Nanocarriers for Delivery of Oligonucleotides to the CNS
Abstract
:1. Introduction
- Protect the nucleic acid against enzymatic degradation,
- Non-toxic and low immunogenicity,
- Selective internalisation into the target cells of the CNS,
- Release of the nucleic acid and appropriate expression or activity in that cell type.
- The nanocarriers must not be cleared through the kidney, or removed by mononuclear phagocytes in the liver and spleen, before they have had time to interact with the brain endothelium,
- They should selectively target the brain endothelium, in comparison with endothelium in other tissues,
- They must cross the brain endothelial cells with their nucleic acid cargo intact and in sufficient quantity to have a biological effect.
2. Barriers for Nanoparticle Transport into the CNS
- Paracellular movement through the junctions between endothelial cells.
- Direct movement across the apical plasma membrane, cytosolic transport or diffusion across the cell and transfer across the basal plasma membrane.
- Vesicular transcytosis—endocytosis at the apical (blood) surface of the endothelium and exocytosis at the basal (brain) surface.
3. Oligonucleotides for Treatment of CNS Disease
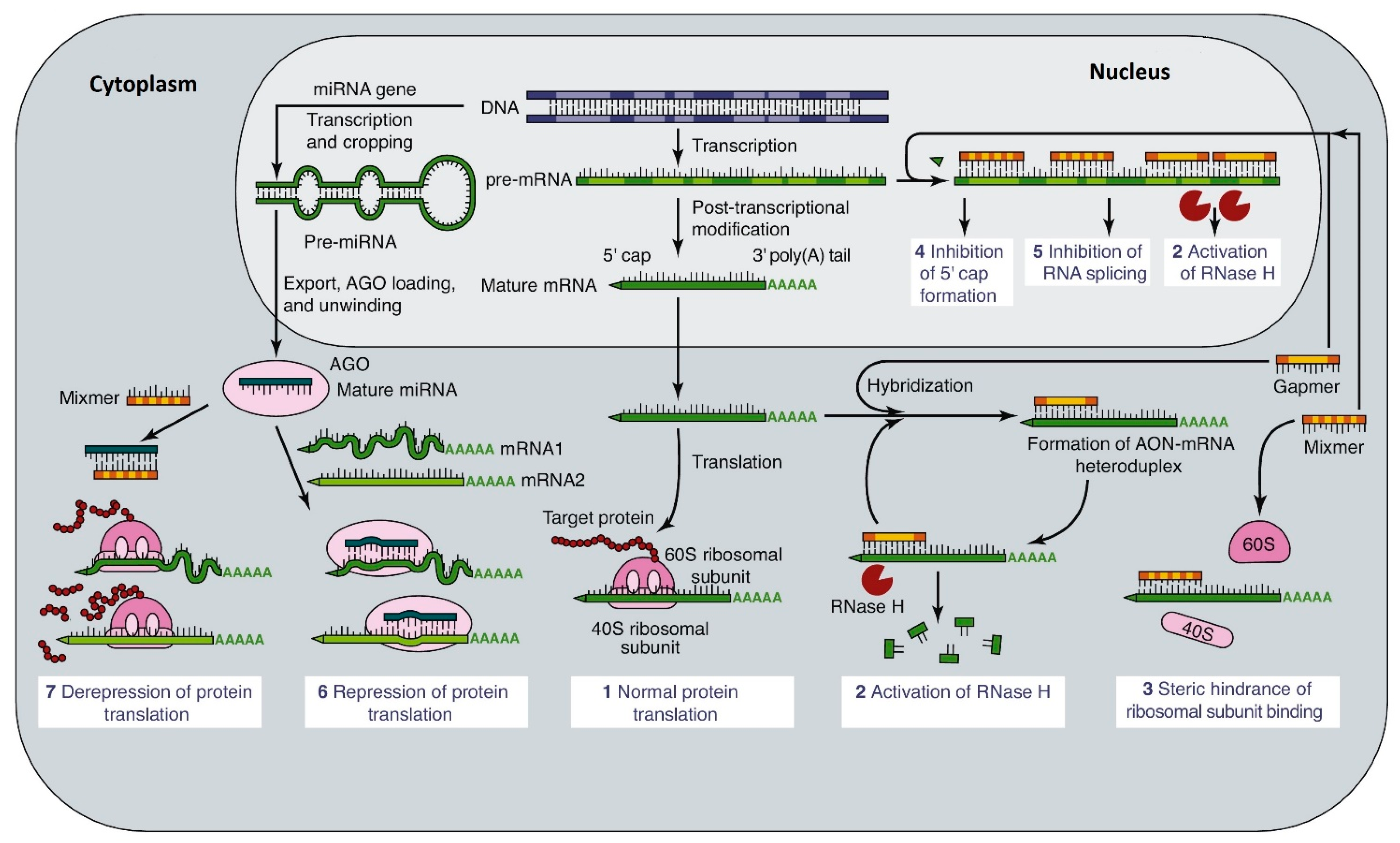
3.1. Actions and Modifications of Oligonucleotides
- Altering splicing of the primary RNA transcript, to include or exclude specific exons.
- Increasing the degradation of mRNA by inhibiting methylation of the 5′-cap on mRNA.
- Inhibiting the nuclear export of mRNA by preventing addition of the poly-A tail. This also promotes its breakdown by 3′ exonucleases.
- Blocking the translation of mRNA by inhibiting ribosome attachment or progress down the mRNA strand.
- Increasing mRNA’s availability by blocking its interaction with long non-coding RNAs (lncRNA).
- Forming DNA-RNA hybrids or dsRNA, which are substrates for RNAse H.
- Promoting the breakdown of mRNA by micro-RNAs (MiRs) or short interfering RNA (siRNA)
- Acting as guide RNA (gRNA) for gene-editing by CRISPR/Cas9.
3.2. Level of Therapeutic Oligonucleotides
4. Nanocarriers for Oligonucleotides
4.1. Smaller Nanocarriers
4.2. Larger Nanocarriers
4.3. Effect of Nanocarrier Size
4.4. Targeting of Oligonucleotide Nanocarriers
5. Tissue Distribution of Oligonucleotide Nanocarriers
5.1. Route of Administration
| Nanocarrier | Cargo | Notes | Ref |
|---|---|---|---|
| Glucose/galactose-coated 2 nm gold core | Thiol-bound ssDNA or dsDNA 20–40 bp | 7–8 nm | [43] |
| Polymer-modified mesoporous silica | Internal, ASOs | 70–200 nm | [44] |
| PEI or amine coated 15 nm iron-oxide core (SPIO) | External, electrostatic-bound ASO | Superparamagnetic 50–60 nm | [45] |
| Aminated, cationic cyclodextrin | Trapped siRNA | 160–180 nm, peptide-targeted | [52] |
| Ca phosphate core, phospholipid shell with bound PEG | ssDNA ASO in core | 30–60 nm | [53] |
| Polyethylene imine (PEI) modified with PEG | Trapped ssDNA ASO | 90–160 nm, insulin/transferrin targeted | [55] |
| Linear PEI/PEG conjugate | siRNA | Fibrillar micelles formed around RNA | [56] |
| Bioreducible lipids modified with cholesterol/DOPE/PEG | ASO in core | 150–500 nm with ASO | [61] |
| Tri-poly phosphate-modified chitosan/PEG conjugate | Encapsulated ssDNA ASO | 170 nm nanoparticle + TfR antibody = 784 nm | [59] |
| Liposome—cationic lipid (DOTAP) and cholesterol ± PEG | Encapsulated siRNA | Conjugated peptide targeting AcChR | [62] |
| Liposome—cationic lipid mixture | siRNA duplex | 50–60 nm | [65] |
| Chitosan | siRNA | 103–205 nm | [87] |
| Peptide-tagged, chitosan/PEG | siRNA-biotin | 5–10 nm | [86] |
| Lipochitoplex—chitosan core/liposome shell | DNA in chitosan core | Chitosan core 65 nm Lipochitoplex 99 nm | [88] |
| Polyion complex micelle + modified poly-L-lysine and PEG | ASO in core | 45 nm targeting GLUT1 | [73] |
| Cationic lipid mixtures | Ribonucleoprotein—gRNA/DNA, Cas9 | <200 nm, dependent on formulation | [26] |
5.2. Subcellular Localisation of Nanocarriers and Oligonucleotides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simonato, M.; Bennett, J.; Boulis, N.M.; Castro, M.G.; Fink, D.J.; Goins, W.F.; Gray, S.J.; Lowenstein, P.R.; Vandenberghe, L.H.; Wilson, T.J.; et al. Progress in gene therapy for neurological disorders. Nat. Rev. Neurol. 2013, 9, 277–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingusci, S.; Verlangia, G.; Soukupova, M.; Zucchini, S.; Simonato, M. Gene therapy tools for brain diseases. Front. Pharmacol. 2019, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Ravina, B.M.; Bankiewicz, K.S.; Paul, S.M.; Sah, D.W.Y. Gene therapy for neurological disorders: Progress and prospects. Nat. Rev. Drug Discov. 2018, 17, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV gene transfer to the nervous system: A clinical reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef] [Green Version]
- Lykken, E.L.; Shyng, C.; Ewards, R.J.; Rozenberg, A.; Gray, S.J. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J. Neurodev. Disord. 2018, 10, 16–25. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Pulst, S.M. Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 2018, 15, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Khorkova, O.; Wahlestedt, C. Oligonucleotide therapies for disorders of the nervous system. Nat. Biotechnol. 2017, 35, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.J.; Kalburgi, S.N.; McCown, T.J.; Samulski, R.J. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013, 20, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayant, R.D.; Sosa, D.; Kaushik, A.; Atluri, V.; Vashist, A.; Tomitaka, A.; Nair, M. Current status of non-viral gene therapy for CNS disorders. Expert Opin. Drug Deliv. 2016, 13, 1433–1445. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. Blood-brain barrier and delivery of protein and gene therapeutics to brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. J. Control. Release 2016, 240, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-Coated Gold Nanoparticles Transfer across Human Brain Endothelium and Enter Astrocytes In Vitro. PLoS ONE 2013, 8, e81043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface charge, glycocalyx, and blood-brain barrier function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef] [PubMed]
- Yokel, R.A. Nanoparticle brain delivery: A guide to verification methods. Nanomedicine 2020, 15, 409–432. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Pheng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, A.J.; Norrbom, M.; Chun, S.; Bennett, C.F.; Rigo, F. Nonsense-mediated decay as a terminating mechanism for antisense oligonucleotides. Nucleic Acids Res. 2014, 42, 5871–5879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat. Biomed. Eng. 2017, 1, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, P.H.; Persson, R.; Funder, E.D.; Albaek, N.; Diemer, S.L.; Hansen, D.J.; Møller, M.R.; Papargyri, N.; Christiansen, H.; Hansen, B.R.; et al. Locked nucleic acid: Modality, diversity and drug discovery. Drug Discov. Today 2018, 23, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurster, C.D.; Ludolph, A.C. Antisense oligonucleotides in neurological disorders. Adv. Neurol. Disord. 2018, 11, 1756286418776932. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Cheng, Q.; Min, Y.L.; Olson, E.L.; Siegwart, D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef]
- Amado, D.A.; Davidson, B.L. Gene therapy for ALS: A review. Mol. Ther. 2021, 29, 3345–3358. [Google Scholar] [CrossRef]
- Parente, V.; Corti, S. Advances in spinal muscular atrophy therapeutics. Adv. Neurol. Disord. 2018, 11, 1756285618754501. [Google Scholar] [CrossRef]
- Kingwell, K. Double setback for ASO trials in Huntington disease Nat. Revs. Drug Discov. 2021, 20, 412–413. [Google Scholar] [CrossRef]
- Kordasiewicz, H.B.; Stanek, L.M.; Wancewicz, E.V.; Mazur, C.; McAlonis, M.M.; Pytel, K.A.; Artates, J.W.; Weiss, A.; Cheng, S.H.; Shihabuddin, L.S.; et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuroendocrinology 2012, 74, 1031–1044. [Google Scholar] [CrossRef] [Green Version]
- Imbert, M.; Blandel, F.; Leumann, C.; Garcia, L.; Goyenvalle, A. Lowering mutant huntingtin using tricyclo-DNA antisense oligonucleotides as a therapeutic approach for Huntington’s disease. Nucleic Acid Ther. 2019, 29, 256–265. [Google Scholar] [CrossRef]
- Vázquez-Mojena, Y.; León-Arcia, K.; González-Zaldivar, Y.; Rodríguez-Labrada, R.; Velázquez-Pérez, L. Gene Therapy for Polyglutamine Spinocerebellar Ataxias: Advances, Challenges, and Perspectives. Mov. Disord. 2021, 36, 2731–2744. [Google Scholar] [CrossRef]
- Martier, R.; Konstantinova, P. Gene therapy for neurodegenerative diseases: Slowing down the ticking clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef]
- Scoles, D.R.; Meera, P.; Schneider, M.D.; Paul, S.; Dansithong, W.; Figueroa, K.P.; Hung, G.; Rigo, F.; Bennett, C.F.; Otis, T.S.; et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 2017, 544, 362–366. [Google Scholar] [CrossRef]
- Moore, L.R.; Rajpal, G.; Dillingham, I.T.; Qutob, M.; Blumenstein, K.G.; Gattis, D.; Hung, G.; Kordasiewicz, H.B.; Paulson, H.L.; McLoughlin, H.S. Evaluation of Antisense Oligonucleotides Targeting ATXN3 in SCA3 Mouse Models. Mol. Ther. Nucleic Acids 2017, 7, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Borel, F.; Gernoux, G.; Sun, H.; Stock, R.; Blackwood, M.; Brown, R.H.; Mueller, C. Safe and effective superoxide dismutase 1 silencing using artificial microRNA in macaques. Sci. Transl. Med. 2018, 10, eaau6414. [Google Scholar] [CrossRef] [Green Version]
- d’Ydewalle, C.; Ramos, D.M.; Pyles, N.J.; Ng, S.Y.; Gorz, M.; Pilato, C.M.; Ling, K.; Kong, L.; Ward, A.J.; Rubin, L.L.; et al. The antisense transcript SMN-AS1 regulates SMN expression and is a novel therapeutic target for spinal muscular atrophy. Neuron 2017, 93, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, E.; Lorenzati, M.; Rivetti di Val Cervo, P.; Brussino, A.; Cernigoj, M.; Della Sala, E.; Bartoletti Stella, A.; Ferrero, M.; Caiazzo, M.; Capellari, S.; et al. Allele-specific silencing as treatment for gene duplication disorders: Proof-of-principle in autosomal dominant leukodystrophy. Brain 2019, 142, 1905–1920. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Fatima, N.; Gromnicova, R.; Loughlin, J.; Sharrack, B.; Male, D.K. Gold nanocarriers for transport of oligonucleotides across brain endothelial cells. PLoS ONE 2020, 15, e023661. [Google Scholar] [CrossRef]
- Cha, W.; Fan, R.; Miao, Y.; Zhou, Y.; Qin, C.; Shan, X.; Wan, X.; Li, J. Mesoporous silica nanoparticles as carriers for intracellular delivery of nucleic acids and subsequent therapeutic applications. Molecules 2017, 22, 782. [Google Scholar] [CrossRef]
- Yoshida, S.; Duong, C.; Oestergaard, M.; Fazio, M.; Chen, C.; Peralta, R.; Guo, S.; Seth, P.P.; Li, Y.; Beckett, L.; et al. MXD3 antisense oligonucleotide with superparamagnetic iron oxide nanoparticles: A new targeted approach for neuroblastoma. Nanomedicine 2020, 24, 102129. [Google Scholar] [CrossRef]
- Male, D.; Gromnicova, R.; McQuaid, C. Gold nanoparticles for imaging and drug transport to the CNS. Int. Rev. Neurobiol. 2016, 130, 155–198. [Google Scholar]
- Gromnicova, R.; Yilmaz, C.U.; Orhan, N.; Kaya, M.; Davies, H.; Williams, P.; Romero, I.A.; Sharrack, B.; Male, D. Localisation and mobility of glucose-coated gold nanoparticles within the brain. Nanomedicine 2016, 6, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle- based gene delivery. Gene 2006, 13, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Busquets, M.A.; Espargaró, A.; Sabaté, R.; Estelrich, J. Magnetic nanoparticles cross the blood-brain barrier: When physics rises to a challenge. Nanomaterials 2015, 5, 2231–2248. [Google Scholar] [CrossRef]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, M.C.P.; Cronin, M.F.; Cryan, J.F.; O’Driscol, C.M. Modified cyclodextrin-based nanoparticles mediated delivery of siRNA for huntingtin gene silencing across an in vitro BBB model. Eur. J. Pharm. Biopharm. 2021, 169, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Watson, W.; Morsch, M.; Cole, N.J.; Chung, R.S.; Saunders, D.N.; Yerbury, J.J.; Vine, K.L. Improving the delivery of SOD1 antisense oligonucleotides to motor neurons using calcium phosphate-lipid nanoparticles. Front. Mol. Neurosci. 2017, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, S.V.; Batrakova, A.E.V.; Kabanov, A.V. Nanogels for Oligonucleotide Delivery to the Brain. Bioconjug. Chem. 2003, 15, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyam, R.; Ren, Y.; Lee, J.; Braunstein, K.E.; Mao, H.-Q.; Wong, P.C. Intraventricular delivery of siRNA nanoparticles to the central nervous system. Mol. Ther. 2015, 4, E242. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Andrés-Benito, P.; Aso, E.; Romero, I.A.; Roig-Carles, D.; Gromnicova, R.; Espina, M.; Ferrer, I.; García, M.L.; et al. PPARγ agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer’s disease: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 5577–5590. [Google Scholar] [CrossRef] [Green Version]
- Kozlu, S.; Caban, S.; Yerlikaya, F.; Fernandez-Megia, E.; Novoa-Carballal, R.; Riguera, R.; Yemisci, M.; Gürsoy-Ozdemir, Y.; Dalkara, T.; Couvreur, P.; et al. An aquaporin 4 antisense oligonucleotide loaded, brain targeted nanoparticulate system design. Pharmazie 2014, 69, 340–345. [Google Scholar]
- Ramos-Cabrer, P.; Campos, F. Liposomes and nanotechnology in drug development: Focus on neurological targets. Int. J. Nanomed. 2013, 8, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ma, F.; Liu, F.; Chen, J.; Zhao, X.; Xu, Q. Efficient delivery of antisense oligonucleotides using bioreducible lipid nanoparticles in vitro and in vivo. Mol. Ther. Nucleic Acids 2020, 19, 1357–1367. [Google Scholar] [CrossRef]
- Zabel, M.D.; Mollnow, L.; Bender, H. Lipopeptide delivery of siRNA to the central nervous system. Methods Mol. Biol. 2019, 1943, 389–403. [Google Scholar] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Büchel, C.; Kreuter, J. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain. J. Drug Target. 2010, 18, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Bost, J.P.; Barriga, H.; Holme, M.N.; Gallud, A.; Maugeri, M.; Gupta, D.; Lehto, T.; Valadi, H.; Esbjörner, E.K.; Stevens, M.M.; et al. Delivery of oligonucleotide therapeutics: Chemical modifications, lipid nanoparticles, and extracellular vesicles. ACS Nano 2021, 15, 13993–14021. [Google Scholar] [CrossRef] [PubMed]
- Rungta, R.; Choi, H.B.; Lin, P.J.; Ko, R.W.; Ashby, D.; Nair, J.; Manoharan, M.; Cullis, P.R.; MacVicar, B.A. Lipid nanoparticle delivery of siRNA to silence neuronal gene expression in the brain. Mol. Nucleic Acids 2013, 2, E136. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Sharon, A.; Baranes, K.; Motei, M.; Lellouche, J.-P.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood–brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Fatima, N.; Akcan, U.; Kaya, M.; Gromnicova, R.; Loughlin, J.; Sharrack, B.; Male, D. Tissue distribution and cellular localization of gold nanocarriers with bound oligonucleotides. Nanomedicine 2021, 16, 709–720. [Google Scholar] [CrossRef]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Velasco-Aguirre, C.; Morales, F.; Gallardo-Toledo, E.; Guerrero, S.; Giralt, E.; Araya, E.; Kogan, M.J. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: Preclinical approaches. Int. J. Nanomed. 2015, 10, 4919–4936. [Google Scholar]
- Etame, A.B.; Smith, C.A.; Chan, W.; Rutka, J.T. Design and potential application of PEGylated gold nanoparticles with size-dependent permeation through brain microvasculature. Nanomedicine 2011, 7, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Romero, I.A.; Male, D.K.; Slowing, K.; Garcia-Garcia, L.; Torres-Suárez, A. Cannabidiol enhances the passage of lipid nanocapsules across the blood brain barrier both in vitro and in vivo. Mol. Pharm. 2019, 16, 1999–2010. [Google Scholar] [CrossRef]
- Min, H.S.; Kim, H.J.; Naito, M.; Ogura, S.; Toh, K.; Hayashi, K.; Kim, B.S.; Fukushima, S.; Anraku, Y.; Miyata, K.; et al. Systemic brain delivery of antisense oligonucleotides across the blood-brain barrier with a glucose-coated polymeric nanocarrier. Angew. Chem. Int. Ed. Engl. 2020, 59, 8173–8180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S.; et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 2017, 8, 1001. [Google Scholar] [CrossRef] [Green Version]
- Burkhart, A.; Azizi, M.; Thomsen, M.S.; Thomsen, L.B.; Moos, T. Accessing targeted nanoparticles to the brain: The vascular route. Curr. Med. Chem. 2014, 21, 4092–4099. [Google Scholar] [CrossRef]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar]
- McQuaid, C.; Halsey, A.; Dubois, M.; Romero, I.; Male, D. Comparison of polypeptides that bind the transferrin receptor, for targeting gold nanocarriers. PLoS ONE 2021, 16, e0252341. [Google Scholar]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Nagata, T.; Dwyer, C.A.; Yoshida-Tanaka, K.; Ihara, K.; Ohyagi, M.; Kaburagi, H.; Miyata, H.; Ebihara, S.; Yoshioka, K.; Ishii, T.; et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood–brain barrier and knock down genes in the rodent CNS. Nat. Biotechnol. 2021, 39, 1529–1536. [Google Scholar] [CrossRef]
- Huang, R.; Ma, H.; Guo, Y.; Liu, S.; Kuang, Y.; Shao, K.; Li, J.; Liu, Y.; Han, L.; Huang, S.; et al. Angiopep-Conjugated Nanoparticles for Targeted Long-Term Gene Therapy of Parkinson’s Disease. Pharm. Res. 2013, 30, 2549–2559. [Google Scholar] [CrossRef]
- Chan, T.G.; Morse, S.V.; Copping, M.J.; Choi, J.J.; Vilar, R. Targeted delivery of DNA-Au nanoparticles across the blood–brain barrier using focused ultrasound. ChemMedChem 2018, 13, 1311–1314. [Google Scholar] [CrossRef]
- Renner, D.B.; Frey, W.H.I.I.; Hanson, L.R. Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci. Lett. 2012, 513, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Saha, S. Intranasal delivery of chitosan-siRNA nanoparticle formulation to the brain. Methods Mol. Biol. 2014, 1141, 233–247. [Google Scholar]
- Malhotra, M.; Tomaro-Duchesneau, C.; Saha, S.; Prakash, S. Intranasal siRNA delivery to the brain by TAT/MGF tagged pegylated chitosan nanoparticles. J. Pharm. 2013, 2013, 812387. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Enriched chitosan nanoparticles loaded with siRNA are effective in lowering Huntington’s disease gene expression following intranasal administration. Nanomedicine 2020, 24, 102119. [Google Scholar] [CrossRef] [PubMed]
- Baghdan, E.; Pinnareddy, S.R.; Strehlow, B.; Engelhardt, K.; Schäfer, J.; Bakowsky, U. Lipid coated chitosan-DNA particles for enhanced gene delivery. Int. J. Pharm. 2018, 535, 473–479. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Male, D.; Gromnicova, R. Nanocarriers for Delivery of Oligonucleotides to the CNS. Int. J. Mol. Sci. 2022, 23, 760. https://doi.org/10.3390/ijms23020760
Male D, Gromnicova R. Nanocarriers for Delivery of Oligonucleotides to the CNS. International Journal of Molecular Sciences. 2022; 23(2):760. https://doi.org/10.3390/ijms23020760
Chicago/Turabian StyleMale, David, and Radka Gromnicova. 2022. "Nanocarriers for Delivery of Oligonucleotides to the CNS" International Journal of Molecular Sciences 23, no. 2: 760. https://doi.org/10.3390/ijms23020760
APA StyleMale, D., & Gromnicova, R. (2022). Nanocarriers for Delivery of Oligonucleotides to the CNS. International Journal of Molecular Sciences, 23(2), 760. https://doi.org/10.3390/ijms23020760





