The Gestational Effects of Maternal Appetite Axis Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
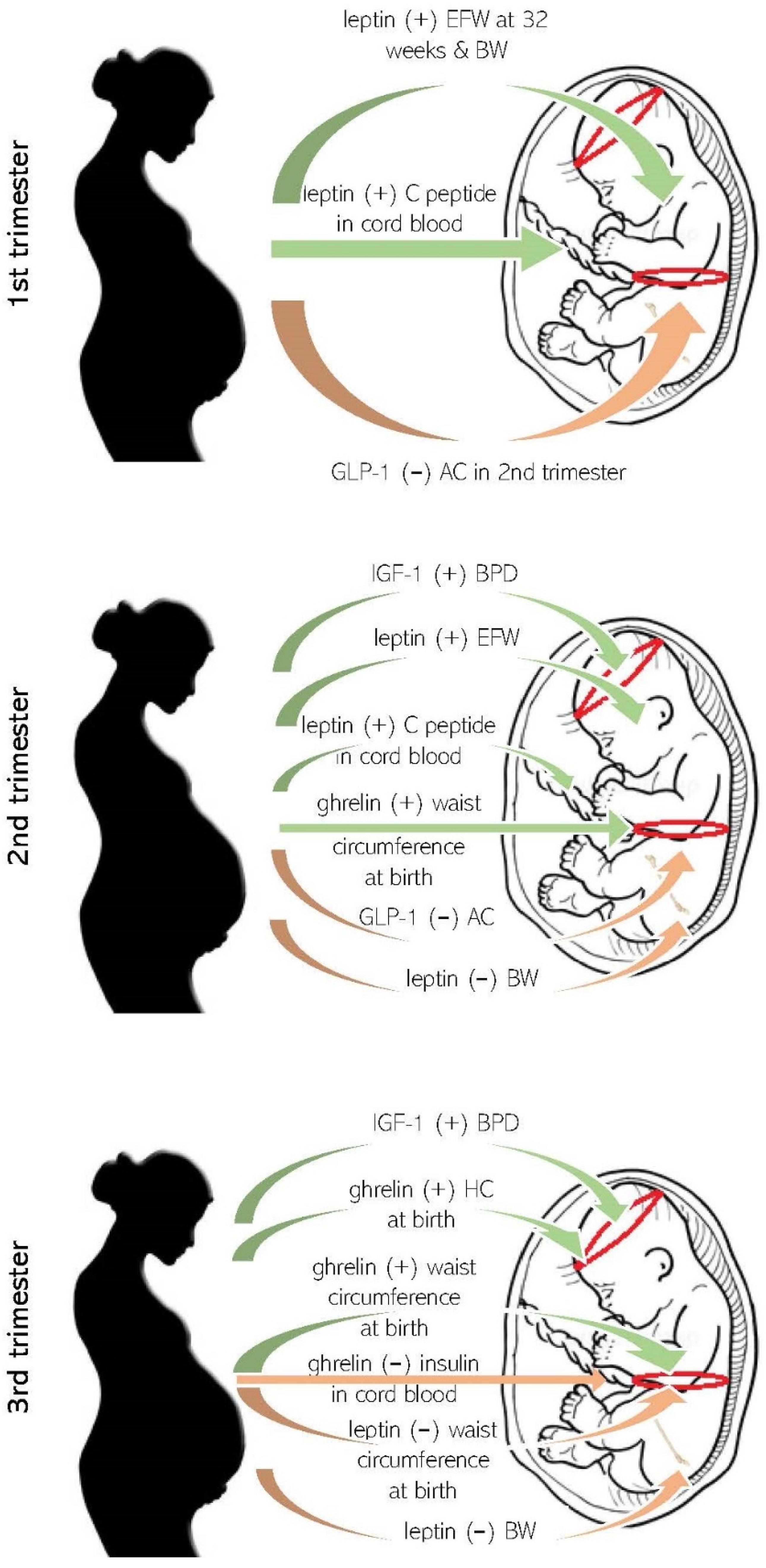
3.1. Maternal Appetite Axis Molecules in Pregnancy and Fetal Growth
3.2. Maternal Appetite Molecules and Anthropometrics at Birth
3.3. Maternal Appetite Molecules and Fetal Metabolism
3.4. Maternal Appetite Axis Molecules during Pregnancy and Possible Metabolic or Endocrine Consequences in Offspring
3.5. Maternal–Fetal Sex–Dependent Biological Competition for Nutrients
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fitzgerald, E.; Hor, K.; Drake, A.J. Maternal Influences on Fetal Brain Development: The Role of Nutrition, Infection and Stress, and the Potential for Intergenerational Consequences. Early Hum. Dev. 2020, 150, 105190. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Perinatal Biomarkers Implying ‘Developmental Origins of Health and Disease’ Consequences in Intrauterine Growth Restriction. Acta Paediatr. 2020, 109, 1317–1322. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Callaghan, B.L.; Kan, J.M.; Richardson, R. The Lasting Impact of Early-Life Adversity on Individuals and Their Descendants: Potential Mechanisms and Hope for Intervention. Genes Brain Behav. 2016, 15, 155–168. [Google Scholar] [CrossRef]
- Kitsiou-Tzeli, S.; Tzetis, M. Maternal Epigenetics and Fetal and Neonatal Growth. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 43–46. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Augustine, R.A.; Grattan, D.R. Hormone Interactions Regulating Energy Balance during Pregnancy. J. Neuroendocrinol. 2010, 22, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Saroha, V.; Symonds, M.E.; Budge, H. Excess Nutrient Supply in Early Life and Its Later Metabolic Consequences. Clin. Exp. Pharmacol. Physiol. 2013, 40, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Maliopoulos, D.; Kasioni, S.; Bargiota, A.; Barber, T.M.; Skevaki, C.; Papassotiriou, I.; Vrachnis, N.; Farmakides, G.; Vlahos, N.F.; et al. Relationship Between Maternal Bone Biomarkers and Fetal Adiposity through Normal Pregnancy. J. Clin. Endocrinol. Metab. 2021, 106, e2647–e2655. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Rawal, S.; Liu, D.; Chen, J.; Tsai, M.Y.; Zhang, C. Maternal Adipokines Longitudinally Measured across Pregnancy and Their Associations with Neonatal Size, Length, and Adiposity. Int. J. Obes. 2019, 43, 1422–1434. [Google Scholar] [CrossRef]
- Mazaki-Tovi, S.; Romero, R.; Kusanovic, J.P.; Vaisbuch, E.; Erez, O.; Than, N.G.; Chaiworapongsa, T.; Nhan-Chang, C.-L.; Pacora, P.; Gotsch, F.; et al. Visfatin in Human Pregnancy: Maternal Gestational Diabetes Vis-à-Vis Neonatal Birthweight. J. Perinat. Med. 2009, 37, 218–231. [Google Scholar] [CrossRef]
- Valsamakis, G.; Kumar, S.; Creatsas, G.; Mastorakos, G. The Effects of Adipose Tissue and Adipocytokines in Human Pregnancy: Adipose Tissue in Pregnancy. Ann. N. Y. Acad. Sci. 2010, 1205, 76–81. [Google Scholar] [CrossRef]
- Valsamakis, G.; Papatheodorou, D.C.; Margeli, A.; Bakoulas, V.; Kapantais, E.; Papassotiriou, I.; Creatsas, G.; Kumar, S.; Mastorakos, G. First Trimester Maternal BMI Is a Positive Predictor of Cord Blood C-Peptide Levels While Maternal Visfatin Levels Is a Negative Predictor of Birth Weight. Hormones 2014, 13, 87–94. [Google Scholar] [CrossRef]
- Kubota, T.; Kamada, S.; Taguchi, M.; Aso, T. Determination of Insulin-like Growth Factor-2 in Feto-Maternal Circulation during Human Pregnancy. Acta Endocrinol. 1992, 127, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, M.; Ruiz-Alcaraz, A.J.; Sanchez-Campillo, M.; Larqué, E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2017, 70, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-C.; Nuyt, A.-M.; Delvin, E.; Audibert, F.; Girard, I.; Shatenstein, B.; Cloutier, A.; Cousineau, J.; Djemli, A.; Deal, C.; et al. Maternal and Fetal IGF-I and IGF-II Levels, Fetal Growth, and Gestational Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1720–1728. [Google Scholar] [CrossRef]
- Boyne, M.S.; Thame, M.; Bennett, F.I.; Osmond, C.; Miell, J.P.; Forrester, T.E. The Relationship among Circulating Insulin-Like Growth Factor (IGF)-I, IGF-Binding Proteins-1 and -2, and Birth Anthropometry: A Prospective Study. J. Clin. Endocrinol. Metab. 2003, 88, 1687–1691. [Google Scholar] [CrossRef]
- Dessì, A.; Pravettoni, C.; Cesare Marincola, F.; Schirru, A.; Fanos, V. The Biomarkers of Fetal Growth in Intrauterine Growth Retardation and Large for Gestational Age Cases: From Adipocytokines to a Metabolomic All-in-One Tool. Expert Rev. Proteom. 2015, 12, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bouhours-Nouet, N.; Boux de Casson, F.; Rouleau, S.; Douay, O.; Mathieu, E.; Bouderlique, C.; Gillard, P.; Limal, J.M.; Descamps, P.; Coutant, R. Maternal and Cord Blood Ghrelin in the Pregnancies of Smoking Mothers: Possible Markers of Nutrient Availability for the Fetus. Horm. Res. Paediatr. 2006, 66, 6–12. [Google Scholar] [CrossRef]
- Klinjampa, R.; Sitticharoon, C.; Souvannavong-Vilivong, X.; Sripong, C.; Keadkraichaiwat, I.; Churintaraphan, M.; Chatree, S.; Lertbunnaphong, T. Placental Neuropeptide Y (NPY) and NPY Receptors Expressions and Serum NPY Levels in Preeclampsia. Exp. Biol. Med. 2019, 244, 380–388. [Google Scholar] [CrossRef]
- Chen, X.; Du, X.; Zhu, J.; Xie, L.; Zhang, Y.; He, Z. Correlations of Circulating Peptide YY and Ghrelin with Body Weight, Rate of Weight Gain, and Time Required to Achieve the Recommended Daily Intake in Preterm Infants. Braz. J. Med. Biol. Res. 2012, 45, 656–664. [Google Scholar] [CrossRef]
- Siahanidou, T.; Mandyla, H.; Vounatsou, M.; Anagnostakis, D.; Papassotiriou, I.; Chrousos, G.P. Circulating Peptide YY Concentrations Are Higher in Preterm than Full-Term Infants and Correlate Negatively with Body Weight and Positively with Serum Ghrelin Concentrations. Clin. Chem. 2005, 51, 2131–2137. [Google Scholar] [CrossRef][Green Version]
- Aktulay, A.; Engin-Ustun, Y.; Yasar, O.; Yilmaz, C.; Erkaya, S.; Ozgu-Erdinc, A. Levels of Glucagon-like Peptide 1 are Decreased in Macrosomic Neonates from Non-Diabetic Mothers. Z. Geburtshilfe Neonatol. 2019, 223, 48–53. [Google Scholar] [CrossRef]
- Valsamakis, G.; Margeli, A.; Vitoratos, N.; Boutsiadis, A.; Sakkas, E.G.; Papadimitriou, G.; Al-Daghri, N.M.; Botsis, D.; Kumar, S.; Papassotiriou, I.; et al. The Role of Maternal Gut Hormones in Normal Pregnancy: Fasting Plasma Active Glucagon-like Peptide 1 Level Is a Negative Predictor of Fetal Abdomen Circumference and Maternal Weight Change. Eur. J. Endocrinol. 2010, 162, 897–903. [Google Scholar] [CrossRef][Green Version]
- Al-Far, H.F.M.; Tjessem, I.H.; Lauszus, F.F. Macrosomia and the IGF System: A Short Review. Focus Sci. 2017, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Lazo-de-la-Vega-Monroy, M.-L.; González-Domínguez, M.; Zaina, S.; Sabanero, M.; Daza-Benítez, L.; Malacara, J.; Barbosa-Sabanero, G. Leptin and Its Receptors in Human Placenta of Small, Adequate, and Large for Gestational Age Newborns. Horm. Metab. Res. 2017, 49, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J.; Russell, J.A. Endocrine Induced Changes in Brain Function during Pregnancy. Brain Res. 2010, 1364, 198–215. [Google Scholar] [CrossRef]
- McMillen, I.C.; MacLaughlin, S.M.; Muhlhausler, B.S.; Gentili, S.; Duffield, J.L.; Morrison, J.L. Developmental Origins of Adult Health and Disease: The Role of Periconceptional and Foetal Nutrition. Basic Clin. Pharmacol. Toxicol. 2008, 102, 82–89. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental Phenotype and the Insulin-like Growth Factors: Resource Allocation to Fetal Growth: The IGFs and Placental Resource Allocation to Fetal Growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef]
- Baldwin, S.; Chung, T.; Rogers, M.; Chard, T.; Wang, H.S. Insulin-like Growth Factor-Binding Protein-1, Glucose Tolerance and Fetal Growth in Human Pregnancy. J. Endocrinol. 1993, 136, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Byrne, J.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Leptin, Fetal Growth and Insulin Resistance in Non-Diabetic Pregnancies. Early Hum. Dev. 2014, 90, 271–274. [Google Scholar] [CrossRef]
- Ruiz-Palacios, M.; Prieto-Sánchez, M.; Ruiz-Alcaraz, A.; Blanco-Carnero, J.; Sanchez-Campillo, M.; Parrilla, J.; Larqué, E. Insulin Treatment May Alter Fatty Acid Carriers in Placentas from Gestational Diabetes Subjects. Int. J. Mol. Sci. 2017, 18, 1203. [Google Scholar] [CrossRef]
- Valsamakis, G.; Papatheodorou, D.C.; Naoum, A.; Margeli, A.; Papassotiriou, I.; Kapantais, E.; Creatsas, G.; Kumar, S.; Mastorakos, G. Neonatal Birth Waist Is Positively Predicted by Second Trimester Maternal Active Ghrelin, a pro-Appetite Hormone, and Negatively Associated with Third Trimester Maternal Leptin, a pro-Satiety Hormone. Early Hum. Dev. 2014, 90, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Osborn, J.F.; Haass, C.; Natale, F.; Spinelli, M.; Scapillati, E.; Spinelli, A.; Pacifico, L. Ghrelin, Leptin, IGF-1, IGFBP-3, and Insulin Concentrations at Birth: Is There a Relationship with Fetal Growth and Neonatal Anthropometry? Clin. Chem. 2008, 54, 550–558. [Google Scholar] [CrossRef]
- Gohlke, B.C.; Huber, A.; Hecher, K.; Fimmers, R.; Bartmann, P.; Roth, C.L. Fetal Insulin-like Growth Factor (IGF)-I, IGF-II, and Ghrelin in Association with Birth Weight and Postnatal Growth in Monozygotic Twins with Discordant Growth. J. Clin. Endocrinol. Metab. 2005, 90, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Yokota, I.; Hosoda, H.; Kotani, Y.; Matsuda, J.; Naito, E.; Ito, M.; Kangawa, K.; Kuroda, Y. Ghrelin Concentration in Cord and Neonatal Blood: Relation to Fetal Growth and Energy Balance. J. Clin. Endocrinol. Metab. 2003, 88, 5473–5477. [Google Scholar] [CrossRef]
- Saylan, F.; Köken, G.; Cosar, E.; Köken, T.; Saylan, A.; Arıöz, D.T.; Sahin, F.; Köken, R.; Yılmazer, M. Maternal and Fetal Leptin and Ghrelin Levels: Relationship with Fetal Growth. Arch. Gynecol. Obstet. 2011, 284, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Perichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Espino y Sosa, S.; Ramírez-González, M.C. Metabolic Markers during Pregnancy and Their Association with Maternal and Newborn Weight Status. PLoS ONE 2017, 12, e0180874. [Google Scholar] [CrossRef]
- Retnakaran, R.; Ye, C.; Hanley, A.J.G.; Connelly, P.W.; Sermer, M.; Zinman, B.; Hamilton, J.K. Effect of Maternal Weight, Adipokines, Glucose Intolerance and Lipids on Infant Birth Weight among Women without Gestational Diabetes Mellitus. CMAJ 2012, 184, 1353–1360. [Google Scholar] [CrossRef]
- Kos, K.; Syn, W.-K.; Lewandowski, K.C.; Bennett, J.; Nwokolo, C.U.; O’Hare, J.P.; Randeva, H. Comparison of Maternal Ghrelin and Leptin in Healthy Mothers and Mothers with Type 1 Diabetes. Diabet. Med. 2008, 25, 1400–1405. [Google Scholar] [CrossRef]
- Misra, V.K.; Straughen, J.K.; Trudeau, S. Maternal Serum Leptin during Pregnancy and Infant Birth Weight: The Influence of Maternal Overweight and Obesity: Maternal Leptin and Infant Birth Weight. Obesity 2013, 21, 1064–1069. [Google Scholar] [CrossRef]
- Clausen, T.; Burski, T.K.; Øyen, N.; Godang, K.; Bollerslev, J.; Henriksen, T. Maternal Anthropometric and Metabolic Factors in the First Half of Pregnancy and Risk of Neonatal Macrosomia in Term Pregnancies. A Prospective Study. Eur. J. Endocrinol. 2005, 153, 887–894. [Google Scholar] [CrossRef]
- Shroff, M.R.; Holzman, C.; Tian, Y.; Evans, R.W.; Sikorskii, A. Mid-Pregnancy Maternal Leptin Levels, Birthweight for Gestational Age and Preterm Delivery. Clin. Endocrinol. 2013, 78, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.R.; Poston, L.; Franco-Sena, A.B.; Moura da Silva, A.A.; Pinto, T.; de Oliveira, L.C.; Kac, G. Maternal Lipids and Leptin Concentrations Are Associated with Large-for-Gestational-Age Births: A Prospective Cohort Study. Sci. Rep. 2017, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, J.; Pintiaux, A.; Van Herck, E.; Hennen, G.; Foidart, J.-M.; Igout, A. Placental GH, IGF-I, IGF-Binding Protein-1, and Leptin during a Glucose Challenge Test in Pregnant Women: Relation with Maternal Body Weight, Glucose Tolerance, and Birth Weight. J. Clin. Endocrinol. Metab. 2002, 87, 2875–2882. [Google Scholar] [CrossRef]
- Verhaeghe, J.; van Bree, R.; Van Herck, E. Maternal Body Size and Birth Weight: Can Insulin or Adipokines Do Better? Metabolism 2006, 55, 339–344. [Google Scholar] [CrossRef]
- Horosz, E.; Bomba-Opon, D.A.; Szymanska, M.; Wielgos, M. Third Trimester Plasma Adiponectin and Leptin in Gestational Diabetes and Normal Pregnancies. Diabetes Res. Clin. Pract. 2011, 93, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, U.; Gulturk, S.; Aker, A.; Guvenal, T.; Imir, G.; Erselcan, T. Correlation between Birth Weight, Leptin, Zinc and Copper Levels in Maternal and Cord Blood. J. Physiol. Biochem. 2007, 63, 121–128. [Google Scholar] [CrossRef]
- Ökdemir, D.; Hatipoğlu, N.; Kurtoğlu, S.; Siraz, Ü.G.; Akar, H.H.; Muhtaroğlu, S.; Kütük, M.S. The Role of Irisin, Insulin and Leptin in Maternal and Fetal Interaction. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 307–315. [Google Scholar] [CrossRef]
- Papadopoulou, F.G.; Mamopoulos, A.M.; Triantos, A.; Constantinidis, T.C.; Papadimas, J.; Assimakopoulos, E.A.; Koliakos, G.; Mamopoulos, M. Leptin Levels in Maternal and Cord Serum: Relationship with Fetal Development and Placental Weight. J. Matern.-Fetal Med. 2000, 9, 298–302. [Google Scholar] [CrossRef]
- Jansson, N.; Nilsfelt, A.; Gellerstedt, M.; Wennergren, M.; Rossander-Hultheén, L.; Powell, T.L.; Jansson, T. Maternal Hormones Linking Maternal Body Mass Index and Dietary Intake to Birth Weight. Am. J. Clin. Nutr. 2008, 87, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M.-L. Early-Life Programming of Adipose Tissue. Nutr. Res. Rev. 2020, 33, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Fattuoni, C.; Noto, A.; Barberini, L.; Dessì, A.; Fanos, V. The Choice of Amniotic Fluid in Metabolomics for the Monitoring of Fetus Health. Expert Rev. Mol. Diagn. 2016, 16, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Orczyk-Pawilowicz, M.; Jawien, E.; Deja, S.; Hirnle, L.; Zabek, A.; Mlynarz, P. Metabolomics of Human Amniotic Fluid and Maternal Plasma during Normal Pregnancy. PLoS ONE 2016, 11, e0152740. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.; Talbot, O.; Kuang, A.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Cord Blood Metabolomics: Association With Newborn Anthropometrics and C-Peptide Across Ancestries. J. Clin. Endocrinol. Metab. 2019, 104, 4459–4472. [Google Scholar] [CrossRef]
- Ma, J.; Luo, J.; He, M.; Bian, X.; Li, J.; Du, Y.; Sun, B.; Chen, H. Umbilical Cord Blood Metabolomics: Association with Intrauterine Hyperglycemia. Pediatr. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Øyri, L.K.L.; Bogsrud, M.P.; Christensen, J.J.; Ulven, S.M.; Brantsæter, A.L.; Retterstøl, K.; Brekke, H.K.; Michelsen, T.M.; Henriksen, T.; Roeters van Lennep, J.E.; et al. Novel Associations between Parental and Newborn Cord Blood Metabolic Profiles in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 2021, 19, 91. [Google Scholar] [CrossRef]
- Delhaes, F.; Giza, S.A.; Koreman, T.; Eastabrook, G.; McKenzie, C.A.; Bedell, S.; Regnault, T.R.H.; de Vrijer, B. Altered Maternal and Placental Lipid Metabolism and Fetal Fat Development in Obesity: Current Knowledge and Advances in Non-Invasive Assessment. Placenta 2018, 69, 118–124. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Arroyo-Jousse, V.; Jaramillo, A.; Castaño-Moreno, E.; Lépez, M.; Carrasco-Negüe, K.; Casanello, P. Adipokines Underlie the Early Origins of Obesity and Associated Metabolic Comorbidities in the Offspring of Women with Pregestational Obesity. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165558. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Duffield, J.A.; McMillen, I.C. Increased Maternal Nutrition Stimulates Peroxisome Proliferator Activated Receptor-γ, Adiponectin, and Leptin Messenger Ribonucleic Acid Expression in Adipose Tissue before Birth. Endocrinology 2007, 148, 878–885. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Meitzner, K.; Ortega-Senovilla, H.; Graf, K.; Vetter, K.; Abou-Dakn, M.; Herrera, E. Differences in the Implications of Maternal Lipids on Fetal Metabolism and Growth between Gestational Diabetes Mellitus and Control Pregnancies. Diabet. Med. 2011, 28, 1053–1059. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and Fetal Lipid Metabolism under Normal and Gestational Diabetic Conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Brunn, M.; Harder, A.; Roepke, K.; Wittrock-Staar, M.; Ziska, T.; Schellong, K.; Rodekamp, E.; Melchior, K.; et al. Hypothalamic Proopiomelanocortin Promoter Methylation Becomes Altered by Early Overfeeding: An Epigenetic Model of Obesity and the Metabolic Syndrome: Nutritionally Induced Alterations of POMC Promoter Methylation. J. Physiol. 2009, 587, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Gali Ramamoorthy, T.; Allen, T.-J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal Overnutrition Programs Epigenetic Changes in the Regulatory Regions of Hypothalamic Pomc in the Offspring of Rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Gugusheff, J.R.; Ong, Z.Y.; Vithayathil, M.A. Pregnancy, Obesity and Insulin Resistance: Maternal Overnutrition and the Target Windows of Fetal Development. Horm. Mol. Biol. Clin. Investig. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Muhlhausler, B.S. The Effect of N-3 LCPUFA Supplementation on Oxidative Stress and Inflammation in the Placenta and Maternal Plasma during Pregnancy. Prostaglandins Leukot. Essent. Fatty Acids 2016, 113, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. Review: The Placenta and Developmental Programming: Balancing Fetal Nutrient Demands with Maternal Resource Allocation. Placenta 2012, 33, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. The Risk of Maternal Nutritional Depletion and Poor Outcomes Increases in Early or Closely Spaced Pregnancies. J. Nutr. 2003, 133, 1732S–1736S. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M. Competition for Nutrients in Pregnant Adolescents: Consequences for Maternal, Conceptus and Offspring Endocrine Systems. J. Endocrinol. 2019, 242, T1–T19. [Google Scholar] [CrossRef]
- Scholl, T.O.; Stein, T.P.; Smith, W.K. Leptin and Maternal Growth during Adolescent Pregnancy. Am. J. Clin. Nutr. 2000, 72, 1542–1547. [Google Scholar] [CrossRef]
- Van Abeelen, A.F.M.; de Rooij, S.R.; Osmond, C.; Painter, R.C.; Veenendaal, M.V.E.; Bossuyt, P.M.M.; Elias, S.G.; Grobbee, D.E.; van der Schouw, Y.T.; Barker, D.J.P.; et al. The Sex-Specific Effects of Famine on the Association between Placental Size and Later Hypertension. Placenta 2011, 32, 694–698. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J.P. Boys Live Dangerously in the Womb. Am. J. Hum. Biol. 2010, 22, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; Painter, R.C.; de Rooij, S.R.; van Abeelen, A.F.M.; Veenendaal, M.V.E.; Osmond, C.; Barker, D.J.P. Effects of Famine on Placental Size and Efficiency. Placenta 2011, 32, 395–399. [Google Scholar] [CrossRef]
- Kisioglu, B.; Nergiz-Unal, R. Potential Effect of Maternal Dietary Sucrose or Fructose Syrup on CD36, Leptin, and Ghrelin-Mediated Fetal Programming of Obesity. Nutr. Neurosci. 2020, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Patro-Małysza, J.; Trojnar, M.; Skórzyńska-Dziduszko, K.E.; Kimber-Trojnar, Ż.; Darmochwał-Kolarz, D.; Czuba, M.; Leszczyńska-Gorzelak, B. Leptin and Ghrelin in Excessive Gestational Weight Gain—Association between Mothers and Offspring. Int. J. Mol. Sci. 2019, 20, 2398. [Google Scholar] [CrossRef] [PubMed]
- Anini, Y.; Brubaker, P.L. Role of Leptin in the Regulation of Glucagon-like Peptide-1 Secretion. Diabetes 2003, 52, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Antony, K.M.; Romezi, M.; Lindgren, K.; Mitchell, K.B.; Venable, S.F.; Racusin, D.A.; Suter, M.A.; Aagaard, K.M. Maternal Metabolic Biomarkers Are Associated with Obesity and Excess Gestational Weight Gain. Am. J. Perinatol. 2021, 38, e173–e181. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Godínez-Martínez, E.; Sánchez-Jiménez, B.; Montiel-Ojeda, D.; Tolentino, M. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients 2017, 9, 1067. [Google Scholar] [CrossRef]
- Josefson, J.L.; Zeiss, D.M.; Rademaker, A.W.; Metzger, B.E. Maternal Leptin Predicts Adiposity of the Neonate. Horm. Res. Paediatr. 2014, 81, 13–19. [Google Scholar] [CrossRef]
- Clifton, V.L. Review: Sex and the Human Placenta: Mediating Differential Strategies of Fetal Growth and Survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Crawford, M.A. Survival of the Fattest: Fat Babies Were the Key to Evolution of the Large Human Brain. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 17–26. [Google Scholar] [CrossRef]
- Desoye, G.; Herrera, E. Adipose Tissue Development and Lipid Metabolism in the Human Fetus: The 2020 Perspective Focusing on Maternal Diabetes and Obesity. Prog. Lipid. Res. 2021, 81, 101082. [Google Scholar] [CrossRef] [PubMed]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of Maternal BMI and Gestational Diabetes Mellitus on Maternal and Cord Blood Metabolome: Results from the PREOBE Cohort Study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef] [PubMed]

| Studies | Sample (N) | Maternal Molecule Studied | Trimester Studied | Main Outcome | Comments |
|---|---|---|---|---|---|
| Kubota et al. | 52 | IGF-1 IGF-2 | Second and third | Positive correlation to fetal BPD * in second and third trimester (r = 0.606, p < 0.001) No correlations | |
| Baldwin et al. | 200 | IGFBP-1 | Second (20–24 weeks) | Inverse correlation to fetal BPD1, AC2, femur3 and tibia4 length and subcutaneous fat5 (r1 = 0.319, p < 0.000, r2 = 0.257, p < 0.005, r3 = 0.288, p < 0.005, r4 = 0.243, p < 0.005, r5 = 0.326, p < 0.005) | No correlation found in third trimester/IGFBP-1 also inversely related with birth weight (r = 0.185, p < 0.05) |
| Walsh et al. | 574 | leptin | Early gestation and at 28 weeks | Correlation to estimated fetal weight at 32 weeks (r = 0.16, p < 0.001) and (r = 0.12, p = 0.008) | Early pregnancy leptin also correlated with neonatal birth weight (r = 0.14, p = 0.001) |
| Ruiz-Palacios et al. | 68 | insulin | Early third | Association to third trimester fetal AC ** (r = 0.266, p = 0.025) | Included GDM *** pregnancies |
| Valsamakis et al. | 55 | Active GLP-1 Total GIP Active ghrelin Total PYY | First | Negative correlation to fetal AC in second trimester (r = −0.55, p = 0.034) No correlations found | Active GLP-1 was the best negative predictor of second trimester AC |
| Studies | Sample (N) | Maternal Molecule Studied | Trimester Studied | Main Outcome | Comments |
|---|---|---|---|---|---|
| Valsamakis et al. | 80 | Activated ghrelin | Second and third | Positive correlation with neonatal waist circumference at birth (second: r = 0.75 and third: r = 0.70, p < 0.001 with p < 0.001) negative correlation with percent total body fat (r = −0.94, p < 0.001) | Ghrelin levels during second trimester were the best positive predictor of birth waist circumference, no relation to birth weight |
| Leptin | Third | Negative correlation with neonatal waist circumference (r = −0.81, p < 0.001) | |||
| Active GLP-1 | Second | Negative correlation with birth weight (r = −0.40, p = 0.03) | |||
| Chiesa et al. | 153 | ghrelin | During labor | Positive correlation with head circumference at birth (B = 0.45 95% CI: = 0.17, 0.73, p < 0.01) | |
| Saylan et al. | 36 | ghrelin | All three trimesters | No relation to birth or placenta weight | All neonates had birth weight within normal range |
| Bouhours-Nouet et al. | 85 | ghrelin | During labor | No correlation to birth or placenta weight | |
| Valsamakis et al. | 55 | Activated GLP-1 | Second | Negative correlation with birth weight (r = −0.50, p = 0.040) | |
| Perichart-Perera et al. | 177 | leptin | Early first | Positive correlation with birth weight (B = 0.007 95% CI: 0.002, 0.011, p = 0.005) | Valid only in normal maternal BMI pregnancies, excluded macrosomic neonates |
| Retnakaran et al. | 472 | leptin | Late second to early third | Negative correlation with birth weight Adj. OR (95% CI) = −3.92 (−6.23 to −1.60) | Leptin was found to be a significant negative predictor of birth weight and large-for-gestational-age neonate |
| Kos et al. | 12 | Free leptin | 30 weeks of gestation | Negative correlation with birth weight (r = −0.63, p < 0.05) | Same negative correlation in type 1 diabetes mellitus pregnancies |
| Misra et al. | 286 | leptin | All three trimesters | No correlation with birth weight | Included pregnancies complicated with hypertensive disorders |
| Studies | Sample (N) | Trimester Studied | Main Outcome | Comments |
|---|---|---|---|---|
| Walsh et al. | 537 | Early gestation | Correlation with EFW * at 34 weeks (β = 0.16, p = 0.02) and neonatal birth weight (r = 0.14, p = 0.001) | No similar significant correlations at 28 weeks of gestation |
| Shroff et al. | 1304 | Second | Elevated leptin predicts an LGA neonate | Included cases with gestational pathology/After adjusting data for maternal BMI, the correlation attenuated but remained significant in preterm births |
| Farias et al. | 199 | First | Positive correlation with birth LGA birth (intercept OR = 3.88; 95% CI: 1.49 to 10.09; p = 0.005) | Maternal log leptin in 1st trimester was also correlated with birth weight, but when model included data adjusted for maternal pre-pregnancy BMI, statistical significance was not reached |
| Verhaeghe et al. | 278 | Late second | Mothers with elevated leptin levels were more likely to give birth to an obese/overweight neonate | Authors stated that leptin levels measurement had no clinical use |
| Clausen T. et al. | 2050 | Early second | No correlation with birth weight | |
| Verhaeghe et al. | 631 | Late second | Negative correlation with birth weight (T = −4.10, p < 0.0001) | Included pregnancies with hypertensive disorders and/or preeclampsia and abnormal OGTT results |
| Lazo-de-la-Vega-Monroy et al. | 60 20 with SGA **, 20 with AGA *** and 20 with LGA neonates | During labor | No relation with LGA neonates | |
| Horosz et al. | 134 86 with GDM and 48 normal pregnancies | Early third trimester | No relation with AC or birth weight | |
| Ozdemir et al. | 88 | Late third trimester (>38 weeks) | No relation with birth weight | Maternal leptin levels were significantly higher in LGA group |
| Ökdemir et al. | 84 | During labor | No relation with anthropometrics at birth | |
| Papadopoulou et al. | 85 | Right after delivery | No correlation with placental or birth weight | No sex differences observed in leptin levels |
| Studies | Sample (N) | Maternal Molecule Studied | Trimester Studied | Main Outcome | Comments |
|---|---|---|---|---|---|
| Valsamakis et al. | 80 | Activated ghrelin | Third | Negative correlation with insulin levels in cord blood (r = −0.82, p < 0.001) | Third trimester activated ghrelin was the best negative predictor of cord blood insulin levels |
| Walsh et al. | 574 | leptin | In early pregnancy and at 28 weeks | Correlated with C peptide levels in cord blood (β = 0.173, p = 0.004 and β = 0.115, p = 0.05 respectively) | Maternal leptin suggested to be utilized as a biomarker of fetal, intrauterine insulin resistance |
| Studies | Sample (N) | Indices Studied | Main Relevant Outcome | Comments |
|---|---|---|---|---|
| Van Abbelen et al. | 860 | Placental size at birth | Maternal undernutrition during gestation reduced placental surface area in men but not in women | Retrospective cohort study |
| Eriksson et al. | 2003 | Placental surface area at birth | Male fetuses grow more rapidly in-utero compared to females | Retrospective study of birth records/energy and nutrients are invested more in brain, but less in placental growth by male fetuses/maternal diet during pregnancy seems to influence growth of only male fetuses |
| Roseboom et al. | 2414 | Placental area and volume | Placentas of male fetuses had less surface area compared to their female counterparts | Retrospective study of birth records/Famine impaired normal placentation especially those in mid- late gestation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimas, A.; Politi, A.; Papaioannou, G.; Barber, T.M.; Weickert, M.O.; Grammatopoulos, D.K.; Kumar, S.; Kalantaridou, S.; Valsamakis, G. The Gestational Effects of Maternal Appetite Axis Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 695. https://doi.org/10.3390/ijms23020695
Dimas A, Politi A, Papaioannou G, Barber TM, Weickert MO, Grammatopoulos DK, Kumar S, Kalantaridou S, Valsamakis G. The Gestational Effects of Maternal Appetite Axis Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(2):695. https://doi.org/10.3390/ijms23020695
Chicago/Turabian StyleDimas, Angelos, Anastasia Politi, George Papaioannou, Thomas M. Barber, Martin O. Weickert, Dimitris K. Grammatopoulos, Sudhesh Kumar, Sophia Kalantaridou, and Georgios Valsamakis. 2022. "The Gestational Effects of Maternal Appetite Axis Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review" International Journal of Molecular Sciences 23, no. 2: 695. https://doi.org/10.3390/ijms23020695
APA StyleDimas, A., Politi, A., Papaioannou, G., Barber, T. M., Weickert, M. O., Grammatopoulos, D. K., Kumar, S., Kalantaridou, S., & Valsamakis, G. (2022). The Gestational Effects of Maternal Appetite Axis Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. International Journal of Molecular Sciences, 23(2), 695. https://doi.org/10.3390/ijms23020695






