Endoplasmic Reticulum Stress and Unfolded Protein Response Signaling in Plants
Abstract
:1. Introduction
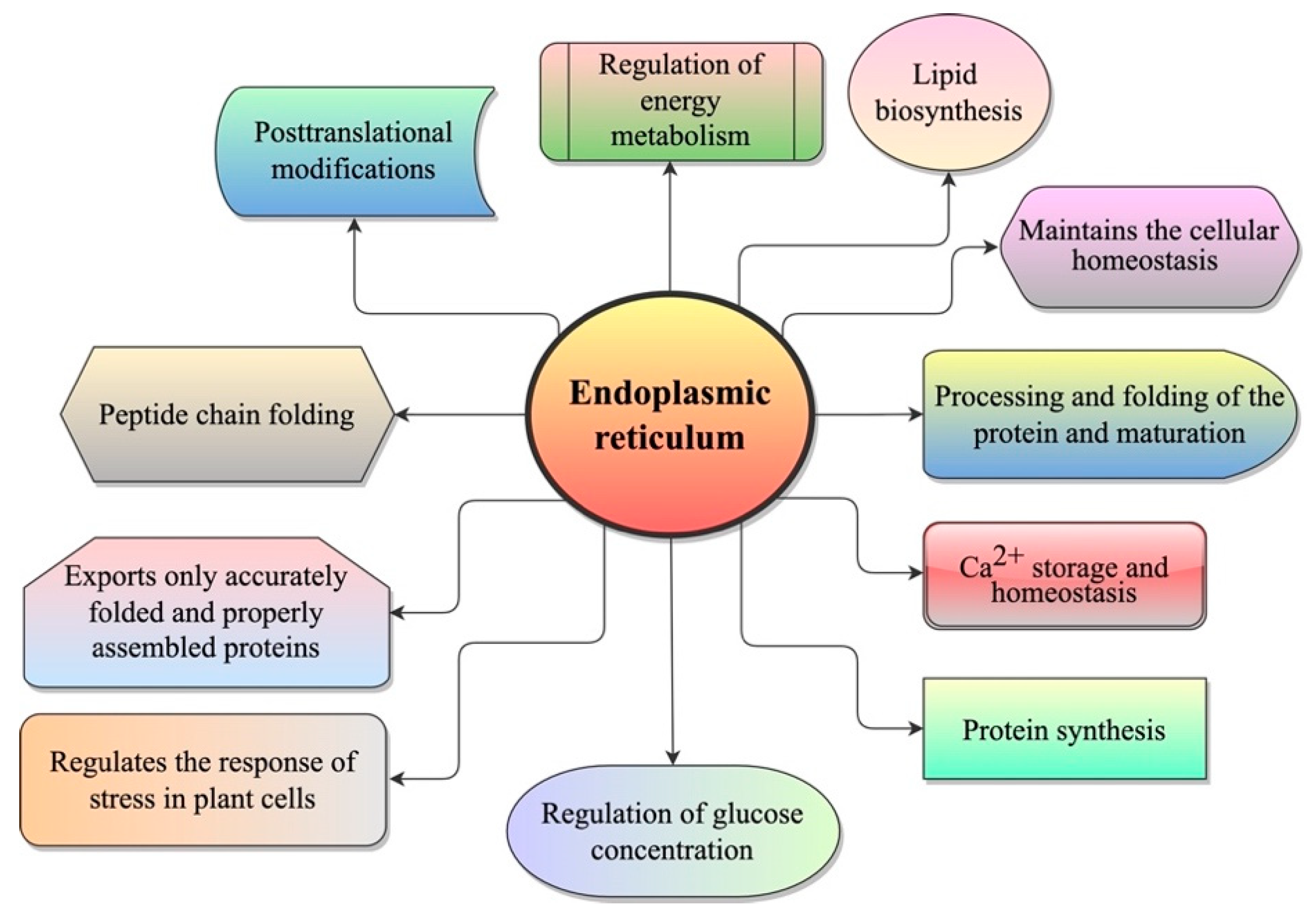
2. Endoplasmic Reticulum (ER)
3. ER Stress
4. Chemical Inducers for the Accumulation of the Unfolded Protein
4.1. Tunicamycin (TM) Stress
4.2. Dithiothreitol (DTT) Stress
5. UPR Signaling in Plant Development
6. UPR Signaling in Different Stresses
| Gene | Function | Stress | Plant/Crop | Reference |
|---|---|---|---|---|
| HRD3A | Defects in HRD3A cause alteration in the UPR, increased plant sensitivity to salt, and retention of ERAD substrates in plant cells. | Salt and ER stresses | Arabidopsis (A. thaliana) | [66] |
| UBC32 | UBC32 affects the stability of barley powdery mildew O (MLO) mutant MLO-12, a known ERAD substrate. | ER stress | Arabidopsis (A. thaliana) | [67] |
| AtOS9 | AtOS9 is an ER-localized glycoprotein and co-expresses with various predicted/known ER chaperones. | ER stress | Arabidopsis (A. thaliana) | [68] |
| NAC103 | ER stress induces the expression of NAC103. Overexpression of NAC103 has pleiotropic effects on plant growth. It plays a crucial role in inducing the expression of some UPR downstream genes under normal growth conditions. | ER stress | Arabidopsis (A. thaliana) | [45] |
| EBS7 | Arabidopsis ethyl methane sulfonate-mutagenized brassinosteroid insensitive 1 suppressor 7 (EBS7) gene observed to be accumulated under ER stress, and its mutations lead to hypersensitivity to salt and ER stresses. | ER and salt stresses | Arabidopsis (A. thaliana) | [69] |
| WRKY75 | WRKY75 is an ER-stress cellular response regulator. Plants expressing WRKY75 show tolerance to salt stress, which connects the ER and abiotic stress responses. | ER and salt stresses | Arabidopsis (A. thaliana) | [71] |
| AtNRP1, AtNRP2 and AtNRPs; (ANAC036 and gVPE) | Loss-of-function of AtNRP1 and AtNRP2 attenuates the cell death caused by ER stress. Osmotic and ER stresses have been shown to induce AtNRPs; (gVPE and ANAC036). | ER stress | Arabidopsis (A. thaliana) | [88] |
| AtHSPR | AtHSPR (A. thaliana Heat Shock Protein Related) is involved in ER stress signaling and cell death caused by salt stress. | ER stress | Arabidopsis (A. thaliana) | [89] |
| SAL1 | SAL1 is a negative regulator of stress signaling and is linked to plant stress responses. Loss-of-function of SAL1 resulted in a significant reduction in ER stress and a significant increase in Cd tolerance. | ER and cadmium (Cd) stresses | Arabidopsis (A. thaliana) | [77] |
| HOP | HSP70-HSP90 organizing protein (HOP) is a member of the cytosolic cochaperones family. HOP3 interacts in vivo with cytosolic HSP70 and HSP90, and with binding immunoglobulin protein (BiP), an HSP70 protein is localized in the ER. | ER stress | Arabidopsis (A. thaliana) | [90] |
| CER9 and HRD1A/1B | Arabidopsis ERAD genes, HRD1A/1B and CER9 might regulate the heat stress response. HRD1A/1B and CER9 collaboratively regulate plant thermos tolerance. | ER and heat stresses | Arabidopsis (A. thaliana) | [70] |
| AtNTL7 | AtNTL7 is a membrane-tethered NAC TF that leads to resistance to ER stress. Overexpression of AtNTL7 exhibits strong resistance to ER stress. | ER stress | Arabidopsis (A. thaliana) | [91] |
| HY5 | Mutation of a main light signaling component, ELONGATED HYPOCOTYL 5 (HY5), leads to ER stress tolerance. HY5 negatively regulates the UPR by competing with bZIP28 for binding to the G-box-like element present in the ER stress response element. | ER stress | Arabidopsis (A. thaliana) | [56] |
| OsDER1 | Suppression or overexpression of OsDER1 results in the activation of UPR and hypersensitivity to ER stress and suppression leads to shrunken and floury seeds. | ER stress | Rice (O. sativa L.) | [49] |
| SPL6 | Mutation of SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 6 (SPL6) up-regulates the expression of IRE1 and persistent UPR, which causes cell death and the abortion of rice apical panicles. | ER stress | Rice (O. sativa L.) | [48] |
| EMR | ERAD-mediating RING finger protein (EMR) plays an essential role in the plant ERAD system, affecting the BR signaling under ER stress conditions. | ER stress | Arabidopsis (A. thaliana) | [92] |
| GAAP1 | GAAP1 (Arabidopsis Golgi anti-apoptotic protein 1) regulates the PCD and UPR. GAAP1 prevents cell death induced by ER stress and encourages the recovery of plant growth by attenuating the UPR process mediated by IRE1 after ER stress relief. | ER stress | Arabidopsis (A. thaliana) | [78,79] |
| BLI | BLISTER (BLI) protein loss-of-function mutation up-regulates the canonical UPR of non-canonical UPR downstream genes, inducing growth retardation and cell death. | ER stress | Arabidopsis (A. thaliana) | [46] |
| hyl1 | HYPONASTIC LEAVES1 (hyl1) mutant plants are more susceptible to TM, which causes ER stress. | ER stress | Arabidopsis (A. thaliana) | [93] |
| FAD2 | The 7 fatty acid desaturases (FADs) desaturate each glycerolipid class differently in plastids and ER. FAD2 mutants have resulted in a hypersensitive response to TM through systematic screening of FAD mutants. | ER stress | Arabidopsis (A. thaliana) | [94] |
| NF-YC14 | NF-YC14 involves in regulating the ER stress response. NF-YC14 overexpression improves plant tolerance to ER stress and increases the expression of downstream genes for ER stress response. | ER stress | Arabidopsis (A. thaliana) | [95] |
| GAAP1, GAAP3, and MAPR3 | Arabidopsis Golgi anti-apoptotic proteins 1 and 3 (GAAP1, 3) resist PCD against ER stress and negatively modulate the IRE1-bZIP60 pathway. Mutations in GAAP1/GAAP3 or/and Membrane-associated progesterone receptor 3 (MAPR3) increase the vulnerability of seedlings to ER stress. | ER stress | Arabidopsis (A. thaliana) | [96] |
| MfSTMIR | MfSTMIR plays a crucial role in salt and ER stress response. The expression of MfSTMIR was observed to be induced by TM and salt. | ER and salt stresses | Sickle medic (Medicago falcata) | [75] |
| SIP1;1, SIP1;2 | A. thaliana aquaporins; SIP1;1, SIP1;2 and SIP2;1 are localized in the ER. The aquaporin SIP2;1 involves alleviating the ER stress. The absence of SIP2;1 reduces pollen tube elongation and pollen germination. | ER stress | Arabidopsis (A. thaliana) | [47] |
| BiP3 | The basal mRNA level of BiP3 is an important gene induced by ER stress in pollen. | ER stress | Arabidopsis (A. thaliana) | [47] |
| PAWH1 and PAWH2 | PAWH1 and PAWH2 are localized in the ER membrane and associated with Hrd1 through EMS-mutagenized Bri1 Suppressor 7 (EBS7). Removal of two PAWHs constitutively triggers the UPR and compromises the resistance to stress. | ER stress | Arabidopsis (A. thaliana) | [97] |
| AtOTU1 | AtOTU1 selectively hydrolyzes various forms of ubiquitin chains. AtOTU1 is required to process plant ERAD substrates. | ER stress | Arabidopsis (A. thaliana) | [98] |
| AtSec62 | Arabidopsis Sec62 (AtSec62) is required for plant development and may function as an ER-phagy receptor in plants. | ER stress | Arabidopsis (A. thaliana) | [99] |
| TIN1 | Transcriptional induction of Tunicamycin induced 1 (TIN1) by ER stress was partially regulated by AtbZIP60. The accumulation of TIN1 protein was observed in response to TM treatment. | ER stress | Arabidopsis (A. thaliana) | [28] |
7. Strategies to Reduce ER Stress
7.1. Unfolded Protein Response (UPR)
7.2. Mechanism of UPR Signaling Pathway in Plants
7.2.1. Regulated IRE-1 Dependent Splicing (RIDS)
7.2.2. ER-Associated Degradation (ERAD)
7.2.3. Autophagy
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AtHSPR | A. thaliana Heat Shock Protein Related |
| AtSec62 | Arabidopsis Sec62 |
| BiP | Binding protein/Binding immunoglobulin protein |
| BiP3 | Binding protein 3 |
| BLI | Golgi-localized protein BLISTER |
| bZIP17 | BASIC LEUCINE ZIPPER 17 |
| bZIP60 | Leucine zipper transcription factor 60 |
| CNX 1-like | Calnexin 1-like |
| CPR | Cytosolic Protein Response |
| CRT | Calreticulin |
| DTT | Dithiothreitol |
| EMR | ERAD-mediating RING finger protein |
| EMS | Ethyl methanesulphonate |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated degradation |
| ERQC | ER protein quality-control system |
| ERSE | ER stress response element |
| ETH | Ethylene |
| FAD2 | FATTY ACID DESATURASE 2 |
| GRP94 | Glucose-regulated protein 94 |
| HOP | HSP70-HSP90 organizing protein |
| HY5 | ELONGATED HYPOCOTYL 5 |
| hyl1 | HYPONASTIC LEAVES1 |
| IRE1 | Inositol requiring enzyme1 |
| MAPR3 | Membrane-associated progesterone receptor 3 |
| MLO | Barley powdery mildew O |
| NPR1 | Nonexpressor of pathogenesis-related (PR) genes 1 |
| PCD | Programmed cell death |
| PDI | Protein disulfide isomerase |
| PPI | Peptidyl-prolyl isomerases |
| PR | Pathogenesis-related |
| RIDD | IRE1-dependent decay of mRNAs |
| RIP | Regulated intramembrane proteolysis |
| ROS | Reactive oxygen species |
| S1P | Site 1 Protease |
| S2P | Site 2 Protease |
| SA | Salicylic acid |
| SAR | Systemic acquired resistance |
| SPL6 | SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 6 |
| TF/TFs | Transcription factor/Transcription factors |
| TM | Tunicamycin |
| UPR | Unfolded protein response |
| UPS | Ubiquitin/proteasome system |
| VIGS | Virus-induced gene silencing |
References
- Millar, A.J. The intracellular dynamics of circadian clocks reach for the light of ecology and evolution. Ann. Rev. Plant Biol. 2016, 67, 595–618. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; Wang, J.-Z.; Dehesh, K. Retrograde signals: Integrators of interorganellar communication and orchestrators of plant development. Ann. Rev. Plant Biol. 2017, 68, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Ma, X.; Guo, H.; Sukiran, N.L.; Guo, B.; Assmann, S.M.; Ma, H. Flower development under drought stress: Morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 2013, 25, 3785–3807. [Google Scholar] [CrossRef] [Green Version]
- Hakim; Ullah, A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; et al. Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J. Communications Between the Endoplasmic Reticulum and Other Organelles During Abiotic Stress Response in Plants. Front. Plant Sci. 2019, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Schwappach, B. From rags to riches—The history of the endoplasmic reticulum. Biochim. Biophys. Acta. Mol. Cell Res. 2013, 1833, 2389–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westrate, L.; Lee, J.; Prinz, W.; Voeltz, G. Form follows function: The importance of endoplasmic reticulum shape. Ann. Rev. Biochem. 2015, 84, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.; Brandizzi, F. Advances in plant ER architecture and dynamics. Plant Physiol. 2018, 176, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Benham, A.M. Endoplasmic Reticulum redox pathways: In sickness and in health. FEBS J. 2019, 286, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kaufman, R.J. The mammalian unfolded protein response. Ann. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Howell, S.H. The unfolded protein response supports plant development and defense as well as responses to abiotic stress. Front. Plant Sci. 2017, 8, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawkar, G.M.; Lee, E.S.; Shelake, R.M.; Park, J.H.; Ryu, S.W.; Kang, C.H.; Lee, S.Y. Activation of the transducers of unfolded protein response in plants. Front. Plant Sci. 2018, 9, 214. [Google Scholar] [CrossRef]
- Merksamer, P.I.; Trusina, A.; Papa, F.R. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 2008, 135, 933–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merksamer, P.I.; Papa, F.R. The UPR and cell fate at a glance. J. Cell Sci. 2010, 123, 1003–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.F.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Autophagy. In Endoplasmic Reticulum; IntechOpen: London, UK, 2018. [Google Scholar]
- Chen, Q.; Yu, F.; Xie, Q. Insights into endoplasmic reticulum-associated degradation in plants. New Phytol. 2020, 226, 345–350. [Google Scholar] [CrossRef]
- Schubert, U.; Anton, L.C.; Gibbs, J.; Norbury, C.C.; Yewdell, J.W.; Bennink, J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 2000, 404, 770–774. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Choi, Y.; Kwon, C.; Yun, H.S. Endoplasmic reticulum stress-induced accumulation of VAMP721/722 requires CALRETICULIN 1 and CALRETICULIN 2 in Arabidopsis. J. Integr. Plant Biol. 2019, 61, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Ruberti, C.; Kim, S.-J.; Stefano, G.; Brandizzi, F. Unfolded protein response in plants: One master, many questions. Curr. Opin. Plant Biol. 2015, 27, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.-J.; Park, J.M. Endoplasmic reticulum plays a critical role in integrating signals generated by both biotic and abiotic stress in plants. Front. Plant Sci. 2019, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Martínez, I.M.; Chrispeels, M.J. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 2003, 15, 561–576. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, H.H.; Brustolini, O.J.; Pimenta, M.R.; Mendes, G.C.; Gouveia, B.C.; Silva, P.A.; Silva, J.C.F.; Mota, C.S.; Soares-Ramos, J.R.; Fontes, E.P. The molecular chaperone binding protein BiP prevents leaf dehydration-induced cellular homeostasis disruption. PLoS ONE 2014, 9, e86661. [Google Scholar] [CrossRef] [Green Version]
- Hauptmann, P.; Riel, C.; Kunz-Schughart, L.A.; Fröhlich, K.U.; Madeo, F.; Lehle, L. Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 2006, 59, 765–778. [Google Scholar] [CrossRef]
- Iwata, Y.; Nishino, T.; Takayama, S.; Koizumi, N. Characterization of a plant-specific gene induced by endoplasmic reticulum stress in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2010, 74, 2087–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 490, pp. 71–92. [Google Scholar]
- McCormack, M.E.; Liu, X.; Jordan, M.R.; Pajerowska-Mukhtar, K.M. An improved high-throughput screening assay for tunicamycin sensitivity in Arabidopsis seedlings. Front. Plant Sci. 2015, 6, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, N.; Ujino, T.; Sano, H.; Chrispeels, M.J. Overexpression of a gene that encodes the first enzyme in the biosynthesis of asparagine-linked glycans makes plants resistant to tunicamycin and obviates the tunicamycin-induced unfolded protein response. Plant Physiol. 1999, 121, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, Y.; Tintor, N.; Lu, X.; Rauf, P.; Pajerowska-Mukhtar, K.; Häweker, H.; Dong, X.; Robatzek, S.; Schulze-Lefert, P. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J. 2009, 28, 3439–3449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häweker, H.; Rips, S.; Koiwa, H.; Salomon, S.; Saijo, Y.; Chinchilla, D.; Robatzek, S.; von Schaewen, A. Pattern recognition receptors require N-glycosylation to mediate plant immunity. J. Biol. Chem. 2010, 285, 4629–4636. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Srivastava, R.; Howell, S.H. Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int. J. Mol. Sci. 2013, 14, 8188–8212. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wang, T.; Zhu, M.; Zhang, L.; Zhang, F.; Jing, E.; Ren, Y.; Wang, Z.; Xin, Z.; Lin, T. Transcriptome and physiological analyses for revealing genes involved in wheat response to endoplasmic reticulum stress. BMC Plant Biol. 2019, 19, 193. [Google Scholar] [CrossRef]
- Rand, J.D.; Grant, C.M. The thioredoxin system protects ribosomes against stress-induced aggregation. Mol. Biol. Cell 2006, 17, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Altuntaş, C.; Terzi, R. Dithiothreitol and PEG Induced Combined Stress May Affect the Expressions of ABA Aldehyde Oxidase, Sucrose Synthase and Proline Metabolic Genes in Maize Seedlings. Phyton 2020, 89, 487. [Google Scholar] [CrossRef]
- Howell, S.H. Endoplasmic reticulum stress responses in plants. Ann. Rev. Plant Biol. 2013, 64, 477–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Yi, P.; Zhang, B.; Xu, C.J.; Liu, Q.Y.; Pi, Z.J.; Xu, X.L.; Chevet, E.; Liu, J.F. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell Signal 2011, 23, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Chen, Y.; Deng, Y.; Brandizzi, F.; Howell, S.H. Elements proximal to and within the transmembrane domain mediate the organelle-to-organelle movement of bZIP28 under ER stress conditions. Plant J. 2012, 70, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Humbert, S.; Howell, S.H. ZmbZIP60 mRNA is spliced in maize in response to ER stress. BMC Res. Notes 2012, 5, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, A.A.; Mukhtar, M.S.; Blanco, F.; Boatwright, J.L.; Moreno, I.; Jordan, M.R.; Chen, Y.; Brandizzi, F.; Dong, X.; Orellana, A. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 2012, 7, e31944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Bassham, D.C.; Howell, S.H. A functional unfolded protein response is required for normal vegetative development. Plant Physiol. 2019, 179, 1834–1843. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. ER-anchored transcription factors bZIP17 and bZIP28 regulate root elongation. Plant Physiol. 2018, 176, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yang, Z.T.; Song, Z.T.; Wang, M.J.; Sun, L.; Lu, S.J.; Liu, J.X. The plant-specific transcription factor gene NAC 103 is induced by b ZIP 60 through a new cis-regulatory element to modulate the unfolded protein response in A rabidopsis. Plant J. 2013, 76, 274–286. [Google Scholar]
- Hong, Z.-H.; Qing, T.; Schubert, D.; Kleinmanns, J.A.; Liu, J.-X. BLISTER-regulated vegetative growth is dependent on the protein kinase domain of ER stress modulator IRE1A in Arabidopsis thaliana. PLoS Genet. 2019, 15, e1008563. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Maeshima, M. The ER-localized aquaporin SIP2; 1 is involved in pollen germination and pollen tube elongation in Arabidopsis thaliana. Plant Mol. Biol. 2019, 100, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Sun, A.-Z.; Chen, S.-T.; Chen, L.-S.; Guo, F.-Q. SPL6 represses signalling outputs of ER stress in control of panicle cell death in rice. Nat. Plants 2018, 4, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Chen, G.; Tian, L. OsDER1 is an ER-associated protein degradation factor that responds to ER stress. Plant Physiol. 2018, 178, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-X.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef] [Green Version]
- Urade, R. The endoplasmic reticulum stress signaling pathways in plants. Biofactors 2009, 35, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Neill, E.M.; Byrd, M.C.; Billman, T.; Brandizzi, F.; Stapleton, A.E. Plant growth regulators interact with elevated temperature to alter heat stress signaling via the Unfolded Protein Response in maize. Sci. Rep. 2019, 9, 10392. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, H.H.; Silva, P.A.; Mendes, G.C.; Brustolini, O.J.; Pimenta, M.R.; Gouveia, B.C.; Valente, M.A.S.; Ramos, H.J.; Soares-Ramos, J.R.; Fontes, E.P. The endoplasmic reticulum binding protein BiP displays dual function in modulating cell death events. Plant Physiol. 2014, 164, 654–670. [Google Scholar] [CrossRef] [Green Version]
- Guan, P.; Wang, J.; Li, H.; Xie, C.; Zhang, S.; Wu, C.; Yang, G.; Yan, K.; Huang, J.; Zheng, C. Sensitive to SALT1, an endoplasmic reticulum-localized chaperone, positively regulates salt resistance. Plant Physiol. 2018, 178, 1390–1405. [Google Scholar] [CrossRef] [Green Version]
- Henriquez-Valencia, C.; Moreno, A.A.; Sandoval-Ibañez, O.; Mitina, I.; Blanco-Herrera, F.; Cifuentes-Esquivel, N.; Orellana, A. bZIP17 and bZIP60 regulate the expression of BiP3 and other salt stress responsive genes in an UPR-independent manner in Arabidopsis thaliana. J. Cell. Biochem. 2015, 116, 1638–1645. [Google Scholar] [CrossRef]
- Nawkar, G.M.; Kang, C.H.; Maibam, P.; Park, J.H.; Jung, Y.J.; Chae, H.B.; Chi, Y.H.; Jung, I.J.; Kim, W.Y.; Yun, D.-J. HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 2084–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kørner, C.J.; Du, X.; Vollmer, M.E.; Pajerowska-Mukhtar, K.M. Endoplasmic reticulum stress signaling in plant immunity—at the crossroad of life and death. Int. J. Mol. Sci. 2015, 16, 26582–26598. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.X.; Howell, S.H. Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol. 2016, 211, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519. [Google Scholar] [CrossRef]
- Angelos, E.; Ruberti, C.; Kim, S.J.; Brandizzi, F. Maintaining the factory: The roles of the unfolded protein response in cellular homeostasis in plants. Plant J. 2017, 90, 671–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelos, E.; Brandizzi, F. NADPH oxidase activity is required for ER stress survival in plants. Plant J. 2018, 96, 1106–1120. [Google Scholar] [CrossRef] [Green Version]
- Tateda, C.; Ozaki, R.; Onodera, Y.; Takahashi, Y.; Yamaguchi, K.; Berberich, T.; Koizumi, N.; Kusano, T. NtbZIP60, an endoplasmic reticulum-localized transcription factor, plays a role in the defense response against bacterial pathogens in Nicotiana tabacum. J. Plant Res. 2008, 121, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, H.; Brandizzi, F.; Verchot, J.; Wang, A. The UPR branch IRE1-bZIP60 in plants plays an essential role in viral infection and is complementary to the only UPR pathway in yeast. PLoS Genet. 2015, 11, e1005164. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Humbert, S.; Liu, J.-X.; Srivastava, R.; Rothstein, S.J.; Howell, S.H. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7247–7252. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Srivastava, R.; Quilichini, T.D.; Dong, H.; Bao, Y.; Horner, H.T.; Howell, S.H. IRE 1, a component of the unfolded protein response signaling pathway, protects pollen development in Arabidopsis from heat stress. Plant J. 2016, 88, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Cui, F.; Li, Q.; Yin, B.; Zhang, H.; Lin, B.; Wu, Y.; Xia, R.; Tang, S.; Xie, Q. The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Res. 2011, 21, 957–969. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Liu, L.; Zhao, Q.; Zhang, Z.; Li, Q.; Lin, B.; Wu, Y.; Tang, S.; Xie, Q. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Liu, Y.; Xia, Y.; Hong, Z.; Li, J. The Arabidopsis homolog of the mammalian OS-9 protein plays a key role in the endoplasmic reticulum-associated degradation of misfolded receptor-like kinases. Mol. Plant 2012, 5, 929–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, C.; Wang, D.; Su, W.; Liu, L.; Wang, M.; Li, J. EBS7 is a plant-specific component of a highly conserved endoplasmic reticulum-associated degradation system in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 12205–12210. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-M.; Lü, S.-Y.; Li, R.-J. The Arabidopsis endoplasmic reticulum associated degradation pathways are involved in the regulation of heat stress response. Biochem. Biophys. Res. Commun. 2017, 487, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Henríquez-Valencia, C.; Gómez-Páez, M.; Medina, J.; Orellana, A.; Vicente-Carbajosa, J.; Zouhar, J. Identification of novel components of the unfolded protein response in Arabidopsis. Front. Plant Sci. 2016, 7, 650. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Du, H.; Zhang, Z.; Xu, W.; Deng, X. BhbZIP60 from resurrection plant Boea hygrometrica is an mRNA splicing-activated endoplasmic reticulum stress regulator involved in drought tolerance. Front. Plant Sci. 2017, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Irsigler, A.S.; Costa, M.D.; Zhang, P.; Reis, P.A.; Dewey, R.E.; Boston, R.S.; Fontes, E.P. Expression profiling on soybean leaves reveals integration of ER-and osmotic-stress pathways. BMC Genom. 2007, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xin, Z.; Yu, X.; Ma, C.; Liang, W.; Zhu, M.; Cheng, Q.; Li, Z.; Niu, Y.; Ren, Y. Osmotic stress induced cell death in wheat is alleviated by tauroursodeoxycholic acid and involves endoplasmic reticulum stress–related gene expression. Front. Plant Sci. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Chen, H.; Duan, M.; Zhu, F.; Wen, J.; Dong, J.; Wang, T. Medicago falcata MfSTMIR, an E3 ligase of endoplasmic reticulum-associated degradation, is involved in salt stress response. Plant J. 2019, 98, 680–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Mizukado, S.; Fujita, Y.; Ichikawa, T.; Nakazawa, M.; Seki, M.; Matsui, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Identification of stress-tolerance-related transcription-factor genes via mini-scale Full-length cDNA Over-eXpressor (FOX) gene hunting system. Biochem. Biophys. Res. Commun. 2007, 364, 250–257. [Google Scholar] [CrossRef]
- Xi, H.; Xu, H.; Xu, W.; He, Z.; Xu, W.; Ma, M. A SAL1 loss-of-function Arabidopsis mutant exhibits enhanced cadmium tolerance in association with alleviation of endoplasmic reticulum stress. Plant Cell Physiol. 2016, 57, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wang, W.; Fan, W.; Wang, Z.; Zhu, M.; Tang, X.; Wu, W.; Yang, X.; Shao, X.; Sun, Y. Arabidopsis GAAP1 and GAAP3 modulate the unfolded protein response and the onset of cell death in response to ER stress. Front. Plant Sci. 2018, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, X.; Zhu, M.; Tang, X.; Wang, Z.; Guo, K.; Zhou, Y.; Sun, Y.; Zhang, W.; Li, X. Arabidopsis GAAP1 to GAAP3 play redundant role in cell death inhibition by suppressing the upregulation of salicylic acid pathway under endoplasmic reticulum stress. Front. Plant Sci. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Wang, X.; Auwerx, J. Systems phytohormone responses to mitochondrial proteotoxic stress. Mol. Cell 2017, 68, 540–551.e545. [Google Scholar] [CrossRef]
- Poór, P.; Czékus, Z.; Tari, I.; Ördög, A. The multifaceted roles of plant hormone salicylic acid in endoplasmic reticulum stress and unfolded protein response. Int. J. Mol. Sci. 2019, 20, 5842. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Aung, K.; Rolčík, J.; Walicki, K.; Friml, J.; Brandizzi, F. Inter-regulation of the unfolded protein response and auxin signaling. Plant J. 2014, 77, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, K.A.; Matthus, E.; Swarbreck, S.M.; Davies, J.M. Calcium-mediated abiotic stress signaling in roots. Front. Plant Sci. 2016, 7, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depaepe, T.; Hendrix, S.; van Rensburg, H.C.J.; Van den Ende, W.; Cuypers, A.; Van Der Straeten, D. At the Crossroads of Survival and Death: The Reactive Oxygen Species–Ethylene–Sugar Triad and the Unfolded Protein Response. Trends Plant Sci. 2021, 26, 338–351. [Google Scholar] [CrossRef]
- Xu, Z.; Song, N.; Ma, L.; Wu, J. IRE1-bZIP60 pathway is required for Nicotiana attenuata resistance to fungal pathogen Alternaria alternata. Front. Plant Sci. 2019, 10, 263. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Ann. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Weaver, N.D.; Kesarwani, M.; Dong, X. Induction of protein secretory pathway is required for systemic acquired resistance. Science 2005, 308, 1036–1040. [Google Scholar] [CrossRef]
- Reis, P.A.; Carpinetti, P.A.; Freitas, P.P.; Santos, E.G.; Camargos, L.F.; Oliveira, I.H.; Silva, J.C.F.; Carvalho, H.H.; Dal-Bianco, M.; Soares-Ramos, J.R. Functional and regulatory conservation of the soybean ER stress-induced DCD/NRP-mediated cell death signaling in plants. BMC Plant Biol. 2016, 16, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zhang, P.; Wang, C. AtHSPR may function in salt-induced cell death and ER stress in Arabidopsis. Plant Signal. Behav. 2016, 11, e1197462. [Google Scholar] [CrossRef]
- Fernández-Bautista, N.; Fernández-Calvino, L.; Muñoz, A.; Castellano, M.M. HOP3, a member of the HOP family in Arabidopsis, interacts with BiP and plays a major role in the ER stress response. Plant Cell Environ. 2017, 40, 1341–1355. [Google Scholar] [CrossRef]
- Chi, Y.H.; Melencion, S.M.B.; Alinapon, C.V.; Kim, M.J.; Lee, E.S.; Paeng, S.K.; Park, J.H.; Nawkar, G.M.; Jung, Y.J.; Chae, H.B. The membrane-tethered NAC transcription factor, AtNTL7, contributes to ER-stress resistance in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 488, 641–647. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, C.H.; Nawkar, G.M.; Lee, E.S.; Paeng, S.K.; Chae, H.B.; Chi, Y.H.; Kim, W.Y.; Yun, D.J.; Lee, S.Y. EMR, a cytosolic-abundant ring finger E3 ligase, mediates ER-associated protein degradation in Arabidopsis. N. Phytol. 2018, 220, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Hirata, R.; Mishiba, K.-i.; Koizumi, N.; Iwata, Y. Deficiency in the double-stranded RNA binding protein HYPONASTIC LEAVES1 increases sensitivity to the endoplasmic reticulum stress inducer tunicamycin in Arabidopsis. BMC Res. Notes 2019, 12, 580. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.C.; Nakamura, Y.; Kanehara, K. Membrane lipid polyunsaturation mediated by FATTY ACID DESATURASE 2 (FAD 2) is involved in endoplasmic reticulum stress tolerance in Arabidopsis thaliana. Plant J. 2019, 99, 478–493. [Google Scholar] [CrossRef]
- Wang, L.; Mei, X.P.; Nan, J.; Liu, C.X.; Zhou, L.; Cai, Y.L. Overexpression of ZmNF-YC14 confers plant ER stress tolerance and ABA sensitivity in Arabidopsis. Acta Physiol. Plant. 2019, 41, 138. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, X.; Wang, Z.; Xu, W.; Zhou, Y.; Wang, W.; Li, X.; Li, R.; Guo, K.; Sun, Y. Arabidopsis GAAPs interacting with MAPR3 modulate the IRE1-dependent pathway upon endoplasmic reticulum stress. J. Exp. Bot. 2019, 70, 6113–6125. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, C.; Chen, Y.; Wang, Y.; Wang, D.; Liu, X.; Wang, M.; Mao, J.; Zhang, J.; Xing, W. PAWH1 and PAWH2 are plant-specific components of an Arabidopsis endoplasmic reticulum-associated degradation complex. Nat. Commun. 2019, 10, 3492. [Google Scholar] [CrossRef]
- Zang, Y.; Gong, Y.; Wang, Q.; Guo, H.; Xiao, W. Arabidopsis OTU 1, a linkage-specific deubiquitinase, is required for endoplasmic reticulum-associated protein degradation. Plant J. 2020, 101, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ye, H.; Cui, Y.; Jiang, L. AtSec62 is critical for plant development and is involved in ER-phagy in Arabidopsis thaliana. J. Integr. Plant Biol. 2020, 62, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Cantizano, N.; Ko, D.K.; Angelos, E.; Pu, Y.; Brandizzi, F. Functional Diversification of ER Stress Responses in Arabidopsis. Trends Biochem. Sci. 2020, 45, 123–136. [Google Scholar] [CrossRef]
- Liu, Y.; Burgos, J.S.; Deng, Y.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 2012, 24, 4635–4651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Tuteja, N. Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant Signal. Behav. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.; Li, Z.; Russo, G.; Tang, J.; Bi, R.; Muppirala, U.; Chudalayandi, S.; Severin, A.; He, M.; Vaitkevicius, S.I. Response to persistent ER stress in plants: A multiphasic process that transitions cells from Prosurvival activities to cell death. Plant Cell 2018, 30, 1220–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, A.A.; Orellana, A. The physiological role of the unfolded protein response in plants. Biol. Res. 2011, 44, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Alcântara, A.; Seitner, D.; Navarrete, F.; Djamei, A. A high-throughput screening method to identify proteins involved in unfolded protein response of the endoplasmic reticulum in plants. Plant Methods 2020, 16, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Brandizzi, F.; Benning, C.; Larkin, R.M. A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 16398–16403. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Deng, Y.; Howell, S.H. Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front. Plant Sci. 2014, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-X.; Howell, S.H. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 2010, 22, 782–796. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Martinez, I.M.; Kimata, Y.; Kohno, K.; Sano, H.; Chrispeels, M.J. Molecular characterization of two Arabidopsis Ire1 homologs, endoplasmic reticulum-located transmembrane protein kinases. Plant Physiol. 2001, 127, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, C.Y.; Back, S.H.; Clark, R.L.; Peisach, D.; Xu, Z.; Kaufman, R.J. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. USA 2006, 103, 14343–14348. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Takahashi, H.; Wakasa, Y.; Kawakatsu, T.; Takaiwa, F. Identification of a cis-element that mediates multiple pathways of the endoplasmic reticulum stress response in rice. Plant J. 2013, 74, 248–257. [Google Scholar] [CrossRef]
- Mishiba, K.-i.; Nagashima, Y.; Suzuki, E.; Hayashi, N.; Ogata, Y.; Shimada, Y.; Koizumi, N. Defects in IRE1 enhance cell death and fail to degrade mRNAs encoding secretory pathway proteins in the Arabidopsis unfolded protein response. Proc. Natl. Acad. Sci. USA 2013, 110, 5713–5718. [Google Scholar] [CrossRef] [Green Version]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef]
- Römisch, K. Endoplasmic reticulum–associated degradation. Annu. Rev. Cell Dev. Biol. 2005, 21, 435–456. [Google Scholar] [CrossRef]
- Hoseki, J.; Ushioda, R.; Nagata, K. Mechanism and components of endoplasmic reticulum-associated degradation. J. Biochem. 2010, 147, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; De Leonardis, A.; Guerra, D.; Di Fonzo, N.; Cattivelli, L.; Mastrangelo, A. The E3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Orenstein, S.J.; Cuervo, A.M. Chaperone-mediated autophagy: Molecular mechanisms and physiological relevance. Semin. Cell Dev. Biol. 2010, 21, 719–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. In Autophagy in Infection and Immunity; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–32. [Google Scholar]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-w.; Li, J.; Bao, J.-k. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Floyd, B.E.; Morriss, S.C.; MacIntosh, G.C.; Bassham, D.C. What to Eat: Evidence for Selective Autophagy in Plants F. J. Integr. Plant Biol. 2012, 54, 907–920. [Google Scholar] [CrossRef]
- Bassham, D.C.; Laporte, M.; Marty, F.; Moriyasu, Y.; Ohsumi, Y.; Olsen, L.J.; Yoshimoto, K. Autophagy in development and stress responses of plants. Autophagy 2006, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H. Autophagy: A self-eating mechanism for maintaining cellular homeostasis. Chin. Sci. Bull. 2016, 61, 3903–3906. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Ann. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Y.; Bassham, D.C. Links between ER stress and autophagy in plants. Plant Signal. Behav. 2013, 8, e24297. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Proshad, R.; Kormoker, T.; Tusher, T.R. Autophagy-mediated Nutrient Recycling and Regulation in Plants: A Molecular View. J. Plant Biol. 2019, 62, 307–319. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Grumati, P.; Dikic, I.; Stolz, A. ER-phagy at a glance. J. Cell Sci 2018, 131, jcs217364. [Google Scholar] [CrossRef] [Green Version]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 2014, 53, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Pu, Y.; Yu, X.; Gregory, B.D.; Srivastava, R.; Howell, S.H.; Bassham, D.C. IRE1B degrades RNAs encoding proteins that interfere with the induction of autophagy by ER stress in Arabidopsis thaliana. Autophagy 2018, 14, 1562–1573. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manghwar, H.; Li, J. Endoplasmic Reticulum Stress and Unfolded Protein Response Signaling in Plants. Int. J. Mol. Sci. 2022, 23, 828. https://doi.org/10.3390/ijms23020828
Manghwar H, Li J. Endoplasmic Reticulum Stress and Unfolded Protein Response Signaling in Plants. International Journal of Molecular Sciences. 2022; 23(2):828. https://doi.org/10.3390/ijms23020828
Chicago/Turabian StyleManghwar, Hakim, and Jianming Li. 2022. "Endoplasmic Reticulum Stress and Unfolded Protein Response Signaling in Plants" International Journal of Molecular Sciences 23, no. 2: 828. https://doi.org/10.3390/ijms23020828







