Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives
Abstract
:1. Introduction
1.1. GSH Synthesis and Relationship to GSH-Derivatives
1.2. CaSR Function in Parathyroid, Kidney, and Other Tissues
1.3. Amino Acid and Peptide Modulation of CaSR Activity
2. CaSR Structure, including Ca, Calcimimetic, Calcilytic, and Peptide Binding Sites
3. Associations of Ca with Glutathionergic Metabolism
4. Proposed Role of Glutathionergics in Regulating CaSR Function
5. Medical Implications
6. Conclusions
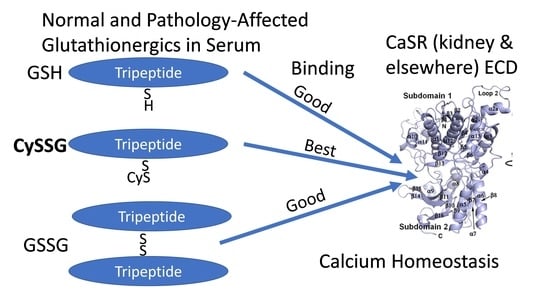
- Glutathionergic compounds can produce biological responses through receptor binding mechanisms on CaSR. Most discussions of the roles of GSH and its derivatives focus almost exclusively on their functions in regulating the redox states of cells and in detoxifying chemicals (e.g., Lu [92]). The idea that glutathionergics may also function as ligands that bind to receptors such as CaSR via structures that are not necessarily affected by their redox state broadens the types of roles that they can have. Furthermore, receptor function involves reversible binding, in contrast to covalent detoxification mechanisms.
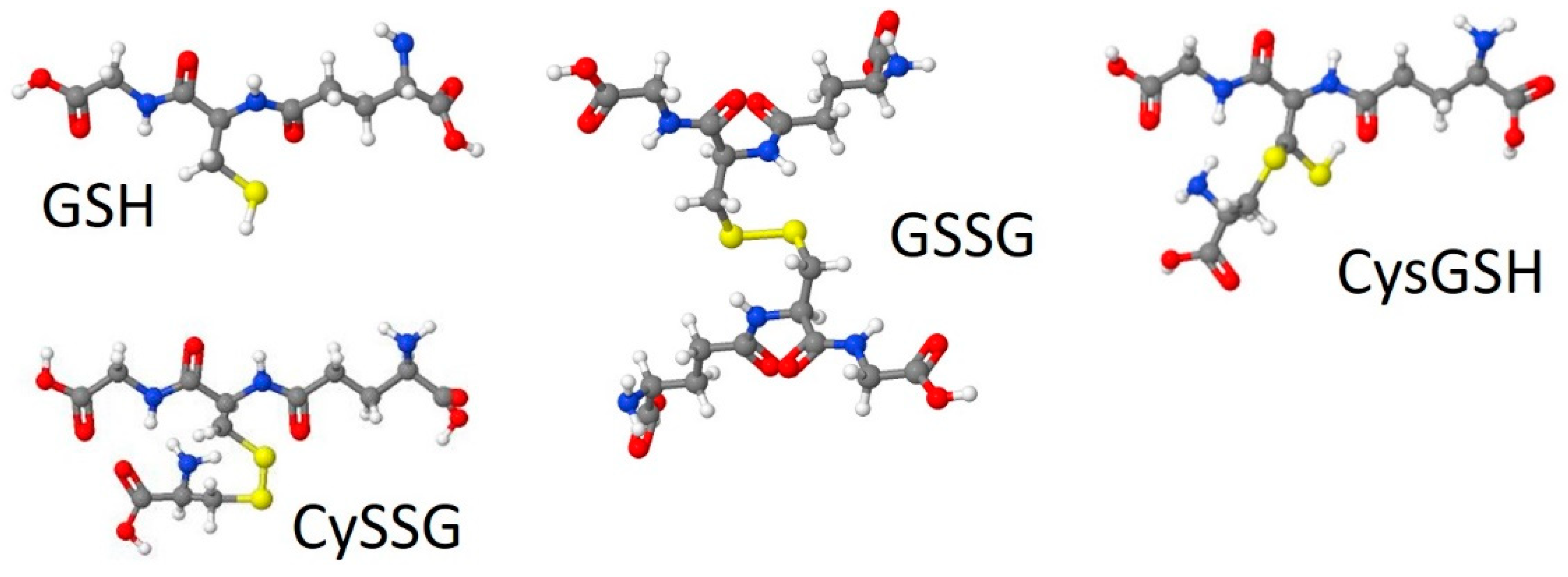
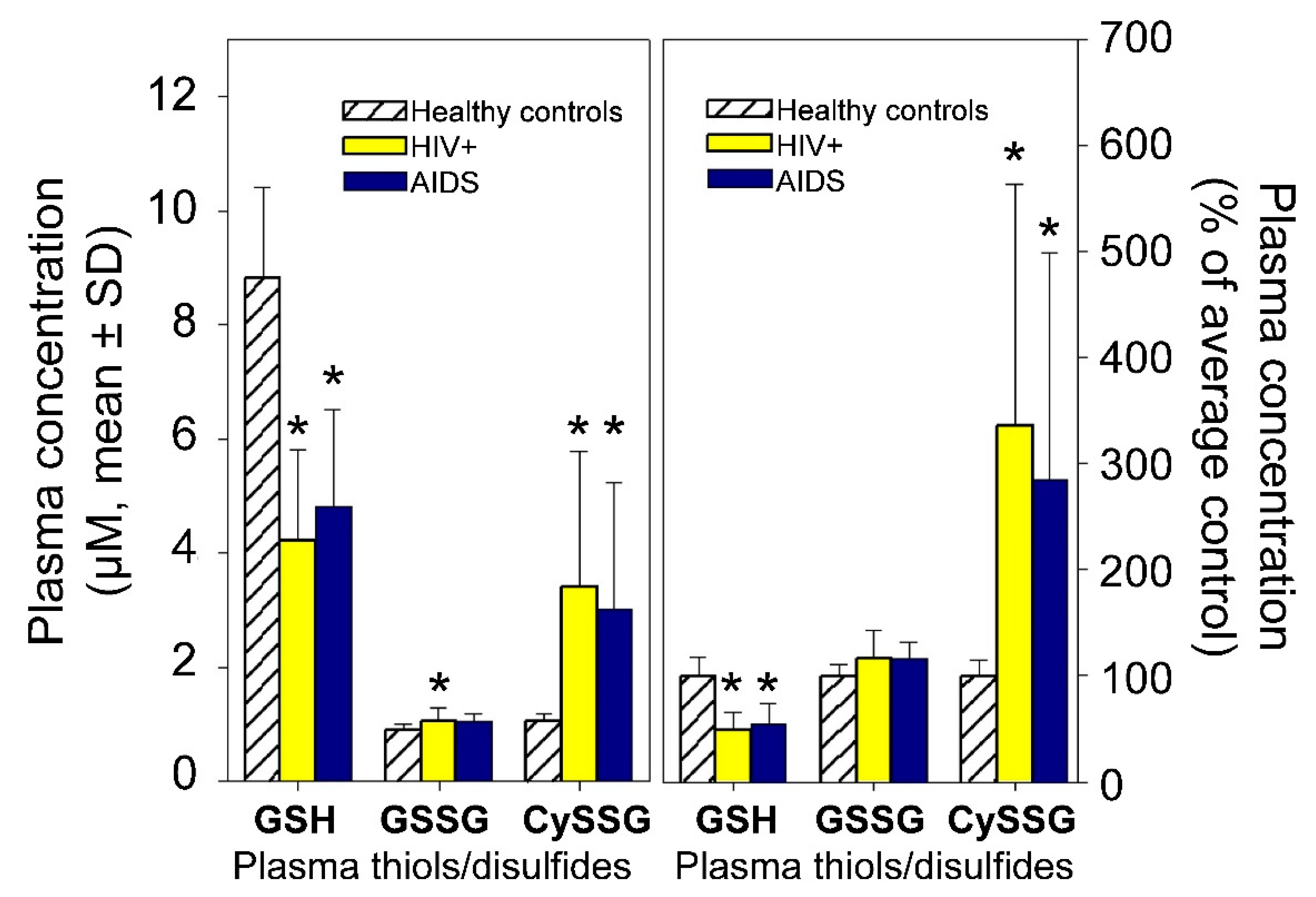
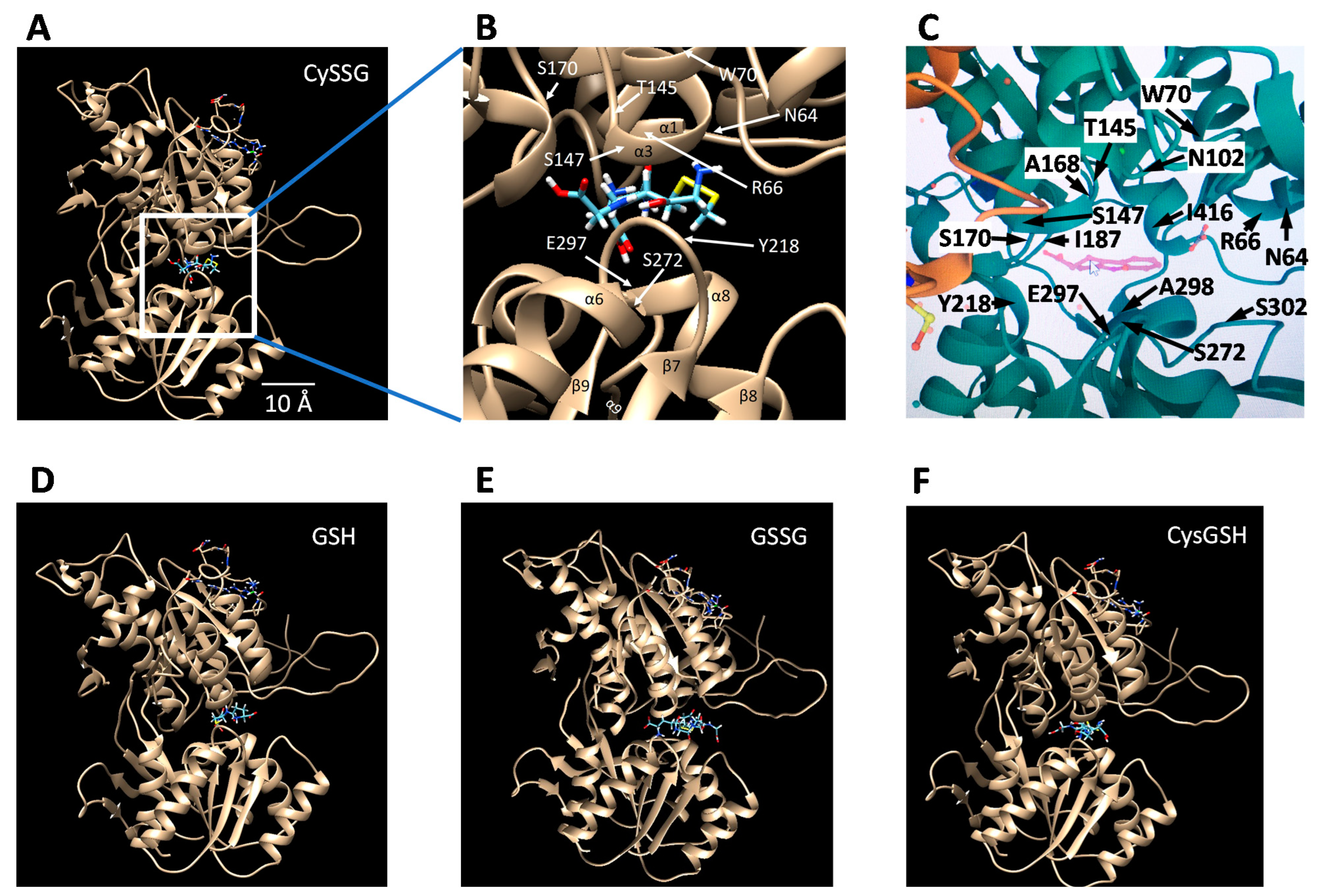
- A role for plasma CySSG. CySSG is present in plasma at concentrations greater than GSSG and nearly as high as GSH; in some medical conditions (e.g., HIV/AIDS) the ratio of CySSG to GSH changes as much as 5-fold. The higher affinity calculated in this paper for CySSG binding to CaSR, compared to GSH and GSSG, suggests that CySSG may even be the preferred ligand at the peptide binding site on CaSR. However, the presence and possible functions of CySSG have gone largely uncommented upon, even in the publications with the most extensive human plasma measurements of CySSG [10,15], despite CySSG-specific significant changes and correlations in the texts of the papers. CySSG is known to stimulate an important receptor-mediated response in invertebrate reproduction [19]. The proposal here is that CySSG has important functions in mammalian Ca homeostasis.
- Glutathionergics may be lead compounds for new regulators of CaSR activity for clinical treatments. As noted above, the presence of a cysteine residue attached to a peptide backbone, found in CySSG, CysGSH, and etelcalcetide, may point the way to further development of compounds that sensitize or antagonize CaSR through actions in the amino acid/peptide binding pocket of the CaSR ECD. A variety of disease conditions associated with changes in CaSR activity and affected by GSH and its derivatives may be subject to treatment with new drugs derived from this understanding.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.H.; Yao, Y.; Kuang, D.H.; Hampson, D.R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 2006, 281, 8864–8870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Robertson, M.J.; Rahman, S.N.; Seven, A.B.; Zhang, C.S.; Meyerowitz, J.G.; Panova, O.; Hannan, F.M.; Thakker, R.V.; Brauner-Osborne, H.; et al. Asymmetric activation of the calcium-sensing receptor homodimer. Nature 2021, 595, 455–459. [Google Scholar] [CrossRef]
- Geng, Y.; Mosyak, L.; Kurinov, I.; Zuo, H.; Sturchler, E.; Cheng, T.C.; Subramanyam, P.; Brown, A.P.; Brennan, S.C.; Mun, H.-C.; et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 2016, 5, e13662. [Google Scholar] [CrossRef]
- Ling, S.; Shi, P.; Liu, S.; Meng, X.; Zhou, Y.; Sun, W.; Chang, S.; Zhang, X.; Zhang, L.; Shi, C.; et al. Structural mechanism of cooperative activation of the human calcium-sensing receptor by Ca2+ ions and L-tryptophan. Cell Res. 2021, 31, 383–394. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Zou, J.; Miller, C.L.; Gorkhali, R.; Yang, J.-Y.; Schilmiller, A.; Wang, S.; Huang, K.; Brown, E.M.; et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2016, 2, e1600241. [Google Scholar] [CrossRef] [Green Version]
- Jocelyn, P.C. The standard redox potential of cysteine-cystine from the thiol-disulfide exchange reaction with glutathione and lipoic acid. Eur. J. Biochem. 1967, 2, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.A.; Mannervik, B. The reduction of the L-cysteine-glutathione mixed disulfide in rat liver. Involvement of an enzyme catalyzing thiol-disulfide interchange. FEBS Lett. 1970, 7, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Ormstad, K.; Jones, D.P.; Orrenius, S. Characteristics of glutathione biosynthesis by freshly isolated rat-kidney cells. J. Biol. Chem. 1980, 255, 175–181. [Google Scholar] [CrossRef]
- Reed, D.J.; Ellis, W.W.; Meck, R.A. The inhibition of gamma-glutamyl-transferase transpeptidase and glutathione metabolism of isolated rat-kidney cells by L-(alpha-S,5s)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125, NSC-163501). Biochem. Biophys. Res. Commun. 1980, 94, 1273–1277. [Google Scholar] [CrossRef]
- Jones, D.P.; Mody, V.C.; Carlson, J.L.; Lynn, M.J.; Sternberg, P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free. Radic. Biol. Med. 2002, 33, 1290–1300. [Google Scholar] [CrossRef]
- Kleinman, W.A.; Richie, J.P. Status of glutathione and other thiols and disulfides in human plasma. Biochem. Pharmacol. 2000, 60, 19–29. [Google Scholar] [CrossRef]
- Lash, L.H.; Jones, D.P. Distribution of oxidized and reduced forms of glutathione and cysteine in rat plasma. Arch. Biochem. Biophys. 1985, 240, 583–592. [Google Scholar] [CrossRef]
- Ookhtens, M.; Mittur, A.V.; Erhart, N.A. Changes in plasma glutathione concentrations, turnover, and disposal in developing rats. Am. J. Physiol. 1994, 266, R979–R988. [Google Scholar] [CrossRef]
- Stein, A.F.; Dills, R.L.; Klaassen, C.D. High-performance liquid-chromatographic analysis of glutathione and its thiol and disulfide degradation products. J. Chromatogr. 1986, 381, 259–270. [Google Scholar] [CrossRef]
- Walmsley, S.L.; Winn, L.M.; Harrison, M.L.; Uetrecht, J.P.; Wells, P.G. Oxidative stress and thiol depletion in plasma and peripheral blood lymphocytes from HIV-infected patients: Toxicological and pathological implications. Aids 1997, 11, 1689–1697. [Google Scholar] [CrossRef]
- Hardege, J.D.; Müller, C.T.; Beckmann, M. A waterborne female sex pheromone in the ragworm Nereis succinia (Annelida, Polychaeta). Polych. Res. 1997, 17, 18–21. [Google Scholar]
- Ram, J.L.; Fei, X.; Danaher, S.M.; Lu, S.; Breithaupt, T.; Hardege, J.D. Finding females: Pheromone-guided reproductive tracking behavior by male Nereis succinea in the marine environment. J. Exp. Biol. 2008, 211, 757–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, J.L.; Hardege, J.D. Nereis succinea nuptial behavior: Does size matter? Invertebr. Reprod. Dev. 2005, 48, 89–94. [Google Scholar] [CrossRef]
- Ram, J.L.; Müller, C.T.; Beckmann, M.; Hardege, J.D. The spawning pheromone cysteine-glutathione disulfide (‘nereithiones’) arouses a multicomponent nuptial behaviour and electrophysiological activity in Nereis succinea males. FASEB J. 1999, 13, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef]
- Ward, D.T. Calcium receptor-mediated intracellular signalling. Cell Calcium 2004, 35, 217–228. [Google Scholar] [CrossRef]
- Conigrave, A.D.; Mun, H.C.; Lok, H.C. Aromatic L-amino acids activate the calcium-sensing receptor. J. Nutr. 2007, 137, 1524S–1527S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, D.; Valenti, G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016, 12, 414–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, P.A. VAMP-2/3 mediates cAMP-induced translocation of NKCC2 to the apical membrane of the thick ascending limb. J. Am. Soc. Nephrol. 2003, 14, 9A. [Google Scholar]
- Ortiz, P.A. cAMP stimulates NaCl absorption by increasing NKCC2 trafficking to the apical membrane of thick ascending limbs: Role of VAMP-2/3. Hypertension 2004, 44, 500. [Google Scholar]
- Ortiz, P.A. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: Role of VAMP. Am. J. Physiol.-Renal Physiol. 2006, 290, F608–F616. [Google Scholar] [CrossRef]
- Wang, W.H.; Lu, M. Effect of arachidonic-acid on activity of the apical K+ channel in the thick ascending limb of the rat-kidney. J. Gen. Physiol. 1995, 106, 727–743. [Google Scholar] [CrossRef]
- Wang, W.H.; Lu, M.; Hebert, S.C. Cytochrome P-450 metabolites mediate extracellular Ca2+-induced inhibition of apical K+ channels in the TAL. Am. J. Physiol.-Cell Physiol. 1996, 271, C103–C111. [Google Scholar] [CrossRef]
- Wang, D.R.; An, S.J.; Wang, W.H.; McGiff, J.C.; Ferreri, N.R. CaR-mediated COX-2 expression in primary cultured mTAL cells. Am. J. Physiol.-Renal Physiol. 2001, 281, F658–F664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranieri, M.; Di Mise, A.; Centrone, M.; D’Agostino, M.; Tingskov, S.J.; Venneri, M.; Pellegrino, T.; Difonzo, G.; Caponio, F.; Norregaard, R.; et al. Olive Leaf Extract (OLE) impaired vasopressin-induced aquaporin-2 trafficking through the activation of the calcium-sensing receptor. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Atchison, D.K.; Beierwaltes, W.H. The influence of extracellular and intracellular calcium on the secretion of renin. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Hou, J. Claudin-14 Underlies Ca++-Sensing Receptor–Mediated Ca++Metabolism via NFAT-microRNA–Based Mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.M.; MacLeod, R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001, 81, 239–297. [Google Scholar] [CrossRef]
- Canaff, L.; Petit, J.L.; Kisiel, M.; Watson, P.H.; Gascon-Barre, M.; Hendy, G.N. Extracellular calcium-sensing receptor is expressed in rat hepatocytes—Coupling to intracellular calcium mobilization and stimulation of bile flow. J. Biol. Chem. 2001, 276, 4070–4079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.J.; Yang, X.; He, J.L.; Liu, J.J.; Yang, S.M.; Dong, H. Important roles of the Ca2+ -sensing receptor in vascular health and disease. Life Sci. 2018, 209, 217–227. [Google Scholar] [CrossRef]
- Li, G.-W.; Wang, Q.-S.; Hao, J.-H.; Xing, W.-J.; Guo, J.; Li, H.-Z.; Bai, S.-Z.; Li, H.-X.; Zhang, W.-H.; Yang, B.-F.; et al. The functional expression of extracellular calcium-sensing receptor in rat pulmonary artery smooth muscle cells. J. Biomed. Sci. 2011, 18, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitsou-Mylona, I.; Burns, C.J.; Squires, P.E.; Persaud, S.J.; Jones, P.M. A Role for the Extracellular Calcium-Sensing Receptor in Cell-Cell Communication in Pancreatic Islets of Langerhans. Cell. Physiol. Biochem. 2008, 22, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Squires, P.E.; Harris, T.E.; Persaud, S.J.; Curtis, S.B.; Buchan, A.M.; Jones, P.M. The extracellular calcium-sensing receptor on human beta-cells negatively modulates insulin secretion. Diabetes 2000, 49, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Amino, Y.; Wakabayashi, H.; Akashi, S.; Ishiwatari, Y. Structural analysis and taste evaluation of γ-glutamyl peptides comprising sulfur-containing amino acids. Biosci. Biotechnol. Biochem. 2018, 82, 383–394. [Google Scholar] [CrossRef]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the Calcium-sensing Receptor in Human Taste Perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Laffitte, A.; Gibbs, M.; de Alvaro, C.H.; Addison, J.; Lonsdale, Z.N.; Giribaldi, M.G.; Rossignoli, A.; Vennegeerts, T.; Winnig, M.; Klebansky, B.; et al. Kokumi taste perception is functional in a model carnivore, the domestic cat (Felis catus). Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, A.D.; Quinn, S.J.; Brown, E.M. L-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4814–4819. [Google Scholar] [CrossRef] [Green Version]
- Wellendorph, P.; Brauner-Osborne, H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br. J. Pharmacol. 2009, 156, 869–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.K.; Yi, P.; Zhao, W.J.; Wu, Y.L.; Acher, F.; Pin, J.P.; Liu, J.F.; Rondard, P. Illuminating the allosteric modulation of the calcium-sensing receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 21711–21722. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Ding, Z.; Cui, Q.; Han, L.; Kou, Y.; Zhang, W.; Wang, H.; Jia, X.; Dai, M.; et al. Structural insights into the activation of human calcium sensing receptor. eLife 2021, 10, e68578. [Google Scholar] [CrossRef]
- Akizawa, T.; Ikejiri, K.; Kondo, Y.; Endo, Y.; Fukagawa, M. Evocalcet: A New Oral Calcimimetic for Dialysis Patients with Secondary Hyperparathyroidism. Ther. Apher. Dial. 2020, 24, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.T.; Riccardi, D. New concepts in calcium-sensing receptor pharmacology and signalling. Br. J. Pharmacol. 2012, 165, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrel, C.; Kessler, A.; Dauban, P.; Dodd, R.H.; Rognan, D.; Ruat, M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J. Biol. Chem. 2004, 279, 18990–18997. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.L.; Wang, Z.Y.; Chen, X.Z.; Ren, Y.; Lu, X.H.; Xing, Y.F.; Lu, J.; Chang, S.H.; Zhang, X.; Shen, Y.Q.; et al. Structural basis for activation and allosteric modulation of full-length calcium-sensing receptor. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Gavai, A.V.; Vaz, R.J.; Mikkilineni, A.B.; Roberge, J.Y.; Liu, Y.L.; Lawrence, R.M.; Corte, J.R.; Yang, W.; Bednarz, M.; Dickson, J.K.; et al. Discovery of novel 1-arylmethyl pyrrolidin-2-yl ethanol amines as calcium-sensing receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 5478–5482. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.F.; Roberge, J.Y.; Ma, Z.P.; Liu, Y.L.; Lawrence, R.M.; Rotella, D.P.; Seethala, R.; Feyen, J.H.M.; Dickson, J.K. Discovery and structure-activity relationships of 2-benzylpyrrolidine-substituted aryloxypropanols as calcium-sensing receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 1225–1228. [Google Scholar] [CrossRef]
- Yarova, P.L.; Huang, P.; Schepelmann, M.W.; Bruce, R.; Ecker, R.; Nica, R.; Telezhkin, V.; Traini, D.; Dos Reis, L.G.; Kidd, E.J.; et al. Characterization of Negative Allosteric Modulators of the Calcium-Sensing Receptor for Repurposing as a Treatment of Asthma. J. Pharmacol. Exp. Ther. 2021, 376, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Fujioka, A.; Konno, M.; Inoue, A.; Yamada, H.; Hirota, Y. Pharmacology of Parsabiv® (etelcalcetide, ONO-5163/AMG 416), a novel allosteric modulator of the calcium-sensing receptor, for secondary hyperparathyroidism in hemodialysis patients. Eur. J. Pharmacol. 2019, 842, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.T.; Hunter, T.; Walter, S.; Dong, J.; Maclean, D.; Baruch, A.; Subramanian, R.; Tomlinson, J.E. Critical Cysteine Residues in Both the Calcium-Sensing Receptor and the Allosteric Activator AMG 416 Underlie the Mechanism of Action. Mol. Pharmacol. 2015, 88, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Miller, C.L.; Gorkhali, R.; Zou, J.; Huang, K.; Brown, E.M.; Yang, J.J. Molecular Basis of the Extracellular Ligands Mediated Signaling by the Calcium Sensing Receptor. Front. Physiol. 2016, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera: A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teshima, K.; Yamamoto, A.; Yamaoka, K.; Honda, Y.; Honda, S.; Sasaki, T.; Kojima, S. Involvement of calcium ion in elevation of mRNA for gamma-glutamylcysteine synthetase (gamma-GCS) induced by low-dose gamma-rays. Int. J. Radiat. Biol. 2000, 76, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.B.; Zhang, Q.; Woods, C.G.; Wong, V.; Collins, S.; Andersen, M.E. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol. Appl. Pharmacol. 2008, 226, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takagi, Y.; Osanai, H.; Li, L.; Takeuchi, M.; Katoh, Y.; Kobayashi, M.; Yamamoto, M. Pi class glutathione S-transferase genes are regulated by Nrf 2 through an evolutionarily conserved regulatory element in zebrafish. Biochem. J. 2005, 388, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, J.B.; Bai, Y.S.; Reece, J.M.; Williams, J.; Liu, D.X.; Freeman, M.L.; Fahl, W.E.; Shugar, D.; Liu, J.; Qu, W.; et al. Molecular mechanism of human Nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 2007, 42, 1797–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.H.; Rong, X.F.; Li, D.; Cai, L.; Rao, J.; Lu, Y. Inhibition of Cartilage Acidic Protein 1 Reduces Ultraviolet B Irradiation Induced-Apoptosis through P38 Mitogen-Activated Protein Kinase and Jun Amino-Terminal Kinase Pathways. Cell. Physiol. Biochem. 2016, 39, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Lauterburg, B.H.; Adams, J.D.; Mitchell, J.R. Hepatic Glutathione Homeostasis in the Rat: Efflux Accounts for Glutathione Turnover. Hepatology 1984, 4, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, G.A.; Fariss, M.W.; Olafsdottir, K.; Reed, D.J. A role of vitamin E in protection against cell injury—Maintenance of intracellular glutathione precursors and biosynthesis. Eur. J. Biochem. 1987, 166, 241–247. [Google Scholar] [CrossRef]
- Abdel-Magied, N.; Elkady, A.A.; Abdel Fattah, S.M. Effect of Low-Level Laser on Some Metals Related to Redox State and Histological Alterations in the Liver and Kidney of Irradiated Rats. Biol. Trace Elem. Res. 2020, 194, 410–422. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States. 2021. Available online: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (accessed on 21 August 2021).
- US Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Executive Summary; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019. Available online: https://www.usrds.org/media/2371/2019-executive-summary.pdf (accessed on 21 August 2021).
- Poon, G. Cinacalcet hydrochloride (Sensipar). Bayl. Univ. Med. Cent. 2005, 18, 181–184. [Google Scholar] [CrossRef]
- Trivedi, R.; Mithal, A.; Chattopadhyay, N. Recent updates on the calcium-sensing receptor as a drug target. Curr. Med. Chem. 2008, 15, 178–186. [Google Scholar] [CrossRef]
- Stollenwerk, B.; Iannazzo, S.; Cooper, K.; Belozeroff, V. Exploring the potential value of improved care for secondary hyperparathyroidism with a novel calcimimetic therapy. J. Med. Econ. 2017, 20, 1110–1115. [Google Scholar] [CrossRef]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; Kewalramani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism. JAMA 2017, 317, 156. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Ottlewski, I.; Shehzad, W.; Riaz, A.; Ijaz, S.; Tufail, A.; Ammara, H.; Mane, S.; Shril, S.; Hildebrandt, F.; et al. Sequencing the CaSR locus in Pakistani stone formers reveals a novel loss-of-function variant atypically associated with nephrolithiasis. BMC Med. Genom. 2021, 14. [Google Scholar] [CrossRef]
- Gonzalez, C.; Ariceta, G.; Langman, C.B.; Zibaoui, P.; Escalona, L.; Dominguez, L.F.; Rosas, M.A. Hypercalciuria is the main renal abnormality finding in Human Immunodeficiency Virus-infected children in Venezuela. Eur. J. Pediatrics 2008, 167, 509–515. [Google Scholar] [CrossRef]
- Teichmann, J.; Stephan, E.; Lange, U.; Discher, T.; Friese, G.; Lohmeyer, J.; Stracke, H.; Bretzel, R.G. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J. Infect. 2003, 46, 221–227. [Google Scholar] [CrossRef]
- Koide, S.; Kugiyama, K.; Sugiyama, S.; Nakamura, S.; Fukushima, H.; Honda, O.; Yoshimura, M.; Ogawa, H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasornotor dysfunction and myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Andrews, N.P.; Padder, F.A.; Husain, M.; Quyyumi, A.A. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J. Am. Coll. Cardiol. 1999, 34, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Romani, R.B.; Raqeeb, A.; Laforenza, U.; Scaffino, M.F.; Moccia, F.; Avelino-Cruz, J.E.; Oldani, A.; Coltrini, D.; Milesi, V.; Taglietti, V.; et al. Cardiac Microvascular Endothelial Cells Express a Functional Ca2+-Sensing Receptor. J. Vasc. Res. 2009, 46, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T. The calcium-sensing receptor in bone. J. Bone Miner. Metab. 2008, 26, 301–311. [Google Scholar] [CrossRef]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Hajimahmoodi, M.; Jamshidi, A.R.; Sajadian, Z. Determination of plasma gluthatione reductase enzyme activity in osteoporotic women. Daru 2008, 16, 51–54. [Google Scholar]
- Zhou, M.Y.; Cheng, L.; Chen, L.; Gu, Y.J.; Wang, Y. Calcium-sensing receptor in the development and treatment of pulmonary hypertension. Mol. Biol. Rep. 2021, 48, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, N.; Patel, R.S.; Eapen, D.J.; Veledar, E.; Al Kassem, H.; Manocha, P.; Khayata, M.; Zafari, A.M.; Sperling, L.; Jones, D.P.; et al. Oxidative Stress Is Associated with Increased Pulmonary Artery Systolic Pressure in Humans. Hypertension 2014, 63, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braidy, N.; Zarka, M.; Jugder, B.E.; Welch, J.; Jayasena, T.; Chan, D.K.Y.; Sachdev, P.; Bridge, W. The Precursor to Glutathione (GSH), gamma-Glutamylcysteine (GGC), Can Ameliorate Oxidative Damage and Neuroinflammation Induced by A beta(40) Oligomers in Human Astrocytes. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, A.; Moreira, P.I.; Oliveira, C.R.; Cardoso, S.M.; Pinton, P.; Pereira, C.F. Amyloid-Beta Disrupts Calcium and Redox Homeostasis in Brain Endothelial Cells. Mol. Neurobiol. 2015, 51, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chen, C.M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Tfelt-Hansen, J.; Chattopadhyay, N. Diverse Roles of Extracellular Calcium-Sensing Receptor in the Central Nervous System. J. Neurosci. Res. 2010, 88, 2073–2082. [Google Scholar] [CrossRef]
- Scannapieco, S.; Picillo, M.; Del Gaudio, L.; Barone, P.; Erro, R. A Novel Phenotype Associated with CaSR-Related Familial Brain Calcifications. Mov. Disord. Clin. Pract. 2020, 7, 701–703. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Gardenal, E.; Gui, L.; Dal Pra, I. Amyloid beta-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143-Further Implications for Alzheimer’s Therapy. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Conley, Y.P.; Mukherjee, A.; Kammerer, C.; DeKosky, S.T.; Kamboh, M.I.; Finegold, D.N.; Ferrell, R.E. Evidence Supporting a Role for the Calcium-Sensing Receptor in Alzheimer Disease. Am. J. Med. Genet. Part B-Neuropsychiatr. Genet. 2009, 150, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2008, 30, 42–59. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goralski, T.; Ram, J.L. Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives. Int. J. Mol. Sci. 2022, 23, 717. https://doi.org/10.3390/ijms23020717
Goralski T, Ram JL. Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives. International Journal of Molecular Sciences. 2022; 23(2):717. https://doi.org/10.3390/ijms23020717
Chicago/Turabian StyleGoralski, Thomas, and Jeffrey L. Ram. 2022. "Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives" International Journal of Molecular Sciences 23, no. 2: 717. https://doi.org/10.3390/ijms23020717
APA StyleGoralski, T., & Ram, J. L. (2022). Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives. International Journal of Molecular Sciences, 23(2), 717. https://doi.org/10.3390/ijms23020717