Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
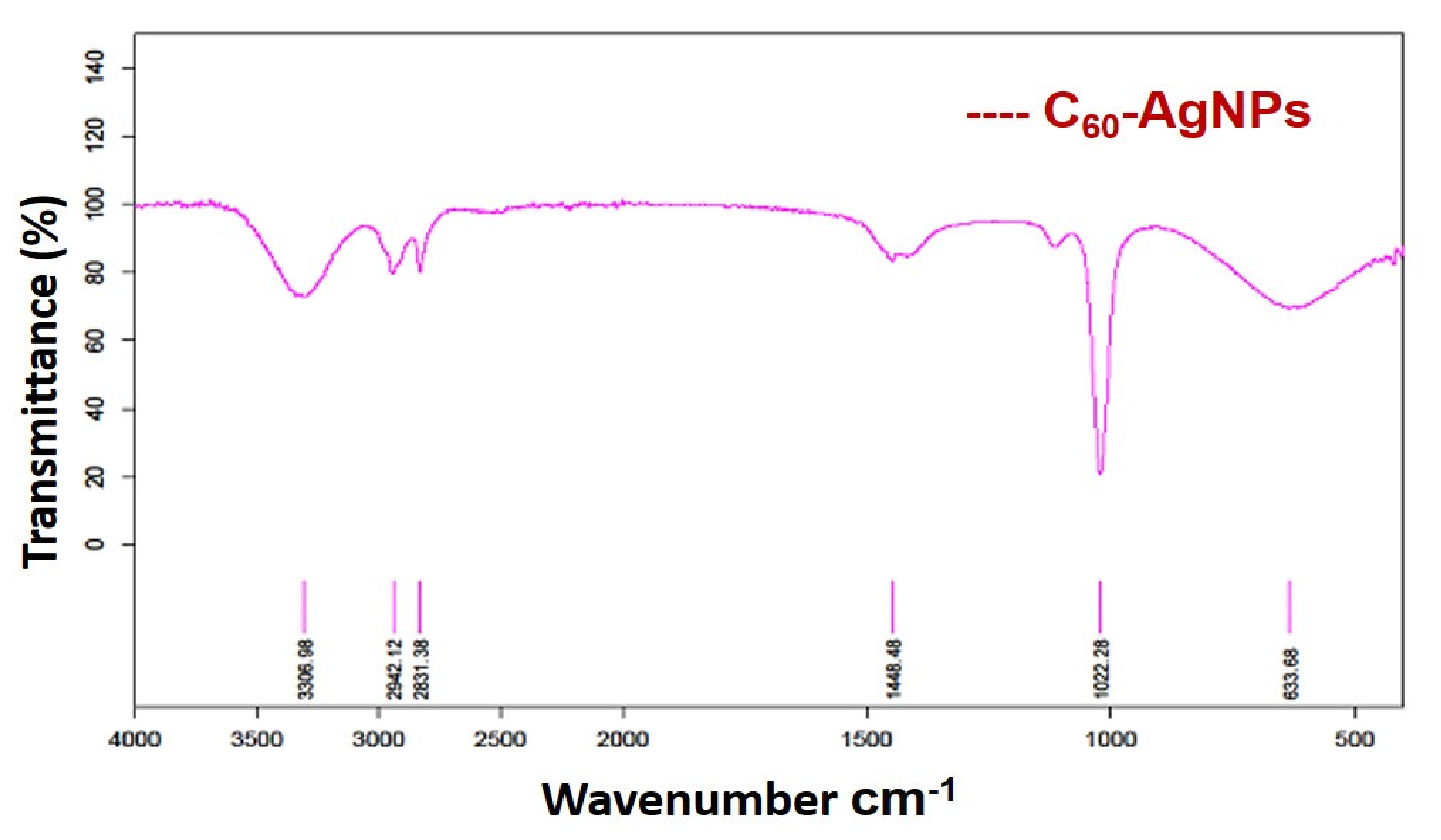
2.2.1. FTIR Analysis
2.2.2. NMR (Nuclear Magnetic Resonance) Analysis
2.2.3. Dynamic Light Scattering (DLS) and Zeta Sizer Analyses
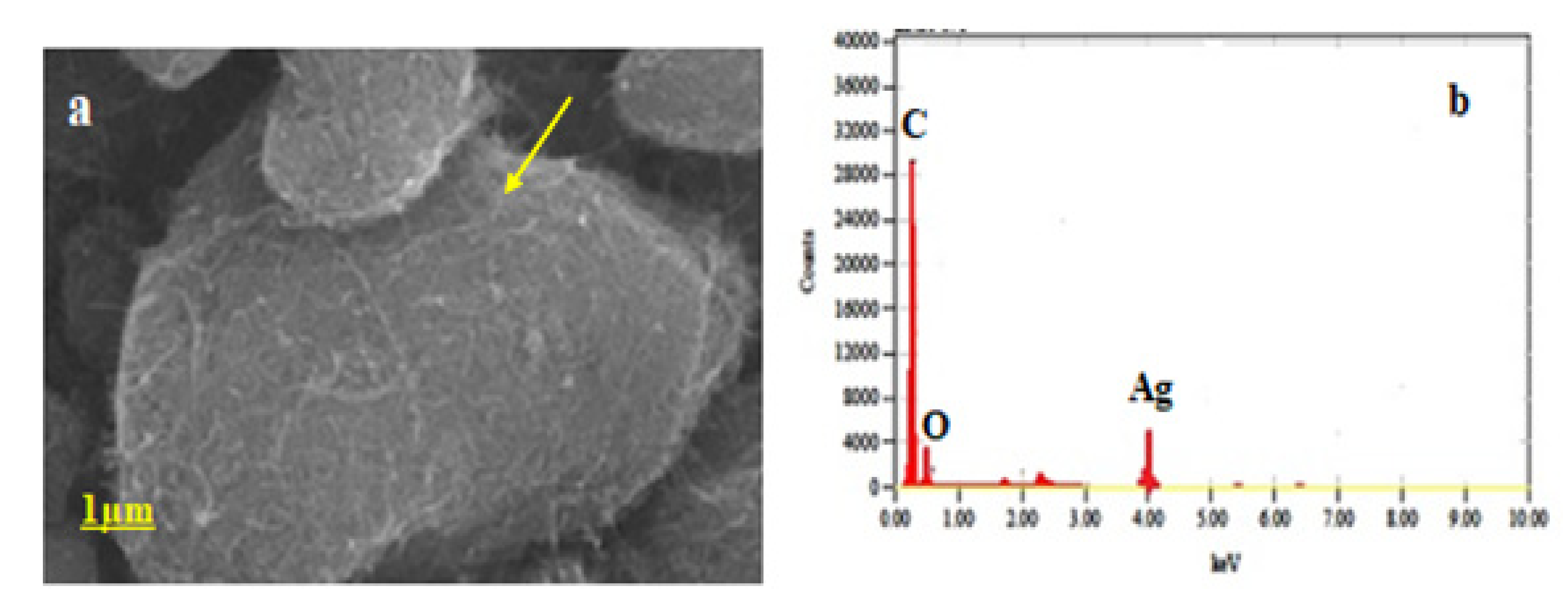
2.2.4. Field Emission-Scanning Electron Microscopy (FE-SEM)
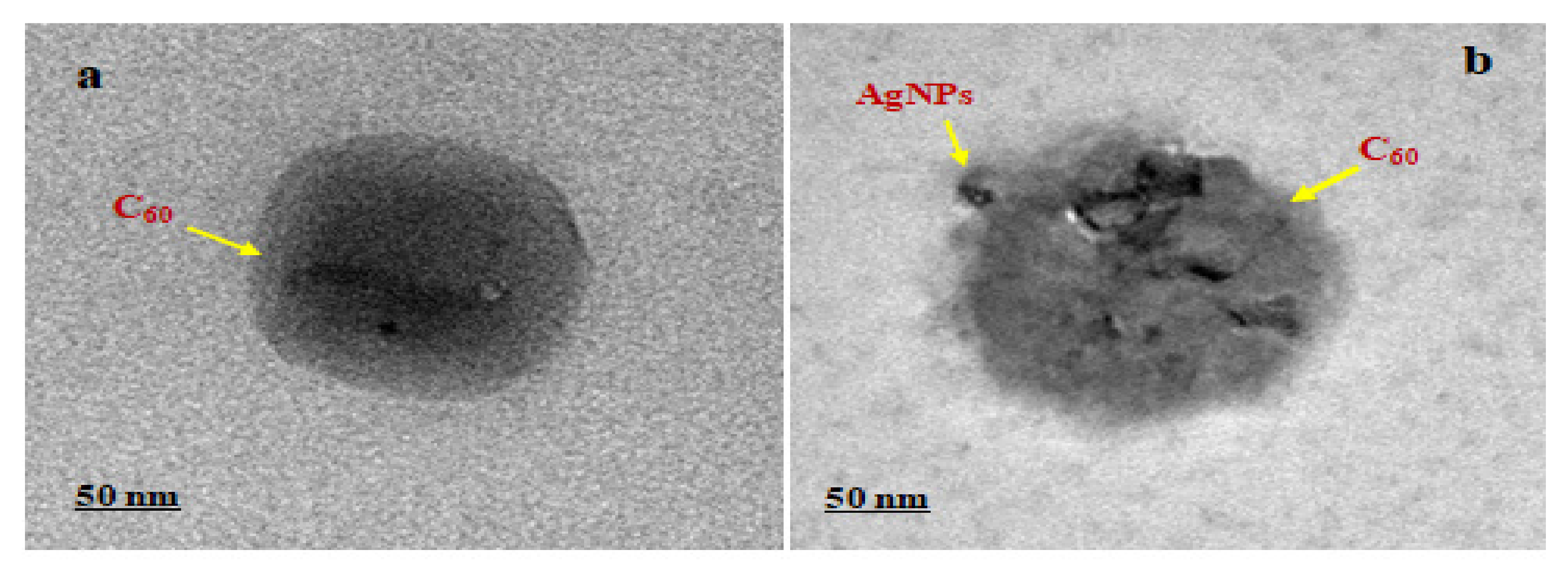
2.2.5. High-Resolution Transmission Electron Microscopy (HR-TEM)
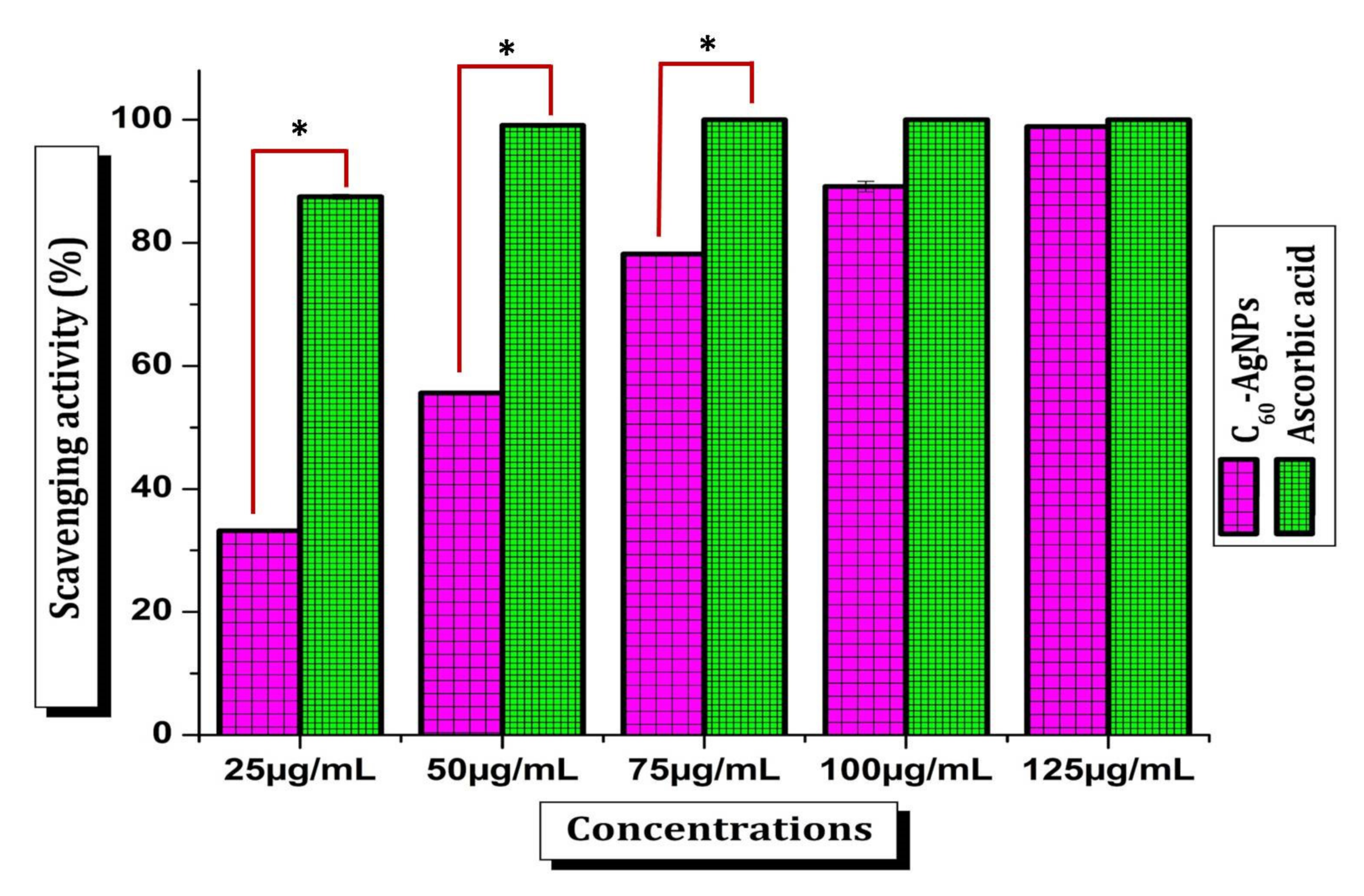
2.2.6. Antioxidant Activity
- AO = absorbance of the control
- A1 = sample absorbance
2.2.7. Antimicrobial Activity
2.2.8. Cytotoxic Activity
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. NMR Spectroscopy
3.3. Field Emission Scanning Electron Microscopy (FE-SEM)
3.4. High-Resolution Transmission Electron Microscopy (HR-TEM)
3.5. DLS and Zeta–Sizer Analyses
3.6. Antioxidant Activity
3.7. Antimicrobial Activity
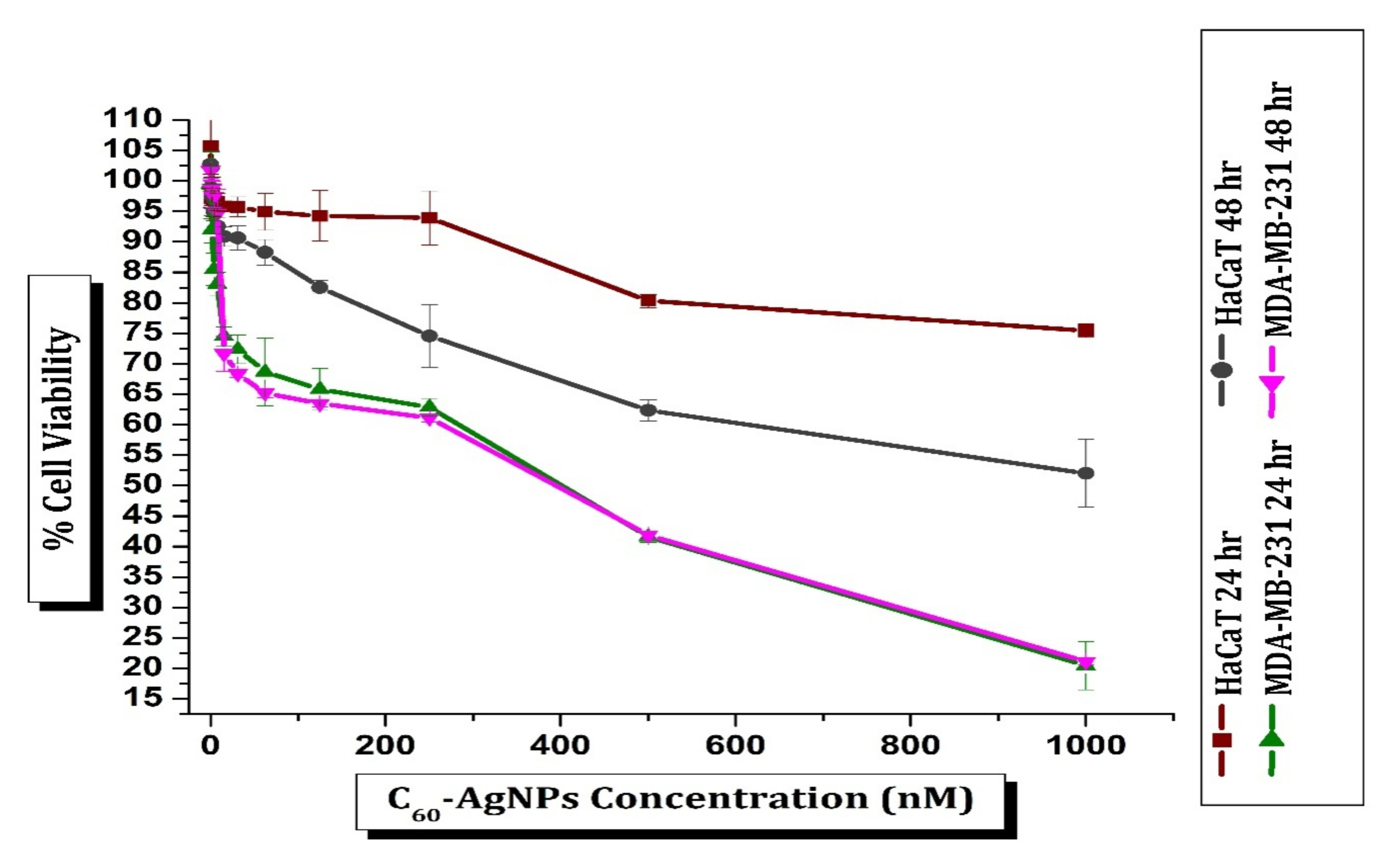
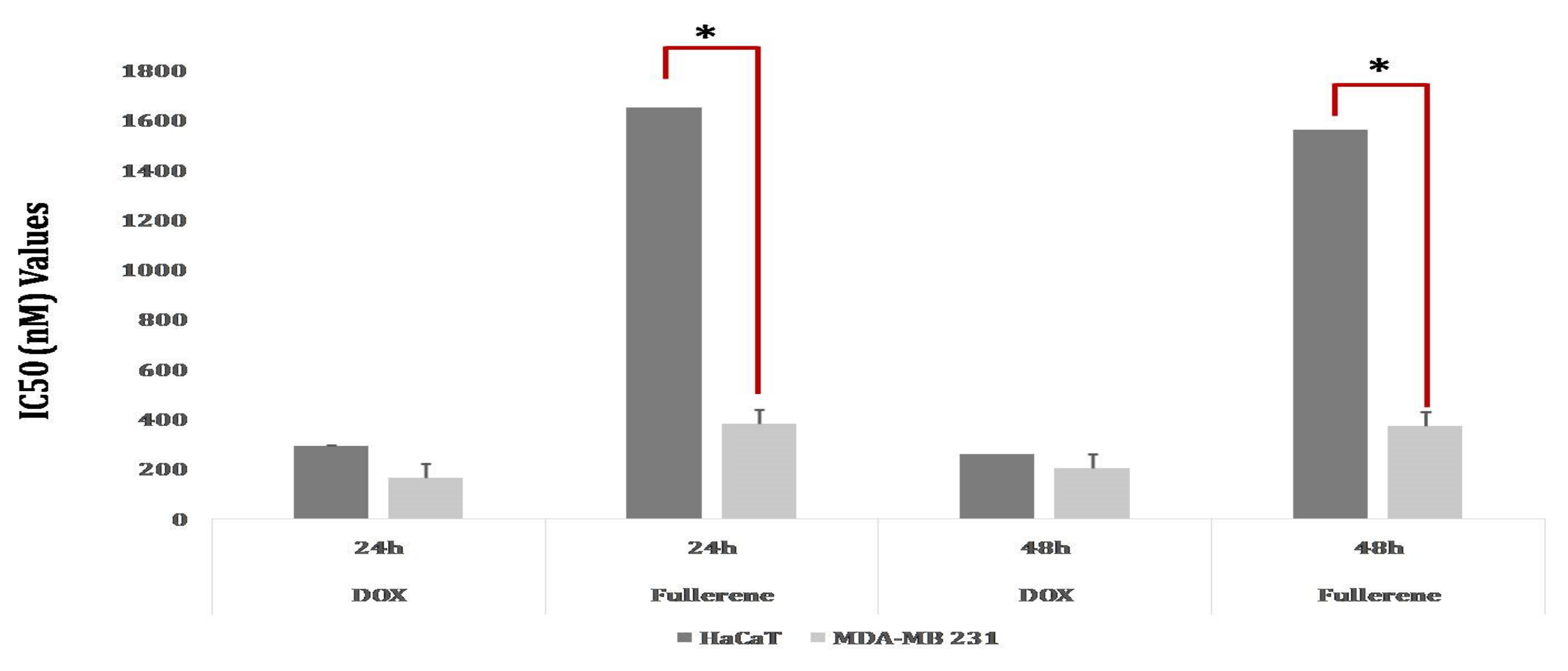
3.8. Cytotoxicity Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Kishore, Y.; Kumar, V.B.; Hashem, A.; Fathi, E.; Allah, A.; Mohanta, D.; Kumar, T. Nutritional assessment study and role of green silver nanoparticles in shelf-life of coconut endosperm to develop as functional food. Saudi J. Biol. Sci. 2020, 27, 1280–1288. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Nguyen, V.-A.; Bui, D.T.; Pham, N.T.; Do, Q.P.; Nguyen, D.T.; Cao, H.H.; Lam, T.D. Graphene Decorated with Silver Nanoparticles as Electrocatalytic Labels in Non-Enzymatic Bisphenol-A Immunosensor. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Chand, K.; Jiao, C.; Lakhan, M.N.; Shah, A.H.; Kumar, V.; Fouad, D.E.; Chandio, M.B.; Maitlo, A.A.; Ahmed, M.; Cao, D. Green synthesis, characterization and photocatalytic activity of silver nanoparticles synthesized with Nigella Sativa seed extract. Chem. Phys. Lett. 2021, 763, 138218. [Google Scholar] [CrossRef]
- Santiago, A.R.P.; Fernandez-Delgado, O.; Gomez, A.; Ahsan, A.; Echegoyen, L. Fullerenes as Key Components for Low-Dimensional (Photo)electrocatalytic Nanohybrid Materials. Angew. Chem. 2021, 133, 124–143. [Google Scholar] [CrossRef]
- Müller, S.; Manger, F.; von Reventlow, L.G.; Colsmann, A.; Wagenknecht, H.-A. Molecular Chromophore-DNA Architectures with Fullerenes: Optical Properties and Solar Cells. Front. Chem. 2021, 9, 78. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, X.; Huang, P.; Fang, P.; Wang, J.; Yang, S.; Wu, K.; Du, P. A supramolecular polymeric heterojunction composed of an all-carbon conjugated polymer and fullerenes. Chem. Sci. 2021, 12, 10506–10513. [Google Scholar] [CrossRef] [PubMed]
- Ibragimov, T.D. Effect of fullerenes C60 on dielectric relaxation, electric conductivity, and electro-optic properties of 4-cyano-4′-pentylbiphenyl. Full-Nanotub. Carbon Nanostructures 2021, 29, 457–463. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Versatile fullerenes as sensor materials. Mater. Today Chem. 2021, 20, 100454. [Google Scholar] [CrossRef]
- Gulumkar, V.; Äärelä, A.; Moisio, O.; Rahkila, J.; Tähtinen, V.; Leimu, L.; Korsoff, N.; Korhonen, H.; Poijärvi-Virta, P.; Mikkola, S.; et al. Controlled Monofunctionalization of Molecular Spherical Nucleic Acids on a Buckminster Fullerene Core. Bioconjug. Chem. 2021, 32, 1130–1138. [Google Scholar] [CrossRef]
- Jaiswal, S.; Tiwari, S.; Bahadur, P.S. Inter-stellar Space Discovery, Spectroscopic Structure identification and Application of C60 Buckminsterfullerene. Int. J. Eng. Res. Gen. Sci. 2021, 9, 12–19. [Google Scholar]
- Thotakura, N.; Raza, K. C60-Fullerenes as an Emerging Cargo Carrier for the Delivery of Anti-Neoplastic Agents: Promises and Challenges. In Micro-and Nanotechnologies-Based Product Development; Mehra, N., Gulbake, A., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2021; pp. 49–56. ISBN 9781003043164. [Google Scholar]
- Barranger, A.; Langan, L.M.; Sharma, V.; Rance, G.A.; Aminot, Y.; Weston, N.J.; Akcha, F.; Moore, M.N.; Arlt, V.M.; Khlobystov, A.N.; et al. Antagonistic Interactions between Benzo[a]pyrene and Fullerene (C60) in Toxicological Response of Marine Mussels. Nanomaterials 2019, 9, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtami, J.; Athira, S.; Mohanan, P. Fullerene C70: A Promising Carbon Cage for Biomedical Applications. Trends Biomater. Artif. Organs 2021, 35, 104–107. [Google Scholar]
- Biby, T.E.; Prajitha, N.; Ashtami, J.; Sakthikumar, D.; Maekawa, T.; Mohanan, P.V. Toxicity of dextran stabilized fullerene C60 against C6 Glial cells. Brain Res. Bull. 2020, 155, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Athira, S.; Biby, E.; Mohanan, P. Dextran stabilized fullerene soot induced toxicity on alveolar epithelial cells (A549 cells). Environ. Res. 2020, 188, 109716. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, Structures and Antioxidant Activities of Fullerenols: Knowledge Learned at the Atomistic Level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and characterization of synthetic melanin-like nanoparticles reinforced chitosan nano-composite films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef]
- Ahmed, E.M. Enhancing thermal, viscoelastic, and optical properties of biodegradable fullerene (C60)/agarose/chitosan composite films for biotechnology. Appl. Phys. A 2021, 127, 481. [Google Scholar] [CrossRef]
- Priya, R.S.; Geetha, D.; Ramesh, P.S. Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs—A comparative study. Ecotoxicol. Environ. Saf. 2016, 134, 308–318. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Bastia, A.K.; Mohanta, T.K. Biosynthesis of Silver Nanoparticles from Protium serratum and Investigation of their Potential Impacts on Food Safety and Control. Front. Microbiol. 2017, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K. Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Rauta, P.R.; Mishra, A.K.; De, D.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; Abd-Allah, E.F.; Mahanta, S.; et al. Development of Graphene Oxide Nanosheets as Potential Biomaterials in Cancer Therapeutics: An In-Vitro Study Against Breast Cancer Cell Line. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4236–4249. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Syed, A.; Ameen, F.; Bastia, A.K.; Mohanta, T.K. Bio-inspired synthesis of silver nanoparticles from leaf extracts of Cleistanthus collinus (Roxb.): Its potential antibacterial and anticancer activities. IET Nanobiotechnol. 2018, 12, 343–348. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A. Sol-gel coating filled with SDS-stabilized fullerene nanoparticles for active corrosion protection of the magnesium alloy. Surf. Coat. Technol. 2021, 419, 127292. [Google Scholar] [CrossRef]
- Branca, C.; Khouzami, K.; Wanderlingh, U.; D’Angelo, G. Effect of intercalated chitosan/clay nanostructures on concentrated pluronic F127 solution: A FTIR-ATR, DSC and rheological study. J. Colloid Interface Sci. 2018, 517, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kuzmany, H.; Winkler, R.; Pichler, T. Infrared spectroscopy of fullerenes. J. Physics Condens. Matter 1995, 7, 6601–6624. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Prudnov, F.A.; Sagdullina, D.K.; Martynov, I.V.; Inasaridze, L.N.; Chernyak, A.V.; Maskaev, A.V.; Kuznetsov, I.E.; Akkuratov, A.V.; Troshin, P.A. Bis(pyrrolidino)[60]fullerenes: Promising photostable fullerene-based acceptors suppressing light-induced absorber degradation pathways. Synth. Met. 2021, 271, 116632. [Google Scholar] [CrossRef]
- Bhattacharjee, R.R.; Dasgupta, U. Seed-mediated synthesis of silver nanoparticles: Tunable surface Plasmon and their facile fabrication. Mater. Today Proc. 2021, 43, 1342–1347. [Google Scholar] [CrossRef]
- Yin, H.; Lin, H.; Zong, Y.; Wang, X.-D. The recent advances in C60 micro/nanostructures and their optoelectronic applications. Org. Electron. 2021, 93, 106142. [Google Scholar] [CrossRef]
- Kim, H.; Ramalingam, M.; Balakumar, V.; Zhang, X.; Gao, W.; Son, Y.-A.; Bradford, P.D. Chemically interconnected ternary AgNP/polypyrrole/functionalized buckypaper composites as high-energy-density supercapacitor electrodes. Chem. Phys. Lett. 2019, 739, 136957. [Google Scholar] [CrossRef]
- Khan, A.; Shkir, M.; Ansari, S.A.; Parveen, N.; AlFaify, S.; El-Toni, A.M.; Gupta, R.K.; Adil, S.F. One-pot flash combustion synthesis of Fe@ NiO nano-composites for supercapacitor applications. Ceram. Int. 2021, 47, 9024–9033. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Zereffa, E.A.; Adimasu, Y. Synthesis and characterization of ZnO/PVA nano-composites for antibacterial and electrochemical applications. Inorg. Nano-Metal Chem. 2021, 51, 1127–1138. [Google Scholar] [CrossRef]
- Mondal, D.; Phukan, G.; Paul, N.; Borah, J. Improved self-heating and optical properties of bifunctional Fe3O4/ZnS nano-composites for magnetic hyperthermia application. J. Magn. Magn. Mater. 2021, 528, 167809. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef]
- Alex, K.V.; Pavai, P.T.; Rugmini, R.; Prasad, M.S.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Tobler, D.J.; Shaw, S.; Benning, L.G. Quantification of initial steps of nucleation and growth of silica nanoparticles: An in-situ SAXS and DLS study. Geochim. Cosmochim. Acta 2009, 73, 5377–5393. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size Dependent and Reactive Oxygen Species Related Nanosilver Toxicity to Nitrifying Bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Wong, K.K.Y.; Ho, C.-M.; Lok, C.-N.; Yu, W.-Y.; Che, C.M.; Chiu, J.-F.; Tam, P.K.H. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem 2006, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Kumar, M.A.P.; Bhushana, N.; Sharma, S. Cinnamon supported facile green reduction of graphene oxide, its dye elimination and antioxidant activities. Mater. Lett. 2015, 151, 93–95. [Google Scholar] [CrossRef]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajeswari, R.; Prabu, H.G. Synthesis Characterization, Antimicrobial, Antioxidant, and Cytotoxic Activities of ZnO Nanorods on Reduced Graphene Oxide. J. Inorg. Organomet. Polym. Mater. 2018, 28, 679–693. [Google Scholar] [CrossRef]
- Elias, L.; Taengua, R.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Carbon Nanomaterials and LED Irradiation as Antibacterial Strategies against Gram-Positive Multidrug-Resistant Pathogens. Int. J. Mol. Sci. 2019, 20, 3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]


| Name of the Test Strain | Mean Zone of Inhibition ± SD (in mm) | Percentage of Inhibition (%) ± SD | MIC IC50 (µg/mL) | ||
|---|---|---|---|---|---|
| C60 | AgNPs | C60-AgNPs | C60-AgNPs | C60-AgNPs | |
| Vibrio cholerae | 12.2 ± 0.08 | 11.4 ± 0.04 | 13.10 ± 0.04 | 84.97 ± 0.68 | 105.44 ± 3.67 |
| Shigelladysenteriae | 13.3 ± 0.00 | 16.3 ± 0.00 | 21.20 ± 0.00 | 99.30 ± 0.37 | 52.72 ± 0.27 |
| Salmonella typhimurium | 12.6 ± 0.00 | 13.1 ± 0.00 | 17.60 ± 0.00 | 92.43 ± 0.09 | 90.10 ± 0.35 |
| Escherichia coli | 13.33 ± 0.05 | 15.23 ± 0.05 | 18.67 ± 0.05 | 93.37 ± 0.76 | 79.54 ± 0.51 |
| Staphylococcus aureus | 11.57 ± 0.05 | 13.47 ± 0.05 | 14.87 ± 0.05 | 86.60 ± 0.73 | 91.15 ± 0.77 |
| Staphylococcus epidermidis | 12.57 ± 0.00 | 13.10 ± 0.00 | 15.23 ± 0.05 | 92.03 ± 0.48 | 84.97 ± 0.68 |
| Bacillus licheniformis | 13.37 ± 0.05 | 14.40 ± 0.00 | 16.20 ± 0.00 | 92.80 ± 0.57 | 81.99 ± 1.37 |
| Candida albicans | 12.60 ± 0.00 | 13.80 ± 0.00 | 17.50 ± 0.00 | 94.90 ± 0.54 | 76.61 ± 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, K.; Mishra, A.K.; Rauta, P.R.; Al-Sehemi, A.G.; Pannipara, M.; Sett, A.; Bratovcic, A.; Avula, S.K.; Mohanta, T.K.; Saravanan, M.; et al. Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections. Int. J. Mol. Sci. 2022, 23, 714. https://doi.org/10.3390/ijms23020714
Biswas K, Mishra AK, Rauta PR, Al-Sehemi AG, Pannipara M, Sett A, Bratovcic A, Avula SK, Mohanta TK, Saravanan M, et al. Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections. International Journal of Molecular Sciences. 2022; 23(2):714. https://doi.org/10.3390/ijms23020714
Chicago/Turabian StyleBiswas, Kunal, Awdhesh Kumar Mishra, Pradipta Ranjan Rauta, Abdullah G. Al-Sehemi, Mehboobali Pannipara, Avik Sett, Amra Bratovcic, Satya Kumar Avula, Tapan Kumar Mohanta, Muthupandian Saravanan, and et al. 2022. "Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections" International Journal of Molecular Sciences 23, no. 2: 714. https://doi.org/10.3390/ijms23020714
APA StyleBiswas, K., Mishra, A. K., Rauta, P. R., Al-Sehemi, A. G., Pannipara, M., Sett, A., Bratovcic, A., Avula, S. K., Mohanta, T. K., Saravanan, M., & Mohanta, Y. K. (2022). Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections. International Journal of Molecular Sciences, 23(2), 714. https://doi.org/10.3390/ijms23020714











