Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments
Abstract
1. Introduction
2. Results
2.1. Temporal Signature of SH-SY5Y Cells Behavior in the Presence or Absence of As2O3 Using Impedancemetry
2.2. Effect of RF Exposure on SH-SY5Y Cell Behavior Assessed by Impedancemetry in the Presence or Absence of As2O3 Co-Exposure
2.3. Effect of RF Exposure on Cortical Astrocytes and Neuron-Glia Co-Culture Cell Behavior Assessed by Impedancemetry in the Presence or Absence of As2O3 Co-Exposure
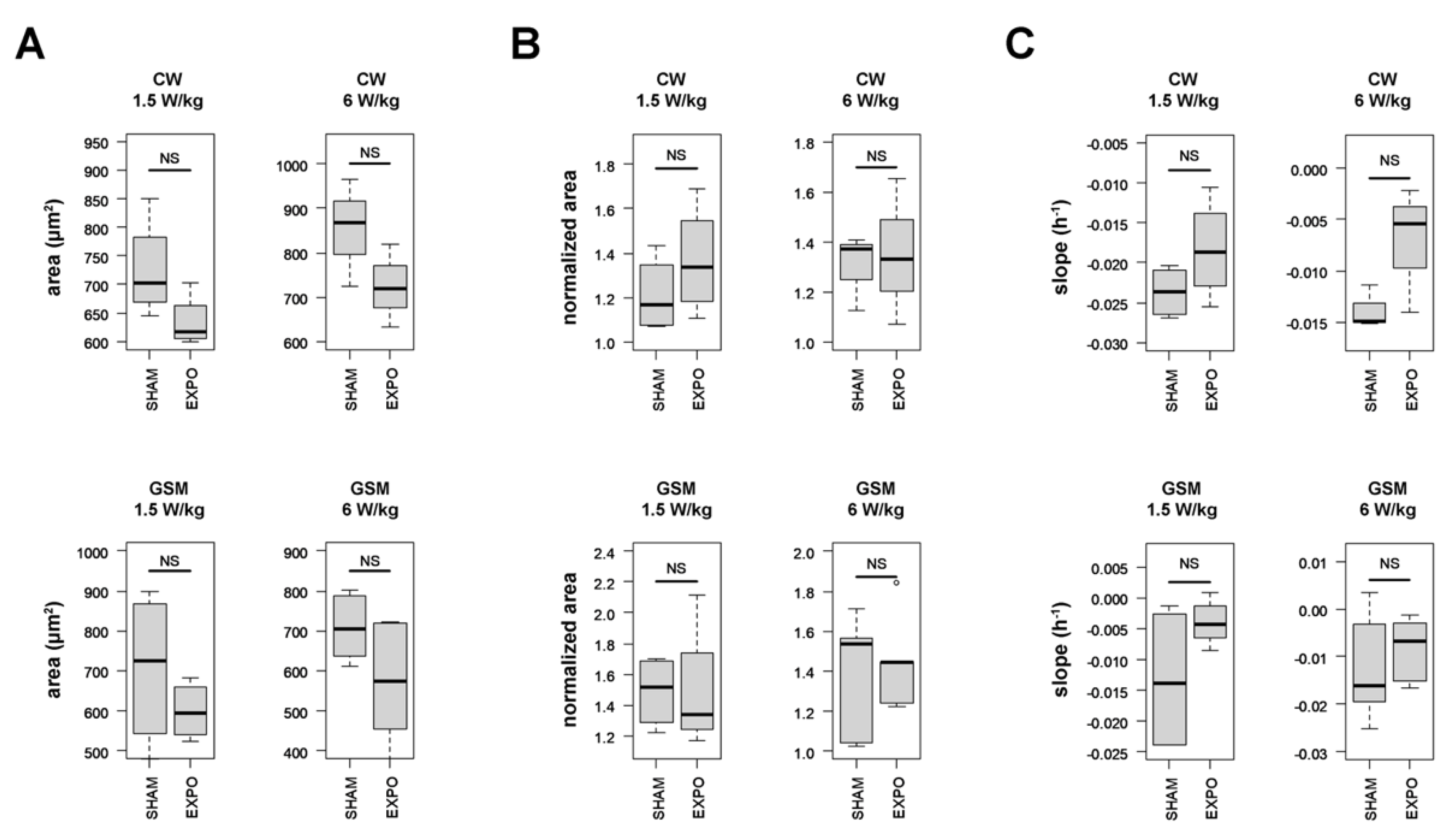
2.4. Characterization of Serum Starvation-Induced Autophagy in HCT116 Colon Cancer Cells Using Digital Holographic Microscopy
2.5. Effect of RF Exposure on the Autophagic Response of HCT116 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cellular Impedance Assay
4.4. Impedancemetry Data Analysis
4.5. Exposure to RF and Holographic Image Acquisition
4.6. DHM Data Analysis
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SSM’s Scientific Council on Electromagnetic Fields. Recent Research on EMF and Health Risk-Fourteenth Report from SSM’s Scientific Council on Electromagnetic Fields, 2019; Swedish Radiation Safety Authority: Stockholm, Sweden, 2020. [Google Scholar]
- ICNIRP. Guidelines for limiting exposure to electromagnetic fields (100 KHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- SCENIHR. Opinion on potential health effects of exposure to electromagnetic fields. Bioelectromagnetics 2015, 36, 480–484. [CrossRef]
- Kostoff, R.N. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol. Rep. 2018, 5, 1169–1172. [Google Scholar] [CrossRef]
- Schuermann, D.; Mevissen, M. Manmade Electromagnetic fields and oxidative stress—Biological effects and consequences for health. IJMS 2021, 22, 3772. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.D.; Moros, E.G.; Brownstein, B.H.; Roti Roti, J.L. The Number of genes changing expression after chronic exposure to code division multiple access or frequency DMA radiofrequency radiation does not exceed the false-positive rate. Proteomics 2006, 6, 4739–4744. [Google Scholar] [CrossRef]
- Chauhan, V.; Qutob, S.S.; Lui, S.; Mariampillai, A.; Bellier, P.V.; Yauk, C.L.; Douglas, G.R.; Williams, A.; McNamee, J.P. Analysis of gene expression in two human-derived cell lines exposedin vitro to a 1.9 GHz Pulse-modulated radiofrequency field. Proteomics 2007, 7, 3896–3905. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, G.; Weng, Y.; Wang, L.; Chiang, H.; Lu, D.; Xu, Z. Effects of global system for mobile communications 1800 MHz radiofrequency electromagnetic fields on gene and protein expression in MCF-7 Cells. Proteomics 2006, 6, 4732–4738. [Google Scholar] [CrossRef]
- Poque, E.; Arnaud-Cormos, D.; Patrignoni, L.; Ruigrok, H.J.; Poulletier De Gannes, F.; Hurtier, A.; Renom, R.; Garenne, A.; Lagroye, I.; Leveque, P.; et al. Effects of radiofrequency fields on RAS and ERK kinases activity in live cells using the bioluminescence resonance energy transfer technique. Int. J. Radiat. Biol. 2020, 96, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Poque, E.; Ruigrok, H.J.; Arnaud-Cormos, D.; Habauzit, D.; Chappe, Y.; Martin, C.; De Gannes, F.P.; Hurtier, A.; Garenne, A.; Lagroye, I.; et al. Effects of radiofrequency field exposure on proteotoxic-induced and heat-induced HSF1 response in live cells using the bioluminescence resonance energy transfer technique. Cell Stress Chaperones 2021, 26, 241–251. [Google Scholar] [CrossRef]
- Ruigrok, H.J.; Arnaud-Cormos, D.; Hurtier, A.; Poque, E.; de Gannes, F.P.; Ruffié, G.; Bonnaudin, F.; Lagroye, I.; Sojic, N.; Arbault, S.; et al. Activation of the TRPV1 thermoreceptor induced by modulated or unmodulated 1800 MHz radiofrequency field exposure. Radiat. Res. 2018, 189, 95–103. [Google Scholar] [CrossRef]
- Croft, L.V.; Mulders, J.A.; Richard, D.J.; O’Byrne, K. Digital holographic imaging as a method for quantitative, live cell imaging of drug response to novel targeted cancer therapies. In Theranostics; Batra, J., Srinivasan, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 2054, pp. 171–183. ISBN 978-1-4939-9768-8. [Google Scholar]
- Kliment, K.; Szekacs, I.; Peter, B.; Erdei, A.; Kurucz, I.; Horvath, R. Label-free real-time monitoring of the BCR-Triggered activation of primary human b cells modulated by the simultaneous engagement of inhibitory receptors. Biosens. Bioelectron. 2021, 191, 113469. [Google Scholar] [CrossRef]
- Wegener, J. Label-Free Monitoring of Cells In Vitro; Bioanalytical Reviews; Wegener, J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, ISBN 978-3-030-32432-2. [Google Scholar]
- Garcia-Fernandez, M.A.; Percherancier, Y.; Lagroye, I.; OConnor, R.P.; Veyret, B.; Arnaud-Cormos, D.; Leveque, P. Dosimetric Characteristics of an EMF delivery system based on a real-time impedance measurement device. IEEE Trans. Biomed. Eng. 2016, 63, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Tsai, C.-W.; Chang, W.-S.; Lin, J.-C.; Hsia, T.-C.; Bau, D.-T. Protective effects of baicalin on arsenic trioxide-induced oxidative damage and apoptosis in human umbilical vein endothelial cells. In Vivo 2021, 35, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bencko, V.; Yan Li Foong, F. The history of arsenical pesticides and health risks related to the use of agent blue. Ann. Agric. Environ. Med. 2017, 24, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Akiyama, K. Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase 3 in vitro. FEBS Lett. 1999, 455, 59–62. [Google Scholar] [CrossRef]
- Karlsson, J.; Øra, I.; Pörn-Ares, I.; Påhlman, S. Arsenic trioxide-induced death of neuroblastoma cells involves activation of bax and does not require P53. Clin. Cancer Res. 2004, 10, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Namgung, U.; Xia, Z. Arsenite-induced apoptosis in cortical neurons is mediated by c-jun n-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J. Neurosci. 2000, 20, 6442–6451. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Wang, L.; Dai, Z.; Yang, K. Arsenic trioxide induces apoptosis and the formation of reactive oxygen species in rat glioma cells. Cell Mol. Biol. Lett. 2018, 23, 13. [Google Scholar] [CrossRef]
- Soueid, M.; Dobbelaar, M.C.F.; Bentouati, S.; Bardet, S.M.; O’Connor, R.P.; Bessières, D.; Paillol, J.; Leveque, P.; Arnaud-Cormos, D. Delivery devices for exposure of biological cells to nanosecond pulsed electric fields. Med. Biol. Eng. Comput. 2018, 56, 85–97. [Google Scholar] [CrossRef]
- Kohler, S.; Ticaud, N.; Iordache, M.-M.; Moisescu, M.G.; Savopol, T.; Leveque, P.; Arnaud-Cormos, D. Setup for simultaneous microwave heating and real-time spectrofluorometric measurements in biological systems. Prog. Electromagn. Res. 2014, 145, 229–240. [Google Scholar] [CrossRef][Green Version]
- Nefzi, A.; Carr, L.; Dalmay, C.; Pothier, A.; Leveque, P.; Arnaud-Cormos, D. Microdosimetry Using rhodamine B within macro- and microsystems for radiofrequency signals exposures of biological samples. IEEE Trans. Microw. Theory Tech. 2020, 68, 1142–1150. [Google Scholar] [CrossRef]
- Priault, M.; Hue, E.; Marhuenda, F.; Pilet, P.; Oliver, L.; Vallette, F.M. Differential dependence on beclin 1 for the regulation of pro-survival autophagy by Bcl-2 and Bcl-XL in HCT116 colorectal cancer cells. PLoS ONE 2010, 5, e8755. [Google Scholar] [CrossRef]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.; O’Carroll, S.; Mez, E.; Angel, C.; Graham, E. Application of XCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef]
- Diemert, S.; Dolga, A.M.; Tobaben, S.; Grohm, J.; Pfeifer, S.; Oexler, E.; Culmsee, C. Impedance measurement for real time detection of neuronal cell death. J. Neurosci. Methods 2012, 203, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-Deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef]
- Joubert, V.; Leveque, P.; Rametti, A.; Collin, A.; Bourthoumieu, S.; Yardin, C. Microwave Exposure of neuronal cells in vitro: Study of apoptosis. Int. J. Radiat. Biol. 2006, 82, 267–275. [Google Scholar] [CrossRef]
- Kayhan, H.; Esmekaya, M.A.; Saglam, A.S.Y.; Tuysuz, M.Z.; Canseven, A.G.; Yagci, A.M.; Seyhan, N. Does MW Radiation affect gene expression, apoptotic level, and cell cycle progression of human SH-SY5Y Neuroblastoma cells? Cell Biochem. Biophys. 2016, 74, 99–107. [Google Scholar] [CrossRef]
- Zielinski, J.; Ducray, A.D.; Moeller, A.M.; Murbach, M.; Kuster, N.; Mevissen, M. Effects of pulse-modulated radiofrequency magnetic field (rf-emf) exposure on apoptosis, autophagy, oxidative stress and electron chain transport function in human neuroblastoma and murine microglial cells. Toxicol. Vitro 2020, 68, 104963. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Min, K.; Jeon, S.; Kim, N.; Pack, J.-K.; Song, K. Continuous exposure to 1.7 GHz LTE electromagnetic fields increases intracellular reactive oxygen species to decrease human cell proliferation and induce senescence. Sci. Rep. 2020, 10, 9238. [Google Scholar] [CrossRef]
- Su, L.; Wei, X.; Xu, Z.; Chen, G. RF-EMF Exposure at 1800 MHz did not elicit dna damage or abnormal cellular behaviors in different neurogenic cells: RF-EMF and DNA damage in neurogenic cells. Bioelectromagnetics 2017, 38, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hoyto, A.; Luukkonen, J.; Juutilainen, J.; Naarala, J. Proliferation, oxidative stress and cell death in cells exposed to 872 MHz radiofrequency radiation and oxidants. Radiat. Res. 2008, 170, 235–243. [Google Scholar] [CrossRef]
- Buttiglione, M.; Roca, L.; Montemurno, E.; Vitiello, F.; Capozzi, V.; Cibelli, G. Radiofrequency Radiation (900 MHz) Induces Egr-1 gene expression and affects cell-cycle control in human neuroblastoma cells. J. Cell Physiol. 2007, 213, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic Cermak, A.M.; Pavicic, I.; Trosic, I. Oxidative stress response in SH-SY5Y cells exposed to short-term 1800 MHz radiofrequency radiation. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2018, 53, 132–138. [Google Scholar] [CrossRef] [PubMed]
- von Niederhäusern, N.; Ducray, A.; Zielinski, J.; Murbach, M.; Mevissen, M. Effects of radiofrequency electromagnetic field exposure on neuronal differentiation and mitochondrial function in SH-SY5Y cells. Toxicol. Vitr. 2019, 61, 104609. [Google Scholar] [CrossRef] [PubMed]
- Calabro, E.; Condello, S.; Curro, M.; Ferlazzo, N.; Caccamo, D.; Magazu, S.; Ientile, R. Modulation of heat shock protein response in SH-SY5Y by mobile phone microwaves. World J. Biol. Chem. 2012, 3, 34–40. [Google Scholar] [CrossRef]
- Falone, S.; Sannino, A.; Romeo, S.; Zeni, O.; Santini, S.; Rispoli, R.; Amicarelli, F.; Scarfì, M.R. Protective effect of 1950 MHz electromagnetic field in human neuroblastoma cells challenged with menadione. Sci. Rep. 2018, 8, 13234. [Google Scholar] [CrossRef]
- Zeni, O.; Romeo, S.; Sannino, A.; Palumbo, R.; Scarfì, M.R. Evidence of bystander effect induced by radiofrequency radiation in a human neuroblastoma cell line. Environ. Res. 2021, 196, 110935. [Google Scholar] [CrossRef]
- Gibot, L.; Kolosnjaj-Tabi, J.; Bellard, E.; Chretiennot, T.; Saurin, Q.; Catrain, A.; Golzio, M.; Vézinet, R.; Rols, M.-P. Evaluations of acute and sub-acute biological effects of narrowband and moderate-band high power electromagnetic waves on cellular spheroids. Sci. Rep. 2019, 9, 15324. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, E.; Kayhan, H.; Kismali, G.; Senturk, F.; Sensoz, M.; Ozturk, G.G.; Sel, T. Effects of radiofrequency radiation on colorectal cancer cell proliferation and inflammation. Turkish J. Biochem. 2021, 46, 0148. [Google Scholar] [CrossRef]
- Terro, F.; Magnaudeix, A.; Crochetet, M.; Martin, L.; Bourthoumieu, S.; Wilson, C.-M.; Yardin, C.; Leveque, P. GSM-900MHz at low dose temperature-dependently downregulates α-synuclein in cultured cerebral cells independently of chaperone-mediated-autophagy. Toxicology 2012, 292, 136–144. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F.; Yang, X.; Shen, H.; Liu, W.; Chen, H.; Jiang, X. The effect of microwave emission from mobile phones on neuron survival in rat central nervous system. Prog. Electromagn. Res. 2008, 82, 287–298. [Google Scholar] [CrossRef][Green Version]
- Joubert, V.; Bourthoumieu, S.; Leveque, P.; Yardin, C. Apoptosis Is induced by radiofrequency fields through the caspase-independent mitochondrial pathway in cortical neurons. Radiat. Res. 2008, 169, 38–45. [Google Scholar] [CrossRef]
- Joubert, V.; Leveque, P.; Cueille, M.; Bourthoumieu, S.; Yardin, C. No apoptosis is induced in rat cortical neurons exposed to gsm phone fields. Bioelectromagnetics 2007, 28, 115–121. [Google Scholar] [CrossRef]
- Su, L.; Yimaer, A.; Xu, Z.; Chen, G. Effects of 1800 MHz RF-EMF Exposure on DNA damage and cellular functions in primary cultured neurogenic cells. Int. J. Radiat. Biol. 2018, 94, 295–305. [Google Scholar] [CrossRef]
- Zuo, W.-Q.; Hu, Y.-J.; Yang, Y.; Zhao, X.-Y.; Zhang, Y.-Y.; Kong, W.; Kong, W.-J. Sensitivity of spiral ganglion neurons to damage caused by mobile phone electromagnetic radiation will increase in lipopolysaccharide-induced inflammation in vitro model. J. Neuroinflammation 2015, 12, 105. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, J.; Li, G.; Zhang, Z.; Xue, J.; Liu, H.; Zhu, H.; Cheng, J.; Liu, Y.; Li, A.; et al. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS ONE 2012, 7, e42332. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Liu, C.; Deng, P.; Zhu, G.; Zhang, L.; He, M.; Lu, Y.; Duan, W.; Pei, L.; et al. Exposure to 1800 MHz radiofrequency radiation impairs neurite outgrowth of embryonic neural stem cells. Sci. Rep. 2014, 4, 5103. [Google Scholar] [CrossRef] [PubMed]
- Eghlidospour, M.; Ghanbari, A.; Mortazavi, S.M.J.; Azari, H. Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem Cells. Anat. Cell Biol. 2017, 50, 115. [Google Scholar] [CrossRef]
- Simkó, M.; Remondini, D.; Zeni, O.; Scarfi, M. Quality matters: Systematic analysis of endpoints related to “cellular life” in vitro data of radiofrequency electromagnetic field exposure. IJERPH 2016, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Alm, K.; El-Schich, Z.; Falck, M.; Gjrloff Wingren, A.; Janicke, B.; Oredsso, S. Cells and Holograms—Holograms and Digital Holographic Microscopy as a Tool to Study the Morphology of Living Cells. In Holography—Basic Principles and Contemporary Applications; Mihaylova, E., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1117-7. [Google Scholar]
- Romeo, S.; Zeni, O.; Sannino, A.; Lagorio, S.; Biffoni, M.; Scarfì, M.R. Genotoxicity of radiofrequency electromagnetic fields: Protocol for a systematic review of in vitro studies. Environ. Internat. 2021, 148, 106386. [Google Scholar] [CrossRef] [PubMed]
- O’Prey, J.; Sakamaki, J.; Baudot, A.D.; New, M.; Van Acker, T.; Tooze, S.A.; Long, J.S.; Ryan, K.M. Application of CRISPR/Cas9 to Autophagy Research. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 588, pp. 79–108. ISBN 978-0-12-809674-1. [Google Scholar]
- El Khoueiry, C.; Moretti, D.; Renom, R.; Camera, F.; Orlacchio, R.; Garenne, A.; Poulletier de Gannes, F.; Poque-Haro, E.; Lagroye, I.; Veyret, B.; et al. Decreased spontaneous electrical activity in neuronal networks exposed to radiofrequency 1800 MHz signals. J. Neurophysiol. 2018, 120, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, C.; Sun, J.; Yang, M.; Zuo, R.; Liu, C.; Lan, W.; Liu, M.; Huang, B.; Zhou, Y. Autophagy mediates serum starvation-induced quiescence in nucleus pulposus stem cells by the regulation of P27. Stem Cell Res. Ther. 2019, 10, 118. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Epstein, R.; Boulier, T.; Robertson, R.E. FreqProf: Frequency Profiles Computing and Plotting. 2016. Available online: https://CRAN.R-project.org/package=FreqProf (accessed on 1 October 2021).
- Gagolewski, M. stringi: Fast and portable character string processing in R. Journal of Statistical Software, 2021. To appear. Available online: https://stringi.gagolewski.com/ (accessed on 1 October 2021).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. 2021. Available online: https://cran.r-project.org/package=PMCMRplus (accessed on 1 October 2021).
- Piepho, H.-P. An algorithm for a letter-based representation of all-pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joushomme, A.; Garenne, A.; Dufossée, M.; Renom, R.; Ruigrok, H.J.; Chappe, Y.L.; Canovi, A.; Patrignoni, L.; Hurtier, A.; Poulletier de Gannes, F.; et al. Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments. Int. J. Mol. Sci. 2022, 23, 658. https://doi.org/10.3390/ijms23020658
Joushomme A, Garenne A, Dufossée M, Renom R, Ruigrok HJ, Chappe YL, Canovi A, Patrignoni L, Hurtier A, Poulletier de Gannes F, et al. Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments. International Journal of Molecular Sciences. 2022; 23(2):658. https://doi.org/10.3390/ijms23020658
Chicago/Turabian StyleJoushomme, Alexandre, André Garenne, Mélody Dufossée, Rémy Renom, Hermanus Johannes Ruigrok, Yann Loick Chappe, Anne Canovi, Lorenza Patrignoni, Annabelle Hurtier, Florence Poulletier de Gannes, and et al. 2022. "Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments" International Journal of Molecular Sciences 23, no. 2: 658. https://doi.org/10.3390/ijms23020658
APA StyleJoushomme, A., Garenne, A., Dufossée, M., Renom, R., Ruigrok, H. J., Chappe, Y. L., Canovi, A., Patrignoni, L., Hurtier, A., Poulletier de Gannes, F., Lagroye, I., Lévêque, P., Lewis, N., Priault, M., Arnaud-Cormos, D., & Percherancier, Y. (2022). Label-Free Study of the Global Cell Behavior during Exposure to Environmental Radiofrequency Fields in the Presence or Absence of Pro-Apoptotic or Pro-Autophagic Treatments. International Journal of Molecular Sciences, 23(2), 658. https://doi.org/10.3390/ijms23020658







