2012, 12, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L.; Prevost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD BacLight: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef]
- Müsken, M.; Di Fiore, S.; Römling, U.; Häussler, S. A 96-well-plate–based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat. Protoc. 2010, 5, 1460–1469. [Google Scholar] [CrossRef]
- Eberhard, J.; Grote, K.; Luchtefeld, M.; Heuer, W.; Schuett, H.; Divchev, D.; Scherer, R.; Schmitz-Streit, R.A.; Langfeldt, D.; Stumpp, N.; et al. Experimental Gingivitis Induces Systemic Inflammatory Markers in Young Healthy Individuals: A Single-Subject Interventional Study. PLoS ONE 2013, 8, e55265. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.; Shah, S.I. Synthesis and Antibacterial Properties of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Hamouda, T.; Baker, J. Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. J. Appl. Microbiol. 2000, 89, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Gordon, O.; Slenters, T.V.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxyl Radical Induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Iram, S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Ahmad, K.; Khan, M.S.; Kim, J. Fruit Derived Potentially Bioactive Bioengineered Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 7711–7726. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Kommerein, N.; Stumpp, S.N.; Müsken, M.; Ehlert, N.; Winkel, A.; Häussler, S.; Behrens, P.; Buettner, F.F.R.; Stiesch, M. An oral multispecies biofilm model for high content screening applications. PLoS ONE 2017, 12, e0173973. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Iwaku, M.; Hoshino, E. Artificial Pseudomonas aeruginosa biofilms and confocal laser scanning microscopic analysis. J. Infect. Chemother. 2001, 7, 87–93. [Google Scholar] [CrossRef]
- Berney, M.; Weilenmann, H.-U.; Egli, T. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 2006, 152, 1719–1729. [Google Scholar] [CrossRef] [Green Version]
- Nebe-Von-Caron, G.; Stephens, P.; Hewitt, C.; Powell, J.; Badley, R. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 2000, 42, 97–114. [Google Scholar] [CrossRef]
- Joux, F.; Lebaron, P. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2000, 2, 1523–1535. [Google Scholar] [CrossRef]
- Sachidanandham, R.; Gin, K.Y.-H.; Poh, C.L. Monitoring of active but non-culturable bacterial cells by flow cytometry. Biotechnol. Bioeng. 2004, 89, 24–31. [Google Scholar] [CrossRef]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.-U.; Egli, T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [Green Version]
- Katsikogianni, M.; Missirlis, Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Perez-Jorge, C.; Lozano, D.; Portal-Núñez, S.; Perez-Tanoira, R.; Conde, A.; Arenas, M.A.; Hernandez-Lopez, J.M.; de Damborenea, J.; Gomez-Barrena, E.; et al. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model. Bone Jt. Res. 2017, 6, 315–322. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic Applications of Antimicrobial Silver-Based Biomaterials in Dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef]

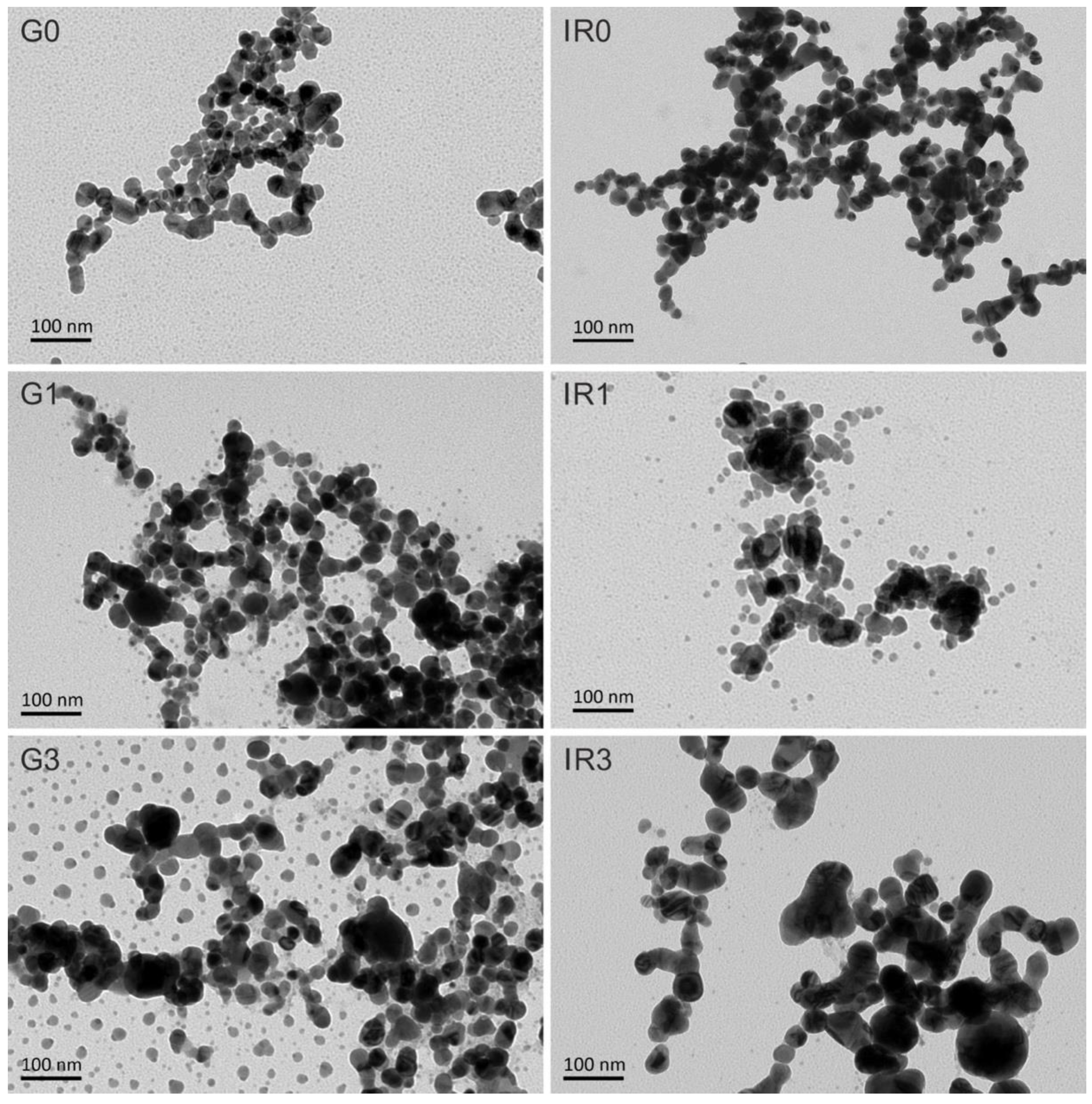
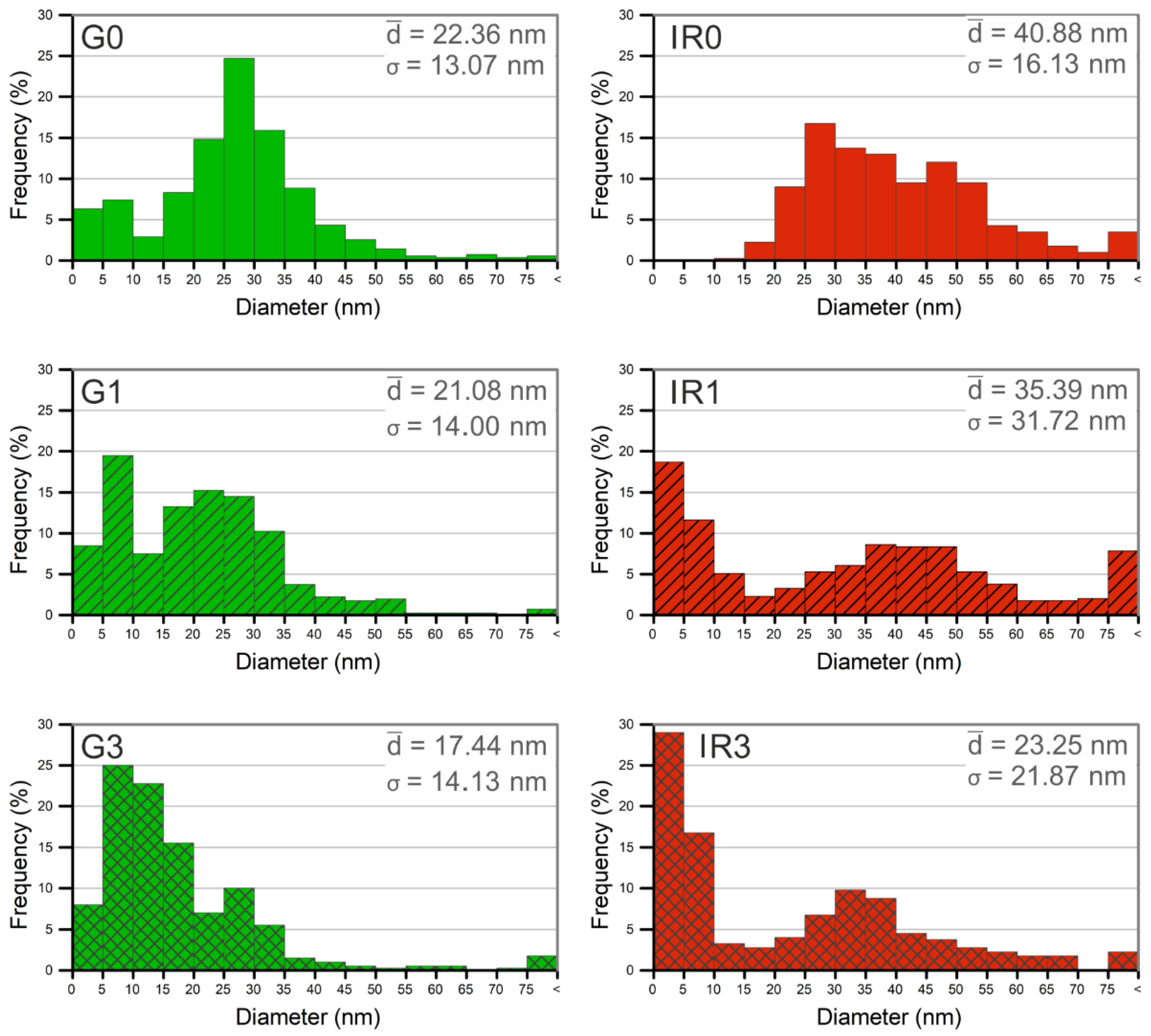
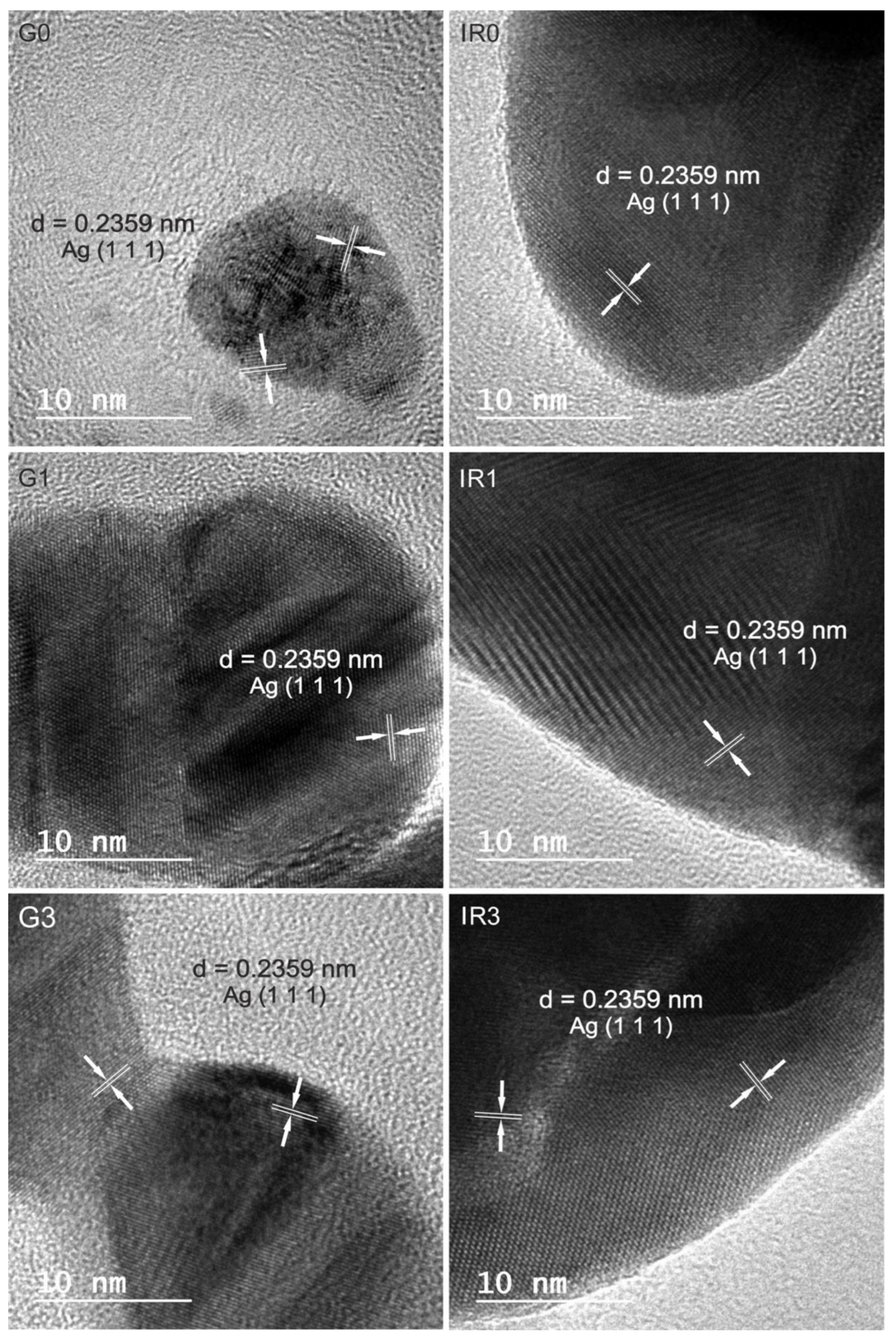
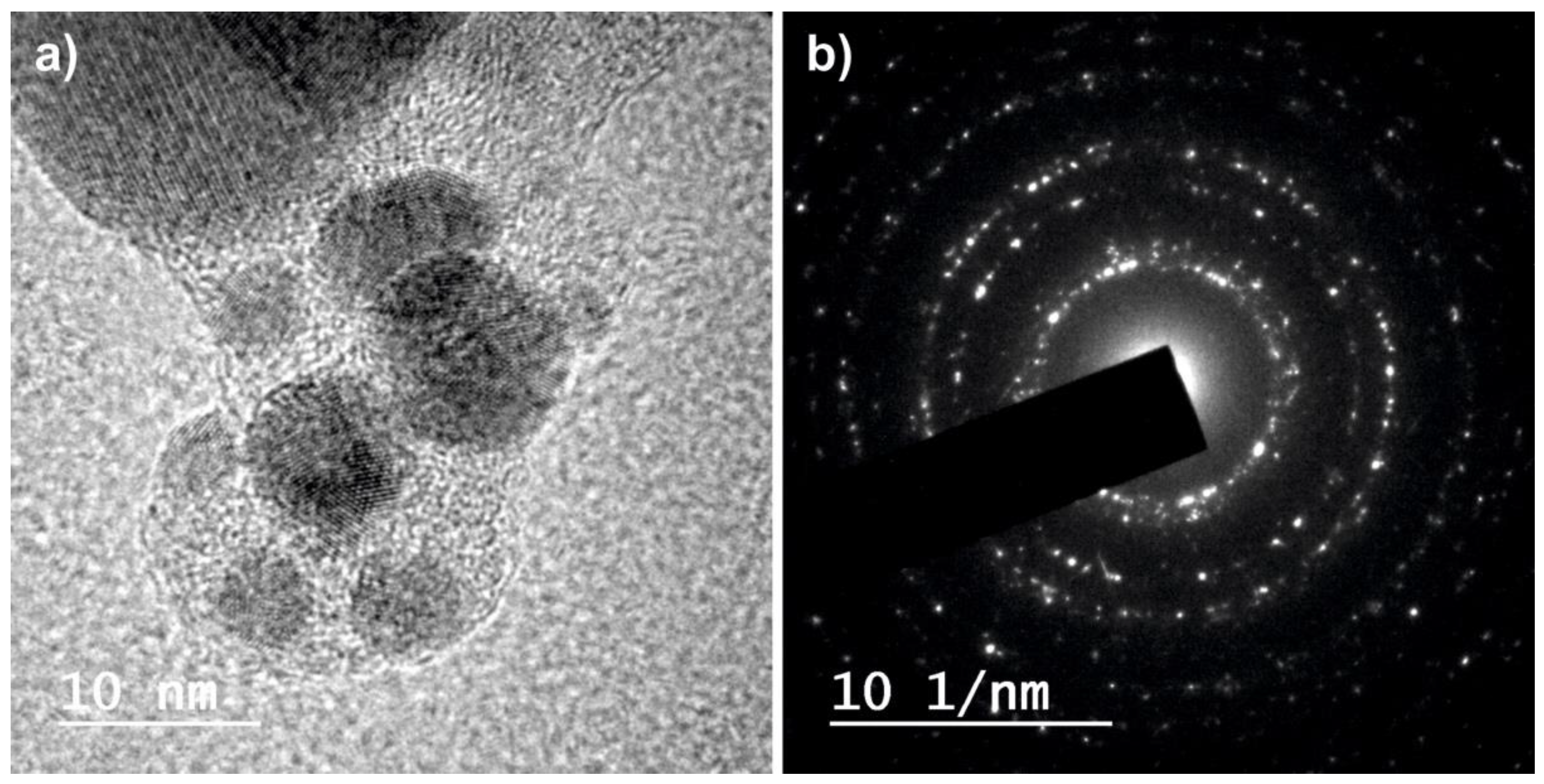




| Sample | G0 | G1 | G3 | IR0 | IR1 | IR3 |
|---|---|---|---|---|---|---|
| Z-Potential (mV) day 0 | −24.5 | −8.75 | −2.33 | −20.7 | −18.9 | −11.8 |
| Measured d-Spacing (nm) | ||||||
|---|---|---|---|---|---|---|
| G0 | G1 | G3 | IR0 | IR1 | IR3 | Ag [hkl] |
| 0.236 | 0.234 | 0.235 | 0.239 | 0.239 | 0.235 | 0.2359 (1 1 1) |
| 0.209 | 0.208 | 0.214 | 0.192 | 0.222 | 0.204 | 0.2044 (2 0 0) |
| - | - | - | 0.155 | - | 0.144 | 0.1445 (2 2 0) |
| - | - | - | - | - | 0.123 | 0.123 (3 1 1) |
| - | 0.116 | - | - | - | 0.118 | 0.11796 (2 2 2) |
| Sample | Laser Source | Treatment |
|---|---|---|
| G0 | Green—nanosecond | As ablated |
| G1 | Green—nanosecond | 1 time re-irradiation |
| G3 | Green—nanosecond | 1 times re-irradiation |
| IR0 | Infrared—picosecond | As ablated |
| IR1 | Infrared—picosecond | 1 time re-irradiation |
| IR3 | Infrared—picosecond | 1 times re-irradiation |
| Laser Source | Wavelength (nm) | Pulse Duration (ns) | Pulse Frequency (kHz) | Pulse Energy (mJ) | Scanning Speed (mm/s) |
|---|---|---|---|---|---|
| Green—nanosecond | 532 | 14 | 20 | 0.26 | 50 |
| Infrared—picosecond | 1064 | 0.8 | 200 | 0.03 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Tanoira, R.; Fernández-Arias, M.; Potel, C.; Carballo-Fernández, R.; Pérez-Castro, S.; Boutinguiza, M.; Górgolas, M.; Lusquiños, F.; Pou, J. Silver Nanoparticles Produced by Laser Ablation and Re-Irradiation Are Effective Preventing Peri-Implantitis Multispecies Biofilm Formation. Int. J. Mol. Sci. 2022, 23, 12027. https://doi.org/10.3390/ijms231912027
Pérez-Tanoira R, Fernández-Arias M, Potel C, Carballo-Fernández R, Pérez-Castro S, Boutinguiza M, Górgolas M, Lusquiños F, Pou J. Silver Nanoparticles Produced by Laser Ablation and Re-Irradiation Are Effective Preventing Peri-Implantitis Multispecies Biofilm Formation. International Journal of Molecular Sciences. 2022; 23(19):12027. https://doi.org/10.3390/ijms231912027
Chicago/Turabian StylePérez-Tanoira, Ramón, Mónica Fernández-Arias, Carmen Potel, Raquel Carballo-Fernández, Sonia Pérez-Castro, Mohamed Boutinguiza, Miguel Górgolas, Fernando Lusquiños, and Juan Pou. 2022. "Silver Nanoparticles Produced by Laser Ablation and Re-Irradiation Are Effective Preventing Peri-Implantitis Multispecies Biofilm Formation" International Journal of Molecular Sciences 23, no. 19: 12027. https://doi.org/10.3390/ijms231912027
APA StylePérez-Tanoira, R., Fernández-Arias, M., Potel, C., Carballo-Fernández, R., Pérez-Castro, S., Boutinguiza, M., Górgolas, M., Lusquiños, F., & Pou, J. (2022). Silver Nanoparticles Produced by Laser Ablation and Re-Irradiation Are Effective Preventing Peri-Implantitis Multispecies Biofilm Formation. International Journal of Molecular Sciences, 23(19), 12027. https://doi.org/10.3390/ijms231912027








