Potential Anti-Metastatic Role of the Novel miR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion
Abstract
:1. Introduction
2. Results
2.1. Novel miR-CT3 and Target Gene Prediction
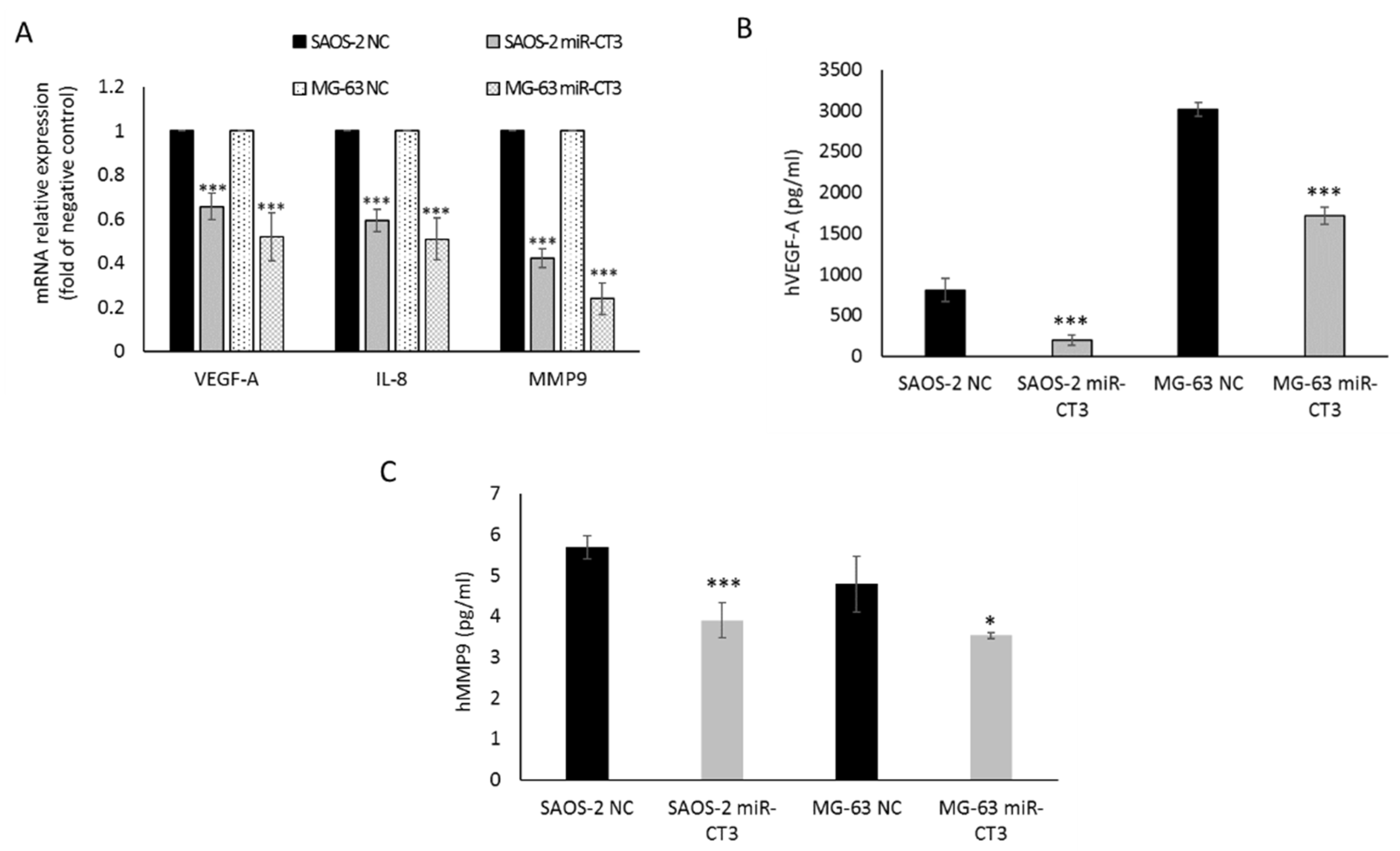
2.2. The Enforced Expression of miR-CT3 Down-Regulates VEGF-A
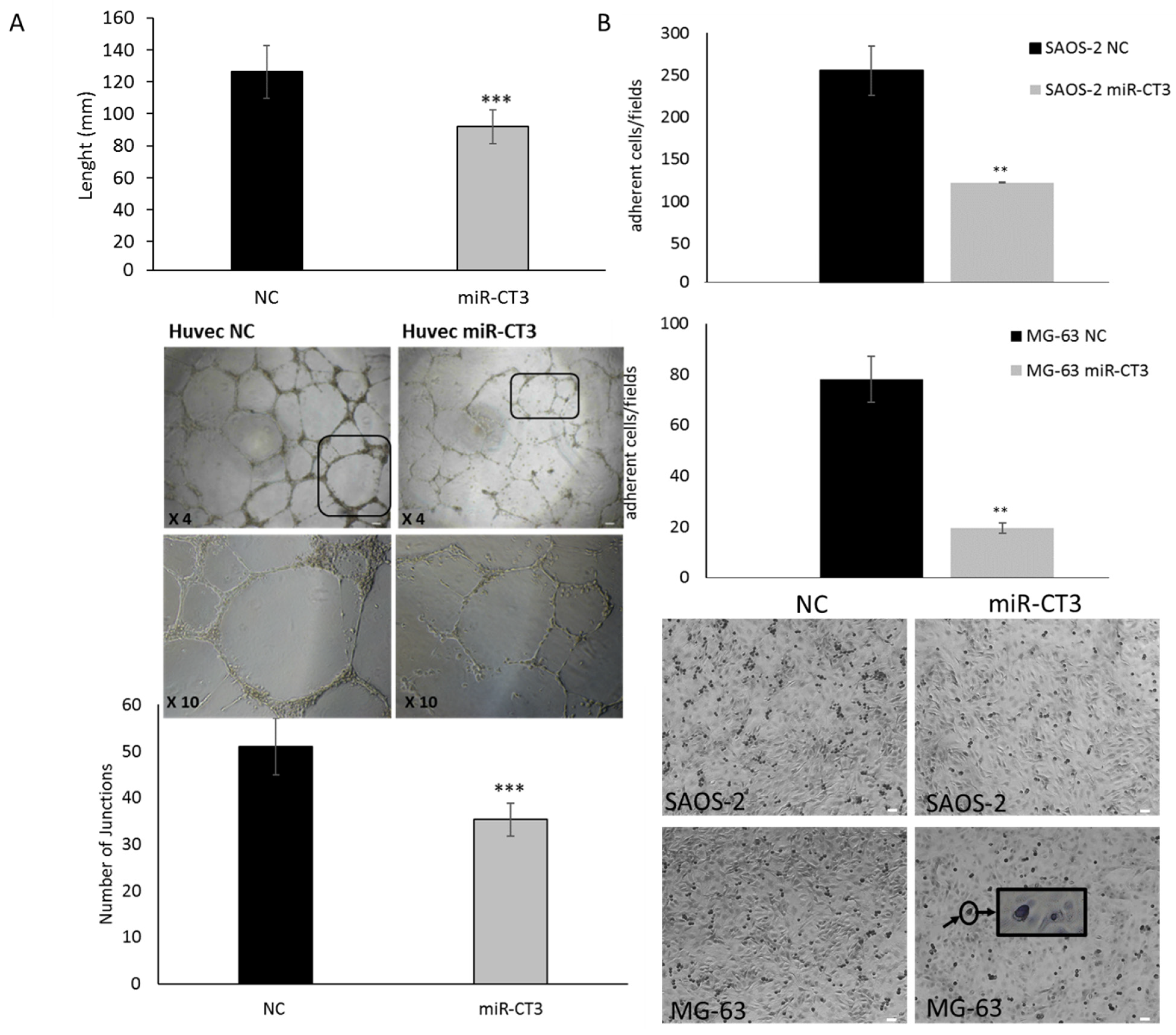
2.3. The Enforced Expression of miR-CT3 Impairs Vessel Formation and Adhesion of OS Cells on Vascular Endothelium
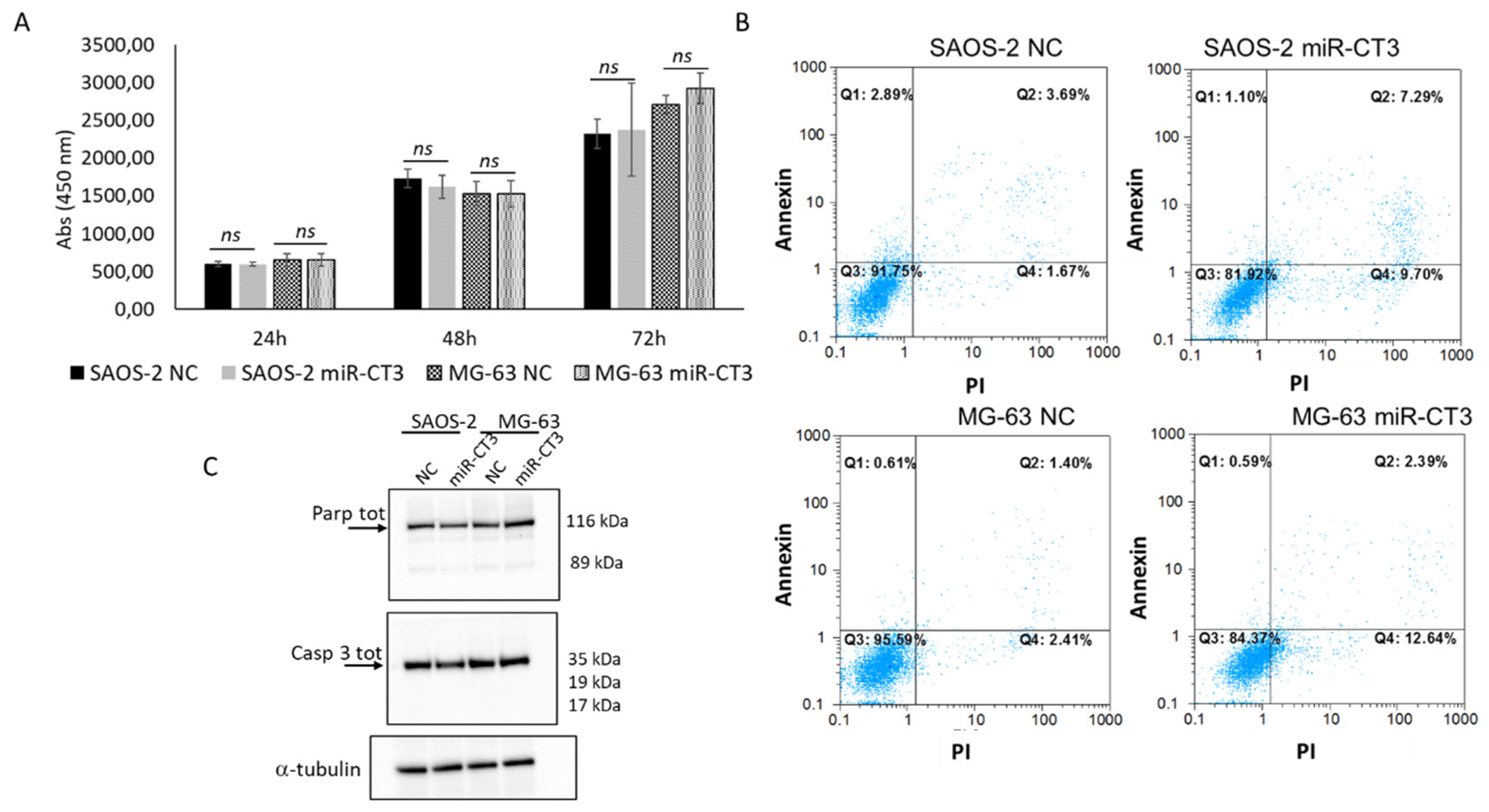
2.4. The Enforced Expression of miR-CT3 Seems Not to Regulate Cell Proliferation and Apoptosis
2.5. MiR-CT3 Reduces Migration and Invasive Ability of OS Cells
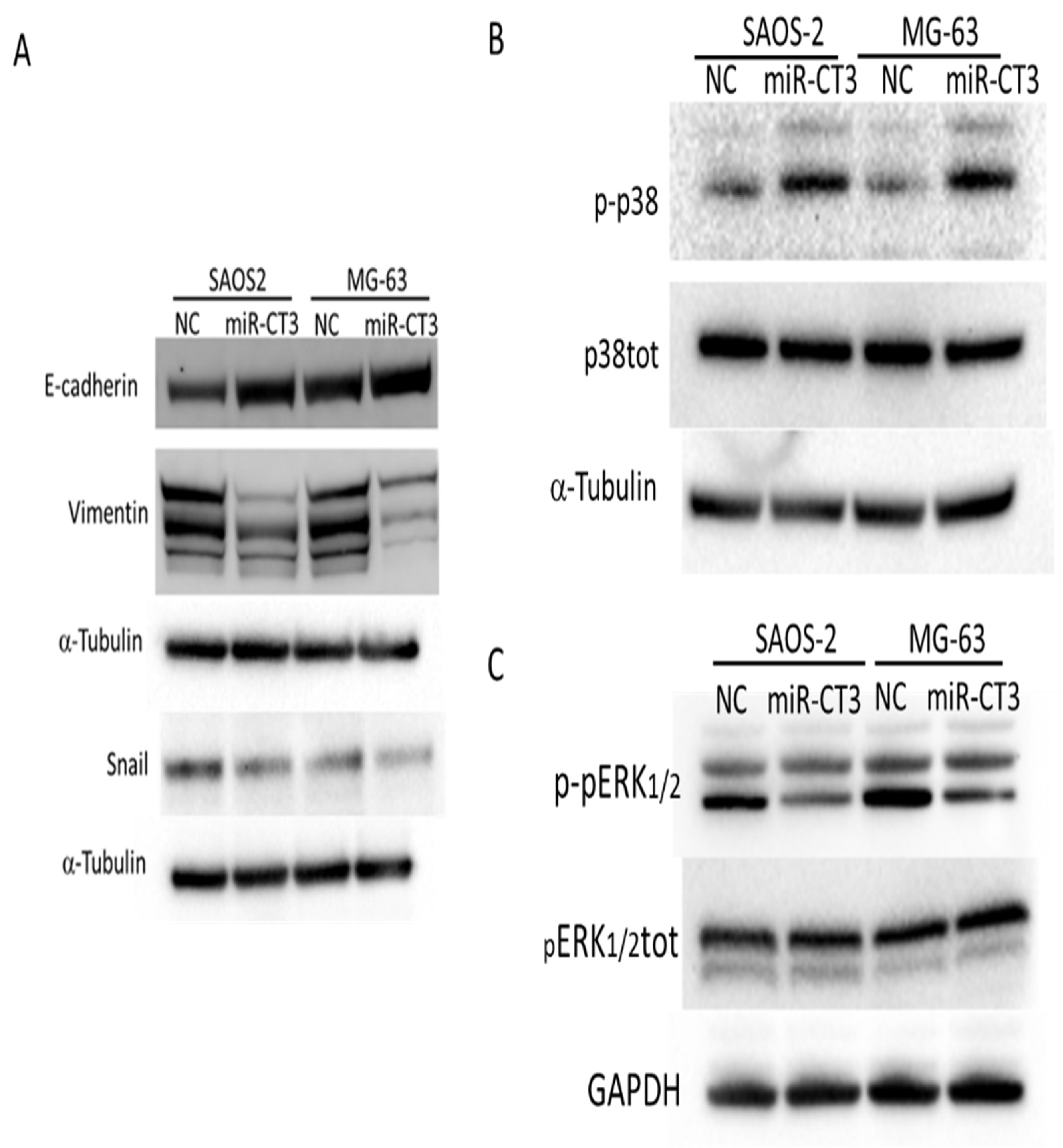
2.6. MiR-CT3 Regulates Expression of Proteins Correlated with Migration
2.7. MiR-CT3 Expression in Metastatic Patient Samples
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Exosome Purification
4.3. Dual-Luciferase Reporter Assay
4.4. Viability Assay (WST-1 Test)
4.5. MiRNA Transfection and qRT-PCR of mRNAs and miRNAs
4.6. Western Blot Analysis
4.7. Tube Formation Assay
4.8. Invasion Assay
4.9. Wound Healing Assay
4.10. Adhesion Assay
4.11. Soft-Agar Colony Formation Assay
4.12. ELISA Assay
4.13. Flow Cytometric Analysis
4.14. Statistical Analysis
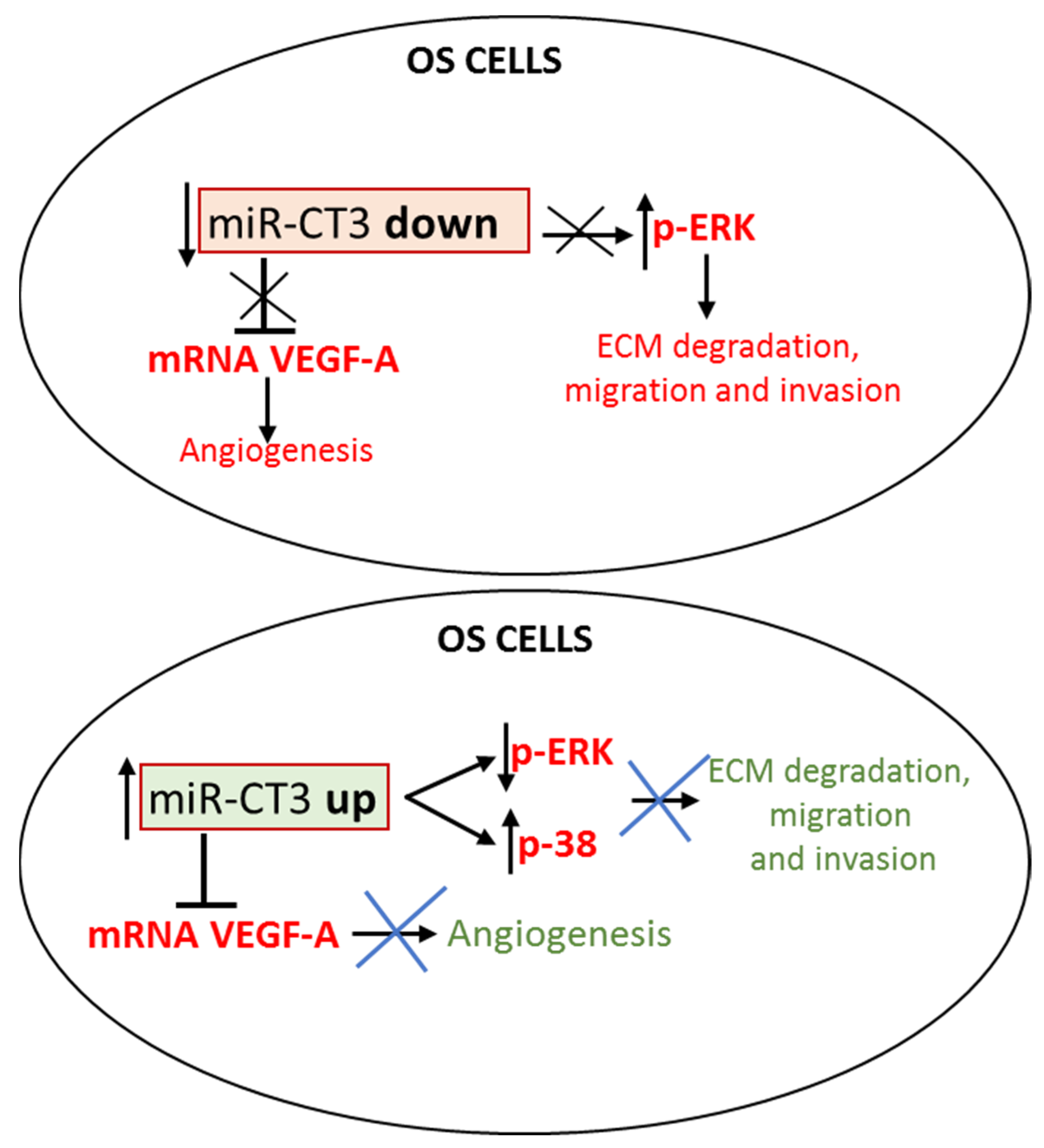
5. Conclusions
6. Ethics Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ottaviani, G.; Jaffe, N. The etiology of osteosarcoma. Cancer Treat Res. 2009, 152, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.P.; Zhang, C.L.; Ma, X.L.; Hu, J.P.; Cai, T.; Zhang, L. Analyzing the Interactions of mRNAs and ncRNAs to predict competing endogenous RNA networks in osteosarcoma chemo-resistance. Mol. Ther. 2019, 27, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasin, N.F.; Abdul Rashid, M.L.; Ajit Singh, V. Survival analysis of osteosarcoma patients: A 15-year experience. J. Orthop. Surg. 2020, 28, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, D.J. GFRA1: A novel molecular target for the prevention of osteosarcoma chemoresistance. Int. J. Mol. Sci. 2018, 19, 1078. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, A.J.; Walkley, C.R. Cells of origin in osteosarcoma: Mesenchymal stem cells or osteoblast committed cells? Bone 2014, 62, 56–63. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular biology of osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Kundu, Z.S. Classification, imaging, biopsy and staging of osteosarcoma. Indian J. Orthop. 2014, 48, 238–246. [Google Scholar] [CrossRef]
- Canale, S.T.; Beaty, J.H. Campbell’s Operative Orthopaedics; Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Kayton, M.L.; Huvos, A.G.; Casher, J.; Abramson, S.J.; Rosen, N.S.; Wexler, L.H.; Meyers, P.; LaQuaglia, M.P. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J. Pediatr. Surg. 2006, 41, 200–206. [Google Scholar] [CrossRef]
- Sasaki, R.; Osaki, M.; Okada, F. MicroRNA-based diagnosis and treatment of metastatic human osteosarcoma. Cancers 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, N.; Puri, A.; Gelderblom, H. Osteosarcoma: Evolution of treatment paradigms. Sarcoma 2013, 2013, 203531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yao, H.; Zheng, Z.; Qiu, G.; Sun, K. MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int. J. Cancer 2011, 128, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Mudduluru, G.; Ceppi, P.; Muppala, S.; Kozlowski, M.; Niklinski, J.; Papotti, M.; Allgayer, H. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer 2012, 130, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, Z.; Xu, J.; Chen, C.; Ge, Q.; Li, P.; Wei, D.; Wu, Z.; Sun, X. MicroRNA-134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J. 2018, 285, 1359–1371. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Junxin, C.; Yongcan, H.; Xiaoguang, L.; Binsheng, Y. Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. J. Bone Oncol. 2020, 25, 100322. [Google Scholar] [CrossRef]
- Llobat, L.; Gourbault, O. Role of MicroRNAs in human osteosarcoma: Future perspectives. Biomedicines 2021, 9, 463. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, L.; De Luca, A.; Costa, V.; Amodio, N.; Carina, V.; Bellavia, D.; Tassone, P.; Pagani, S.; Fini, M.; Alessandro, R.; et al. Circulating biomarkers in osteosarcoma: New translational tools for diagnosis and treatment. Oncotarget 2017, 8, 100831–100851. [Google Scholar] [CrossRef] [Green Version]
- Raimondo, S.; Urzì, O.; Conigliaro, A.; Raimondi, L.; Amodio, N.; Alessandro, R. Emerging insights on the biological impact of extracellular vesicle-associated ncRNAs in multiple myeloma. Noncoding RNA 2020, 6, 30. [Google Scholar] [CrossRef]
- Max, K.E.A.; Bertram, K.; Akat, K.M.; Bogardus, K.A.; Li, J.; Morozov, P.; Ben-Dov, I.Z.; Li, X.; Weiss, Z.R.; Azizian, A.; et al. Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proc. Natl. Acad. Sci. USA 2018, 115, E5334–E5343. [Google Scholar] [CrossRef] [Green Version]
- Otoukesh, B.; Abbasi, M.; Gorgani, H.O.; Farahini, H.; Moghtadaei, M.; Boddouhi, B.; Kaghazian, P.; Hosseinzadeh, S.; Alaee, A. MicroRNAs signatures, bioinformatics analysis of miRNAs, miRNA mimics and antagonists, and miRNA therapeutics in osteosarcoma. Cancer Cell Int. 2020, 20, 254. [Google Scholar] [CrossRef]
- Iftikhar, H.; Carney, G.E. Evidence and potential in vivo functions for biofluid miRNAs: From expression profiling to functional testing: Potential roles of extracellular miRNAs as indicators of physiological change and as agents of intercellular information exchange. Bioessays 2016, 38, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Q.; Papp, G.; Póliska, S.; Szabó, K.; Tarr, T.; Bálint, B.L.; Szodoray, P.; Zeher, M. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PLoS ONE 2017, 12, e0174585. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis 2020, 41, 666–677. [Google Scholar] [CrossRef]
- Cuscino, N.; Raimondi, L.; De Luca, A.; Carcione, C.; Russelli, G.; Conti, L.; Baldi, J.; Conaldi, P.G.; Giavaresi, G.; Gallo, A. Gathering novel circulating exosomal microRNA in osteosarcoma cell lines and possible implications for the disease. Cancers 2019, 11, 1924. [Google Scholar] [CrossRef] [Green Version]
- Assi, T.; Watson, S.; Samra, B.; Rassy, E.; Le Cesne, A.; Italiano, A.; Mir, O. Targeting the VEGF pathway in osteosarcoma. Cells 2021, 10, 1240. [Google Scholar] [CrossRef]
- Li, Y.S.; Liu, Q.; Tian, J.; He, H.B.; Luo, W. Angiogenesis process in osteosarcoma: An updated perspective of pathophysiology and therapeutics. Am. J. Med. Sci. 2019, 357, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, D.; Sun, Y.; Sun, B.; Wang, G.; Trent, J.C.; Araujo, D.M.; Chen, K.; Zhang, W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer 2011, 117, 4925–4938. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.B. Osteosarcomagenesis: Modeling cancer initiation in the mouse. Sarcoma 2011, 2011, 694136. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004, 9, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.M.; Unni, K.K.; McLeod, R.A.; Pritchard, D.J. Low-grade intraosseous osteosarcoma. Cancer 1990, 65, 1418–1428. [Google Scholar] [CrossRef]
- Roessner, A.; Lohmann, C.; Jechorek, D. Translational cell biology of highly malignant osteosarcoma. Pathol. Int. 2021, 71, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Nguemgo Kouam, P.; Bühler, H.; Hero, T.; Adamietz, I.A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat. Oncol. 2019, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Tsai, H.C.; Chou, P.Y.; Wang, S.W.; Chen, H.T.; Lin, Y.M.; Chiang, I.P.; Chang, T.M.; Hsu, S.K.; Chou, M.C.; et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/VEGF-A axis in human osteosarcoma cells. Oncotarget 2016, 7, 4310–4325. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.H.; Tsai, H.C.; Cheng, Y.C.; Lin, C.Y.; Huang, Y.L.; Tsai, C.H.; Xu, G.H.; Wang, S.W.; Fong, Y.C.; Tang, C.H. CTGF promotes osteosarcoma angiogenesis by regulating miR-543/angiopoietin 2 signaling. Cancer Lett. 2017, 391, 28–37. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, Y.; Ke, Z.; Yang, Z.; Zhou, J.; Huang, X.; Wang, L. Down-regulation of miR-183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am. J. Pathol. 2012, 180, 2440–2451. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, M.; Zhao, G.; Ma, Q.; Ma, B.; Qiu, X.; Fan, Q. miR-183 inhibits the metastasis of osteosarcoma via downregulation of the expression of Ezrin in F5M2 cells. Int. J. Mol. Med. 2012, 30, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Jiang, D.; Gong, F.; Huang, Y.; Luo, Y.; Rong, Y.; Wang, J.; Ge, X.; Ji, C.; Fan, J.; et al. miR-210-5p promotes epithelial-mesenchymal transition by inhibiting PIK3R5 thereby activating oncogenic autophagy in osteosarcoma cells. Cell Death Dis. 2020, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Liu, S.; Wu, B.; Zhao, Y.; Chen, B.; Guo, J.; Qiu, S.; Cao, Y.M. miR-19 Promotes cell proliferation, invasion, migration, and EMT by inhibiting SPRED2-mediated autophagy in osteosarcoma cells. Cell Transplant. 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shao, X.; Li, Y.; Gihu, R.; Xie, H.; Zhou, J.; Yan, H. Targeting signaling pathway networks in several malignant tumors: Progresses and challenges. Front. Pharmacol. 2021, 12, 675675. [Google Scholar] [CrossRef]
- Husmann, K.; Arlt, M.J.; Muff, R.; Langsam, B.; Bertz, J.; Born, W.; Fuchs, B. Matrix Metalloproteinase 1 promotes tumor formation and lung metastasis in an intratibial injection osteosarcoma mouse model. Biochim. Biophys. Acta 2013, 1832, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Yong, B.; Luo, C.; Tan, P.; Peng, T.; Shen, J. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World J. Surg. Oncol. 2012, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Cai, L.; Liu, Z.M.; Zhou, X.S. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac. J. Cancer Prev. 2013, 14, 3681–3684. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Xu, Y.; Sun, X.; Ma, Y.; Zhang, Y.; Wang, Y.; Guan, H.; Jia, Z.; Li, Y. miR-17-5p promotes proliferation and epithelial-mesenchymal transition in human osteosarcoma cells by targeting SRC kinase signaling inhibitor 1. J. Cell Biochem. 2019, 120, 5495–5504. [Google Scholar] [CrossRef] [PubMed]
- Koleckova, M.; Ehrmann, J.; Bouchal, J.; Janikova, M.; Brisudova, A.; Srovnal, J.; Staffova, K.; Svoboda, M.; Slaby, O.; Radova, L.; et al. Epithelial to mesenchymal transition and microRNA expression are associated with spindle and apocrine cell morphology in triple-negative breast cancer. Sci. Rep. 2021, 11, 5145. [Google Scholar] [CrossRef]
- Peng, F.; Fan, H.; Li, S.; Peng, C.; Pan, X. MicroRNAs in epithelial-mesenchymal transition process of cancer: Potential targets for chemotherapy. Int. J. Mol. Sci. 2021, 22, 7526. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Beckford, J.; Alachkar, H. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J. Transl. Med. 2018, 16, 170. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef]
- Liu, L.K.; Jiang, X.Y.; Zhou, X.X.; Wang, D.M.; Song, X.L.; Jiang, H.B. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod. Pathol. 2010, 23, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.I.; Peralta-Leal, A.; O’Valle, F.; Rodriguez-Vargas, J.M.; Gonzalez-Flores, A.; Majuelos-Melguizo, J.; López, L.; Serrano, S.; de Herreros, A.G.; Rodríguez-Manzaneque, J.C.; et al. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013, 9, e1003531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Yustein, J.T.; Xu, J. Research models and mesenchymal/epithelial plasticity of osteosarcoma. Cell Biosci. 2021, 11, 94. [Google Scholar] [CrossRef]
- Sharili, A.S.; Allen, S.; Smith, K.; Price, J.; McGonnell, I.M. Snail2 promotes osteosarcoma cell motility through remodelling of the actin cytoskeleton and regulates tumor development. Cancer Lett. 2013, 333, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, X.; Zhao, Y.; Jin, Y.; Zheng, J. G-protein-coupled estrogen receptor suppresses the migration of osteosarcoma cells via post-translational regulation of Snail. J. Cancer Res. Clin. Oncol. 2019, 145, 87–96. [Google Scholar] [CrossRef]
- Yin, K.; Liao, Q.; He, H.; Zhong, D. Prognostic value of twist and E-cadherin in patients with osteosarcoma. Med. Oncol. 2012, 29, 3449–3455. [Google Scholar] [CrossRef]
- Chandhanayingyong, C.; Kim, Y.; Staples, J.R.; Hahn, C.; Lee, F.Y. MAPK/ERK signaling in osteosarcomas, ewing sarcomas and chondrosarcomas: Therapeutic implications and future directions. Sarcoma 2012, 2012, 404810. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luk, F.; Yang, J.L.; Walsh, W.R. Ras/Raf/MEK/ERK pathway is associated with lung metastasis of osteosarcoma in an orthotopic mouse model. Anticancer Res. 2011, 31, 1147–1152. [Google Scholar] [PubMed]
- Chen, H.J.; Lin, C.M.; Lee, C.Y.; Shih, N.C.; Peng, S.F.; Tsuzuki, M.; Amagaya, S.; Huang, W.W.; Yang, J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. 2013, 30, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.D.; Zhu, B.; Li, S.J.; Yuan, T.; Yang, Q.C.; Fan, C.Y. Down-regulation of RPS9 inhibits osteosarcoma cell growth through inactivation of MAPK signaling pathway. J. Cancer 2017, 8, 2720–2728. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhao, H.; Zhang, L.; Shi, X. Silencing of long-non-coding RNA ANCR suppresses the migration and invasion of osteosarcoma cells by activating the p38MAPK signalling pathway. BMC Cancer 2019, 19, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, K.C.B.; Evangelista, A.F.; Leal, L.F.; Souza, C.P.; Vieira, R.A.; Causin, R.L.; Neuber, A.C.; Pessoa, D.P.; Passos, G.A.S.; Reis, R.M.V.; et al. Identification of cell-free circulating microRNAs for the detection of early breast cancer and molecular subtyping. J. Oncol. 2019, 2019, 8393769. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Hornicek, F.J.; Duan, Z. MicroRNA involvement in osteosarcoma. Sarcoma 2012, 2012, 359739. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondi, L.; Gallo, A.; Cuscino, N.; De Luca, A.; Costa, V.; Carina, V.; Bellavia, D.; Bulati, M.; Alessandro, R.; Fini, M.; et al. Potential Anti-Metastatic Role of the Novel miR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion. Int. J. Mol. Sci. 2022, 23, 705. https://doi.org/10.3390/ijms23020705
Raimondi L, Gallo A, Cuscino N, De Luca A, Costa V, Carina V, Bellavia D, Bulati M, Alessandro R, Fini M, et al. Potential Anti-Metastatic Role of the Novel miR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion. International Journal of Molecular Sciences. 2022; 23(2):705. https://doi.org/10.3390/ijms23020705
Chicago/Turabian StyleRaimondi, Lavinia, Alessia Gallo, Nicola Cuscino, Angela De Luca, Viviana Costa, Valeria Carina, Daniele Bellavia, Matteo Bulati, Riccardo Alessandro, Milena Fini, and et al. 2022. "Potential Anti-Metastatic Role of the Novel miR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion" International Journal of Molecular Sciences 23, no. 2: 705. https://doi.org/10.3390/ijms23020705
APA StyleRaimondi, L., Gallo, A., Cuscino, N., De Luca, A., Costa, V., Carina, V., Bellavia, D., Bulati, M., Alessandro, R., Fini, M., Conaldi, P. G., & Giavaresi, G. (2022). Potential Anti-Metastatic Role of the Novel miR-CT3 in Tumor Angiogenesis and Osteosarcoma Invasion. International Journal of Molecular Sciences, 23(2), 705. https://doi.org/10.3390/ijms23020705








