Cancer Therapy by Silver Nanoparticles: Fiction or Reality?
Abstract
:1. Introduction
2. Synthesis and Characterization of AgNPs
3. AgNP–Cancer Interactions
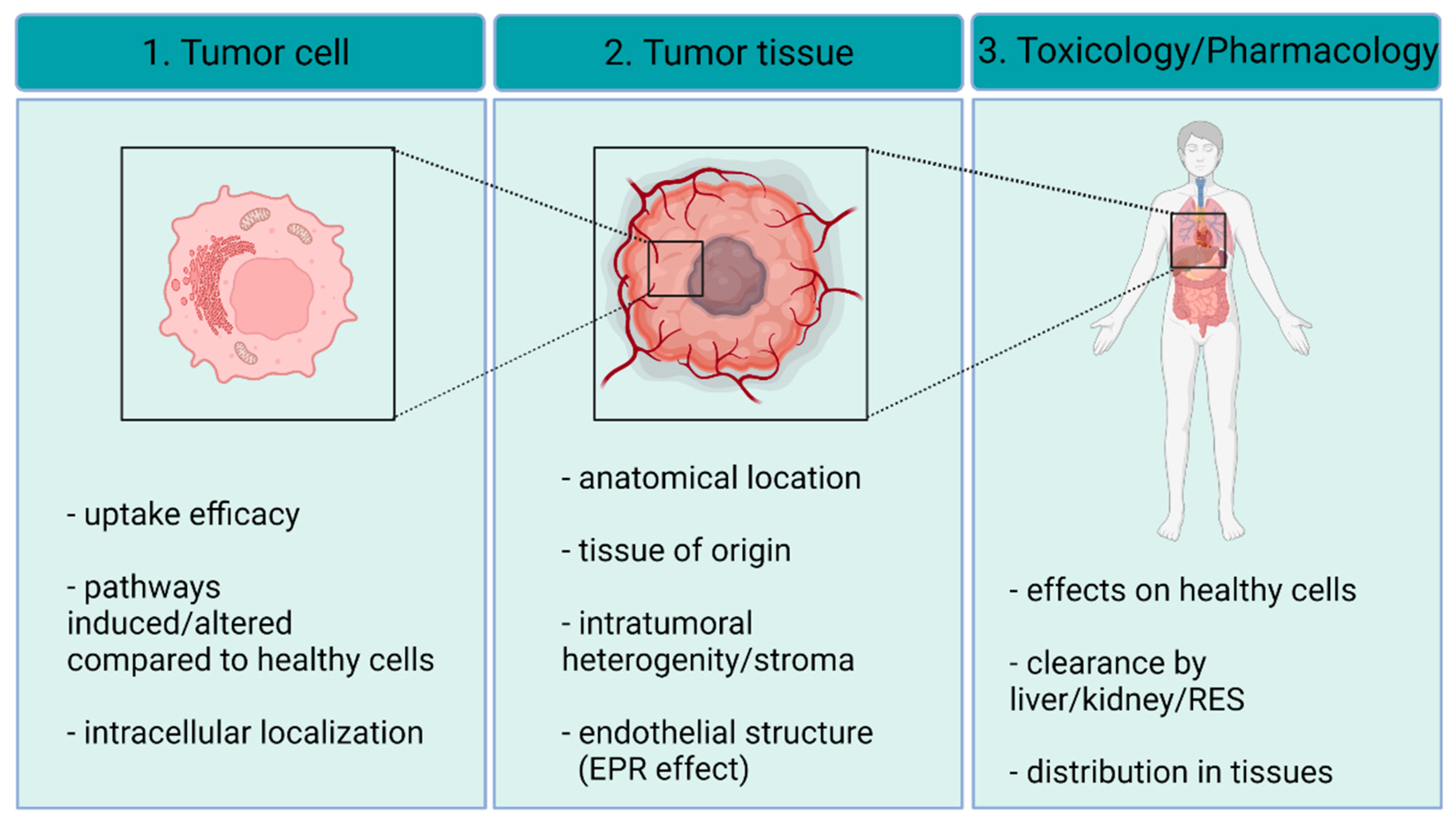
3.1. Targeting AgNPs to the Tumor Tissue
3.2. Interaction of AgNPs with the Tumor Stroma
3.3. Uptake of AgNPs by Cancer Cells
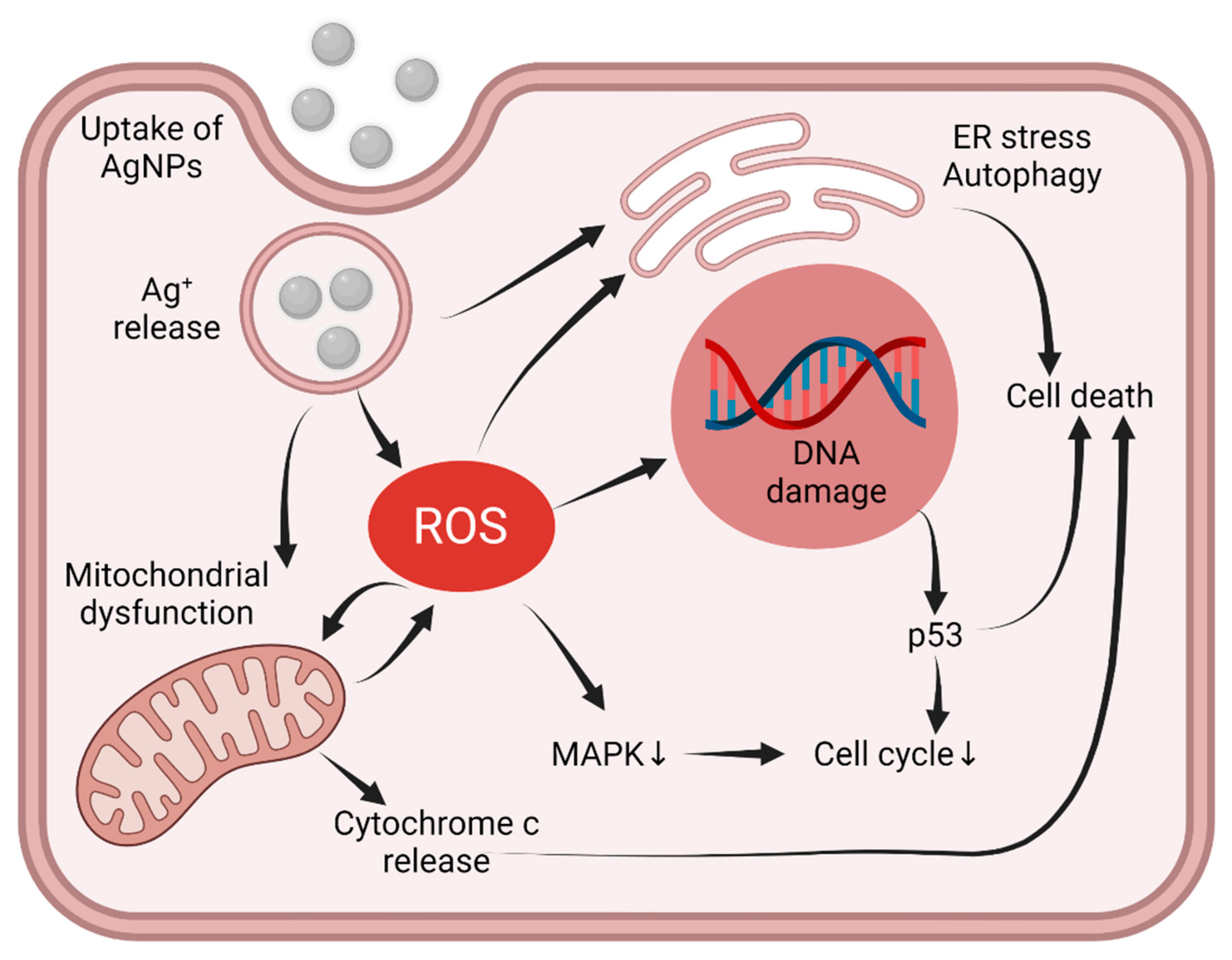
3.4. Intracellular Pathways Triggered by AgNPs
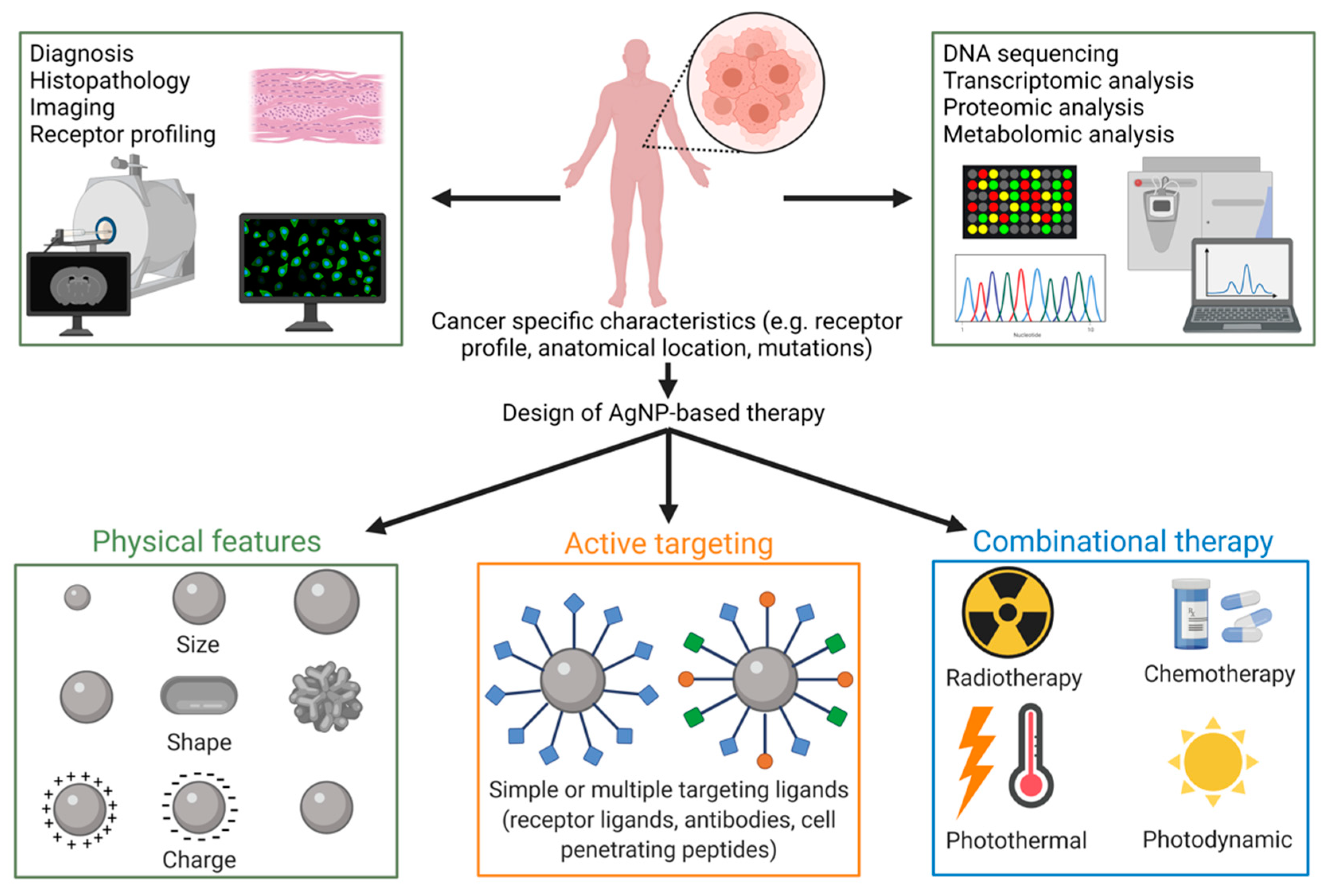
4. Applicability of AgNPs as Anti-Cancer Agents
4.1. Safety Issues: Toxicology on Healthy Tissues
4.2. Tailoring AgNP Surface Chemistry
4.3. Partners in Combination Therapy
4.4. Radio- and Photothermal Therapy
4.5. Therapeutic Strategies Implying Silver-Based Nanoparticles
5. Concluding Remarks—Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.; Hosseinian, F.; Willmore, W. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, I.-L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.-C.; Huang, Y.-J. Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; De Cerain, A.L. Genotoxicity of silver nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Souza, L.R.R.; da Silva, V.S.; Franchi, L.P.; de Souza, T.A.J. Toxic and beneficial potential of silver nanoparticles: The two sides of the same coin. Adv. Exp. Med. Biol. 2018, 1048, 251–262. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. Available online: https://pubmed.ncbi.nlm.nih.gov/26339255 (accessed on 22 December 2021).
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K. UV-Vis Spectroscopy for Characterization of Metal Nanoparticles Formed from Reduction of Metal Ions During Ultrasonic Irradiation. In UV-VIS and Photoluminescence Spectroscopy for Nanomaterials Characterization; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 151–177. [Google Scholar] [CrossRef]
- Vladár, A.E.; Hodoroaba, V.-D. Characterization of nanoparticles by scanning electron microscopy. Charact. Nanoparticles 2020, 7–27. [Google Scholar] [CrossRef]
- Su, D. Advanced electron microscopy characterization of nanomaterials for catalysis. Green Energy Environ. 2017, 2, 70–83. [Google Scholar] [CrossRef]
- Titus, D.; Samuel, E.J.J.; Roopan, S.M. Roopan, Nanoparticle characterization techniques. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–319. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, T.; Ejaz, A.; Abrar, M.; Afsheen, S.; Batool, S.S.; Fahad, M.; Tahir, M.B. Qualitative and quantitative analysis of nanoparticles using laser-induced breakdown spectroscopy (LIBS) and energy dispersive x-ray spectroscopy (EDS). Laser Phys. 2019, 29, 116001. [Google Scholar] [CrossRef]
- Klapetek, P.; Valtr, M.; Nečas, D.; Salyk, O.; Dzik, P. Atomic force microscopy analysis of nanoparticles in non-ideal conditions. Nanoscale Res. Lett. 2011, 6, 514. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Tortowa, NJ, USA, 2011; pp. 63–70. [Google Scholar] [CrossRef]
- Loganathan, S.; Valapa, R.B.; Mishra, R.K.; Pugazhenthi, G.; Thomas, S. Thermogravimetric Analysis for Characterization of Nanomaterials. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]
- Yang, M.-H.; Lin, C.-H.; Chang, L.W.; Lin, P. Application of ICP-MS for the Study of Disposition and Toxicity of Metal-Based Nanomaterials. Breast Cancer 2012, 926, 345–359. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P. Raman Spectroscopy of nanomaterials: How spectra relate to disorder, particle size and mechanical properties. Prog. Cryst. Growth Charact. Mater. 2007, 53, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Korin, E.; Froumin, N.; Cohen, S. Surface Analysis of Nanocomplexes by X-ray Photoelectron Spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R.R. International Journal of Drug Development Passive and Active Targeting in Tumor Microenvironment. Int. J. Drug Dev. Res. 2017, 9, 37–41. [Google Scholar]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.H.; Alzghari, S.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 2016, 232, 255–264. [Google Scholar] [CrossRef]
- Harrington, J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11234875 (accessed on 22 December 2021). [PubMed]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- Scott, D.A.; Drake, R.R. Glycosylation and its implications in breast cancer. Expert Rev. Proteom. 2019, 16, 665–680. [Google Scholar] [CrossRef]
- Pimentel, R.G.C.; Botero, V.R.; Martínez, E.S.M.; García, C.G.; Hinestroza, J.P. Soybean agglutinin-conjugated silver nanoparticles nanocarriers in the treatment of breast cancer cells. J. Biomater. Sci. Polym. Ed. 2016, 27, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Newell, B.; Irudayaraj, J. Folic acid protected silver nanocarriers for targeted drug delivery. J. Biomed. Nanotechnol. 2012, 8, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Zhang, F.; Chen, X.; Zhong, J.; Liu, G.; Tian, Y.; Huang, Q. Uptake of silver nanoparticles by DHA-treated cancer cells examined by surface-enhanced Raman spectroscopy in a microfluidic chip. Lab Chip 2017, 17, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Bhardwaj, V.; Nagasetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Multifunctional surface-enhanced raman spectroscopy-detectable silver nanoparticles for combined photodynamic therapy and pH-triggered chemotherapy. J. Biomed. Nanotechnol. 2016, 12, 2202–2219. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.H.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Silver nanoparticle-embedded graphene oxide-methotrexate for targeted cancer treatment. Colloids Surf. B 2017, 153, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Bathori, G.; Cervenak, L.; Karadi, I. Caveolae-An Alternative Endocytotic Pathway for Targeted Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 30–96. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef] [PubMed]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation Under Biorelevant Conditions. Int. J. Nanomed. 2021, 16, 3021–3040. [Google Scholar] [CrossRef]
- MiclǍuş, T.; Beer, C.; Chevallier, J.; Scavenius, C.; Bochenkov, V.E.; Enghild, J.J.; Sutherland, D.S. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbalinardo, M.; Caicci, F.; Cavallini, M.; Gentili, D. Protein Corona Mediated Uptake and Cytotoxicity of Silver Nanoparticles in Mouse Embryonic Fibroblast. Small 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiee, V.; Mahmoudi, M.; Lou, K.; Cheng, J.; Kraft, M.L. Protein corona significantly reduces active targeting yield. Chem. Commun. 2013, 49, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Quiros, S.; Aragoneses-Cazorla, G.; Garcia-Alcalde, L.; Vallet-Regí, M.; González, B.; Luque-Garcia, J.L. Cancer cell targeting and therapeutic delivery of silver nanoparticles by mesoporous silica nanocarriers: Insights into the action mechanisms using quantitative proteomics. Nanoscale 2019, 11, 4531–4545. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Naddaka, M.; Uboldi, C.; Loudos, G.; Fragogeorgi, E.; Molinari, V.; Pucci, A.; Tsotakos, T.; Psimadas, D.; Ponti, J.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Kovács, D.; Igaz, N.; Marton, A.; Rónavári, A.; Bélteky, P.; Bodai, L.; Spengler, G.; Tiszlavicz, L.; Rázga, Z.; Hegyi, P.; et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J. Nanobiotechnol. 2020, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.F.D.A.; Lins, M.P.; Viana, I.M.M.N.; Dos Santos, J.E.; Smaniotto, S.; Reis, M.D.D.S. Metallic nanoparticles reduce the migration of human fibroblasts in vitro. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Manshian, B.; Jimenez, J.; Himmelreich, U.; Soenen, S.J. Presence of an Immune System Increases Anti-Tumor Effect of Ag Nanoparticle Treated Mice. Adv. Health Mater. 2016, 6, 1601099. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Leo, B.F.; Carranza, C.; Chen, S.; Rivas-Santiago, C.; Porter, A.E.; Ryan, M.P.; Gow, A.; Chung, K.F.; Tetley, T.D.; et al. Modulation of human macrophage responses to mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS ONE 2015, 10, e0143077. [Google Scholar] [CrossRef] [PubMed]
- Reichel, D.; Tripathi, M.; Perez, J.M. Biological effects of nanoparticles on macrophage polarization in the tumor microenvironment. Nanotheranostics 2019, 3, 66–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, M.; Simard, J.-C.; Girard, D. Silver nanoparticles of 70 nm and 20 nm affect differently the biology of human neutrophils. J. Immunotoxicol. 2016, 13, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, M.; Simard, J.-C.; Antoine, F.; Girard, D. Interaction between silver nanoparticles of 20nm (AgNP20) and human neutrophils: Induction of apoptosis and inhibition of de novo protein synthesis by AgNP20 aggregates. J. Appl. Toxicol. 2014, 34, 404–412. [Google Scholar] [CrossRef]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered nanoparticles for drug delivery in cancer therapy. Angew.Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Kang, B.; Afifi, M.M.; Austin, L.A.; El-Sayed, M.A. Exploiting the Nanoparticle Plasmon Effect: Observing Drug Delivery Dynamics in Single Cells via Raman/Fluorescence Imaging Spectroscopy. ACS Nano 2013, 7, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Motte, L.; Olsen, J.C.; Platas-Iglesias, C.; Magzoub, M.; et al. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J. Nanomater. 2017, 2017, 5107485. [Google Scholar] [CrossRef]
- Igaz, N.; Kovács, D.; Rázga, Z.; Konya, Z.; Boros, I.M.; Kiricsi, M. Modulating chromatin structure and DNA accessibility by deacetylase inhibition enhances the anti-cancer activity of silver nanoparticles. Colloids Surf. B 2016, 146, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Sur, I.; Cam, D.; Kahraman, M.; Baysal, A.; Çulha, M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology 2010, 21, 175104. [Google Scholar] [CrossRef]
- Mellman, I.; Yarden, Y. Endocytosis and Cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a016949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Durham, P.; Sayes, C.M.; Lau, B.L.T.; Bruce, E.D. Particle uptake efficiency is significantly affected by type of capping agent and cell line. J. Appl. Toxicol. 2015, 35, 1114–1121. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Kovács, D.; Igaz, N.; Keskeny, C.; Bélteky, P.; Tóth, T.; Gáspár, R.; Madarász, D.; Rázga, Z.; Kónya, Z.; Boros, I.M.; et al. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci. Rep. 2016, 6, 27902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlowski, P.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Gniadek, M.; Labedz, O.; Malewski, T.; Nowakowska, J.; Chodaczek, G.; Celichowski, G.; Grobelny, J.; et al. Tannic acid-modified silver and gold nanoparticles as novel stimulators of dendritic cells activation. Front. Immunol. 2018, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, I.-H. Phagocytosis and endocytosis of silver nanoparticles induce interleukin-8 production in human macrophages. Yonsei Med. J. 2012, 53, 654–657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Y.; Zhang, Y.; Wang, Q.; Huang, Z.; Ding, Q.; Guo, Z.; Zhou, X.; Zhu, L.; Gu, N. The cellular uptake and cytotoxic effect of silver nanoparticles on chronic myeloid leukemia cells. J. Biomed. Nanotechnol. 2014, 10, 669–678. [Google Scholar] [CrossRef]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. British J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åmand, H.L.; Rydberg, H.A.; Fornander, L.H.; Lincoln, P.; Nordén, B.; Esbjörner, E.K. Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2669–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkhani, S.M.; Fard, A.A.; Zakeri-Milani, P.; Mojarrad, S.J.; Valizadeh, H. Enhancing antitumor activity of silver nanoparticles by modification with cell-penetrating peptides. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Osawa, M.; Okabe, S. In Vitro Toxicity of Silver Nanoparticles at Noncytotoxic Doses to HepG2 Human Hepatoma Cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- de Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; de Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomedicine 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Al-Abdan, M.; Albasher, G.; Alarifi, S. Effects of green silver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian, E.; Dizaj, S.M.; Rahimpour, E.; Hasanzadeh, A.; Eftekhari, A.; Hosain Zadegan, H.; Halajzadeh, J.; Ahmadian, H. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater. Sci. Eng. C 2018, 93, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Fahmi, A.; Mahltig, B. Impact of silver nanoparticles and silver ions on innate immune cells. J. Biomed. Nanotechnol. 2014, 10, 1146–1156. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Ferreira, L.B.; Rizoli, C.; Batista, Â.G.; Maróstica Júnior, M.R.; da Silva, E.; Cadore, S.; Durán, N.; Cruz-Höfling, M.A.; de Jesus, M.B. N-Acetylcysteine reverses silver nanoparticle intoxication in rats. Nanotoxicology 2019, 13, 326–338. [Google Scholar] [CrossRef]
- Valenzuela-Salas, M.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxidative Med. Cell. Longev. 2019, 2019, 4528241. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver nanoparticles induce mitochondrial protein oxidation in lung cells impacting cell cycle and proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2019, 256, 113430. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; He, S.; Ma, H.; Jiang, H.; Yan, N.; Zhu, L.; Bang, J.J.; Li, P.A.; Jia, S. Silver nanoparticle exposure causes pulmonary structural damage and mitochondrial dynamic imbalance in the rat: Protective effects of sodium selenite. Int. J. Nanomed. 2020, 15, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Jezek, J.; Cooper, K.F.; Strich, R. Reactive oxygen species and mitochondrial dynamics: The yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, T.; Chen, S.; Qi, S.; Zhang, Z.; Xu, Y. Silver nanoparticles regulate autophagy through lysosome injury and cell hypoxia in prostate cancer cells. J. Biochem. Mol. Toxicol. 2020, 2019, e22474. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.-C.; Durocher, I.; Girard, D. Silver nanoparticles induce irremediable endoplasmic reticulum stress leading to unfolded protein response dependent apoptosis in breast cancer cells. Apoptosis 2016, 21, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, J.M.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Desai, A.; Yan, Y.; Gerson, S.L. Advances in therapeutic targeting of the DNA damage response in cancer. DNA Repair 2018, 66–67, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Rageh, M.M.; El-Gebaly, R.H.; Afifi, M.M. Antitumor activity of silver nanoparticles in Ehrlich carcinoma-bearing mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, H.; Zhou, Z.; Zhao, M.; Chen, R.; Reed, S.H. XPF plays an indispensable role in relieving silver nanoparticle induced DNA damage stress in human cells. Toxicol. Lett. 2018, 2017, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Choi, D.Y.; Song, H.; Park, C.; Kim, J.H.; Hong, K. Cytotoxicity and transcriptomic analysis of silver nanoparticles in mouse embryonic fibroblast cells. Int. J. Mol. Sci. 2018, 19, 3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska, E.; Zauszkiewicz-Pawlak, A.; Wojcik, M.; Inkielewicz-Stepniak, I. Inkielewicz-Stepniak, Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget 2018, 9, 4675–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Shin, D.Y.; Park, Y.J.; Park, C.W.; Oh, S.M.; Chung, K.H. Silver nanoparticles induce p53-mediated apoptosis in human bronchial epithelial (BEAS-2B) cells. J. Toxicol. Sci. 2014, 39, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive Oxygen Species in the Activation of MAP Kinases, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 528. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Perrotta, C.; Maier, J.A. Silver nanoparticles-induced cytotoxicity requires ERK activation in human bladder carcinoma cells. Toxicol. Lett. 2015, 237, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Comfort, K.; Maurer, E.I.; Braydich-Stolle, L.K.; Hussain, S.M. Interference of Silver, Gold, and Iron Oxide Nanoparticles on EGF Signal Transduction in Epithelial Cells Supplementary Experimental. ACS Nano 2011, 5, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.J.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef]
- Limpert, A.S.; Lambert, L.J.; Bakas, N.A.; Bata, N.; Brun, S.N.; Shaw, R.J.; Cosford, N.D.P. Autophagy in Cancer: Regulation by Small Molecules. Trends Pharmacol. Sci. 2018, 39, 1021–1032. [Google Scholar] [CrossRef]
- Mao, B.-H.; Chen, Z.-Y.; Wang, Y.-J.; Yan, S.-J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gopisetty, K.; Kovács, D.; Igaz, N.; Rónavári, A.; Bélteky, P.; Rázga, Z.; Venglovecz, V.; Csoboz, B.; Boros, I.M.; Kónya, Z.; et al. Endoplasmic reticulum stress: Major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, D.; Sun, L.; Huang, Z.; Zhang, X.; Ma, W.; Wu, J.; Xiao, L.; Zhao, Y.; Gu, N. Activation of autophagy by elevated reactive oxygen species rather than released silver ions promotes cytotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in hematopoietic cells. Nanoscale 2017, 9, 5489–5498. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-L.; Jahangiri, A.; DeLay, M.; Aghi, M.K. Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy. Cancer Res. 2012, 72, 4294–4299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.; Yuan, Z.; Zhang, Q.; Long, Z.; Chen, J.; Tang, Z.; Zhu, Y.; Chen, S.; Xu, J.; Yan, M.; et al. Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy 2012, 8, 1798–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fageria, L.; Pareek, V.; Dilip, R.V.; Bhargava, A.; Pasha, S.S.; Laskar, I.R.; Saini, H.; Dash, S.; Chowdhury, R.; Panwar, J. Biosynthesized Protein-Capped Silver Nanoparticles Induce ROS-Dependent Proapoptotic Signals and Prosurvival Autophagy in Cancer Cells. ACS Omega 2017, 2, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, Z.; Wu, H.; Zhou, W.; Jin, P.; Wei, P.; Zhang, Y.; Zheng, F.; Zhang, J.; Xu, J.; et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy 2014, 10, 2006–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizama-Manibusan, B.; Mc Laughlin, B.A. Redox modification of proteins as essential mediators of CNS autophagy and mitophagy. FEBS Lett. 2013, 587, 2291–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Zhang, B.; Huang, W.; Wu, T.; Zhang, T.; Tang, M.; et al. Mitophagy–lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells. Ecotoxicol. Environ. Saf. 2020, 208, 111463. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Yin, X.-M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, M.; Zhang, T.; Du, E.; Liu, Y.; Qi, S.; Xu, Y.; Zhang, Z. Autophagic effects and mechanisms of silver nanoparticles in renal cells under low dose exposure. Ecotoxicol. Environ. Saf. 2018, 166, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Verfaillie, T.; Salazar, M.; Velasco, G.; Agostinis, P. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int. J. Cell Biol. 2010, 2010, 930509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Choi, J.-Y.; Choi, J.; Hyun, J.W. Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int. J. Biochem. Cell Biol. 2012, 44, 224–232. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Christen, V.; Capelle, M.; Fent, K. Silver nanoparticles induce endoplasmatic reticulum stress response in zebrafish. Toxicol. Appl. Pharmacol. 2013, 272, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, A.; Sharif, R.; Ahmad, M.; Masood, M.; Shahid, A.; Anjum, D.H.; Rafique, M.S.; Ghani, S. In vivo evaluation of the biodistribution of intravenously administered naked and functionalised silver nanoparticles in rabbit. IET Nanobiotechnol. 2015, 9, 368–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Hong, G.; He, W.; Zhou, K.; Yang, K.; Li, F.; Chen, G.; Liu, Z.; Dai, H.; et al. Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. Biomaterials 2013, 34, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Chauché, C.; Balharry, D.; Brown, D.M.; Kanase, N.; Boczkowski, J.; Lanone, S.; Stone, V. The role of Kupffer cells in the hepatic response to silver nanoparticles. Nanotoxicology 2014, 8 (Suppl. 1), 149–154. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, T.M.; Khowessah, O.; Motaleb, M.; El-Bary, A.A.; El-Kolaly, M.; Swidan, M.M. I-131 doping of silver nanoparticles platform for tumor theranosis guided drug delivery. Eur. J. Pharm. Sci. 2018, 122, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, U.M.; Mekkawy, I.A.A.; Naguib, M.; Sayed, A.E.-D.H. Silver nanoparticle–induced nephrotoxicity in Clarias gariepinus: Physio-histological biomarkers. Fish Physiol. Biochem. 2019, 45, 1895–1905. [Google Scholar] [CrossRef]
- Park, K.; Park, E.J.; Chun, I.K.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C. Bioavailability and Toxicokinetics of citrate-coated silver nanoparticles in rats. Arch. Pharmacal Res. 2011, 34, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Genter, M.B.; Newman, N.C.; Shertzer, H.G.; Ali, S.F.; Bolon, B. Distribution and systemic effects of intranasally administered 25 nm silver nanoparticles in adult mice. Toxicol. Pathol. 2012, 40, 1004–1013. [Google Scholar] [CrossRef]
- Dan, M.; Wen, H.; Shao, A.; Xu, L. Silver nanoparticle exposure induces neurotoxicity in the rat hippocampus without increasing the blood-brain barrier permeability. J. Biomed. Nanotechnol. 2018, 14, 1330–1338. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kopaeva, M.; Kashkarov, P. Effects of prolonged silver nanoparticle exposure on the contextual cognition and behavior of mammals. Materials 2018, 11, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javurek, B.; Suresh, D.; Spollen, W.G.; Hart, M.L.; Hansen, S.A.; Ellersieck, M.R.; Bivens, N.J.; Givan, S.A.; Upendran, A.; Kannan, R.; et al. Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Cruz, C.; Nuñez, G.A.; Espinoza-Gómez, H.; Flores-López, L.Z. Effect of molecular weight of PEG or PVA as reducing-stabilizing agent in the green synthesis of silver-nanoparticles. Eur. Polym. J. 2016, 83, 265–277. [Google Scholar] [CrossRef]
- Gao, H.; Yang, H.; Wang, C. Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil. Results Phys. 2017, 7, 3130–3136. [Google Scholar] [CrossRef]
- Hanh, T.T.; Thu, N.T.; Quoc, L.A.; Hien, N.Q. Synthesis and characterization of silver/diatomite nanocomposite by electron beam irradiation. Radiat. Phys. Chem. 2017, 139, 141–146. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. In Nanomedicines; Akhyar Farrukh, M., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanciullino, R.; Ciccolini, J.; Milano, G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit. Rev. Oncol. 2013, 88, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloids Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Wang, Y.; Dai, W.; Zou, H.; Wang, S.; Zhang, J.; Pan, J. Salinomycin effectively eliminates cancer stem-like cells and obviates hepatic metastasis in uveal melanoma. Mol. Cancer 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Gurunathan, S.; Zhang, X.-F. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int. J. Nanomed. 2016, 11, 3655–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.-G.; Zhang, S.; Hwang, J.-Y.; Kong, I.-K. Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxidative Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Szoke, K.; Igaz, N.; Spengler, G.; Molnár, J.; Tóth, T.; Madarász, D.; Rázga, Z.; Kónya, Z.; Boros, I.M.; et al. Silver nanoparticles modulate ABC transporter activity and enhance chemotherapy in multidrug resistant cancer. Nanomedicine 2016, 12, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. Combination effect of silver nanoparticles and histone deacetylases inhibitor in human alveolar basal epithelial cells. Molecules 2018, 23, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Liu, D.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Ma, J.; Sun, X.; Chen, Z.; Jiang, X.; Guo, Z.; Huang, L.; Li, Y.; Wang, M.; Wang, C.; et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res. 2009, 19, 1031–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Tan, H.; Zhang, X.; Chen, F.; Zhou, Z.; Hu, X.; Chang, S.; Liu, P.; Zhang, H. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, S922–S930. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Huang, Z.; Chen, Z.; Xu, R.; Wu, H.; Zang, F.; Wang, C.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef]
- Wang, R.; Chen, C.; Yang, W.; Shi, S.; Wang, C.; Chen, J. Enhancement Effect of Cytotoxicity Response of Silver Nanoparticles Combined with Thermotherapy on C6 Rat Glioma Cells. J. Nanosci. Nanotechnol. 2013, 13, 3851–3854. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Guo, Z.; Wang, Z.; Liu, L. Silver Nanocrystals Mediated Combination Therapy of Radiation with Magnetic Hyperthermia on Glioma Cells. J. Nanosci. Nanotechnol. 2012, 12, 8276–8281. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.B.R.; Romero, J.J.; Bertolotti, S.G.; Gonzalez, M.C.; Mártire, D.O. Effect of Silver Nanoparticles on the Photophysics of Riboflavin: Consequences on the ROS Generation. J. Phys. Chem. C 2016, 120, 21967–21975. [Google Scholar] [CrossRef]
- Rivas Aiello, M.B.; Castrogiovanni, D.; Parisi, J.; Azcárate, J.C.; García Einschlag, F.S.; Gensch, T.; Bosio, G.N.; Mártire, D.O. Photodynamic Therapy in HeLa Cells Incubated with Riboflavin and Pectin-coated Silver Nanoparticles. Photochem. Photobiol. 2018, 94, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver nanoparticles as real topical bullets for wound healing. J. Am. Coll. Clin. Wound Spec. 2011, 3, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafaga, A.; Abu-Ahmed, H.M.; El-Khamary, A.N.; Elmehasseb, I.M.; Shaheen, H.M. Enhancement of Equid Distal Limb Wounds Healing by Topical Application of Silver Nanoparticles. J. Equine Vet. Sci. 2018, 61, 76–87. [Google Scholar] [CrossRef]
- Bu, L.L.; Yan, J.; Wang, Z.; Ruan, H.; Chen, Q.; Gunadhi, V.; Bell, R.B.; Gu, Z. Advances in drug delivery for post-surgical cancer treatment. Biomaterials 2019, 219, 119182. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier Andrade, L.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekkawy, A.; El-Mokhtar, M.; Nafady, N.; Yousef, N.; Hamad, M.; El-Shanawany, S.; Ibrahim, E.; Elsabahy, M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: Effect of surface coating and loading into hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Griffin, L.L.; Ali, F.R.; Lear, J.T. Non-melanoma skin cancer. Clin. Med. 2016, 16, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Du, P.; Zhao, H.; Xie, H.; Li, Y.; Yao, L.; Shi, Y.; Hu, L.; Si, S.; Zhang, M.; et al. Ag@TiO2 Nanoprisms with Highly Efficient Near-Infrared Photothermal Conversion for Melanoma Therapy. Chem. Asian J. 2020, 15, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Kadirvelu, K.; Balagurunathan, R. Biocompatible silver, gold and silver/gold alloy nanoparticles for enhanced cancer therapy: In vitro and in vivo perspectives. Nanoscale 2017, 9, 16773–16790. [Google Scholar] [CrossRef] [PubMed]
- Swanner, J.; Sears, J.J.; Singh, R.; Hooker, A.; Donati, G.L.; Furdui, C.M.; Cook, K.L.; Alli, E. Silver nanoparticles selectively treat triple—Negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB BioAdvances 2019, 1, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Curcio, A.; Cabana, S.; Radtke, G.; Bugnet, M.; Kolosnjaj-Tabi, J.; Péchoux, C.; Alvarez-Lorenzo, C.; Botton, G.A.; Silva, A.K.A.; et al. Intracellular Biodegradation of Ag Nanoparticles, Storage in Ferritin, and Protection by a Au Shell for Enhanced Photothermal Therapy. ACS Nano 2018, 12, 6523–6535. [Google Scholar] [CrossRef]
- Hembram, K.C.; Chatterjee, S.; Sethy, C.; Nayak, D.; Pradhan, R.; Molla, S.; Bindhani, B.K.; Kundu, C.N. Comparative and Mechanistic Study on the Anticancer Activity of Quinacrine-Based Silver and Gold Hybrid Nanoparticles in Head and Neck Cancer. Mol. Pharm. 2019, 16, 3011–3023. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, M.; Cui, H.; Gong, J.; Huang, Y.; Wang, J.; Cui, Y.; Dong, W.; Sun, L.; He, H.; et al. Tat-functionalized Ag-Fe3O4 nano-composites as tissue-penetrating vehicles for tumor magnetic targeting and drug delivery. Acta Pharm. Sin. B 2018, 8, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liang, J.; Wu, A.; Chen, Y.; Zhao, P.; Lin, T.; Zhang, M.; Xu, Q.; Wang, J.; Huang, Y. Co-Delivery of Trichosanthin and Albendazole by Nano-Self-Assembly for Overcoming Tumor Multidrug-Resistance and Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 26648–26664. [Google Scholar] [CrossRef]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Rahman, D.S.; Ghosh, S.K.; Sengupta, M. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Adv. Pharm. Bull. 2018, 8, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characterization Methods | Application | References |
|---|---|---|
| UV-Visible spectroscopy (UV-Vis) | Size, shape, stability and surface properties of nanoparticles, purity of sample | [13] |
| Scanning electron microscopy (SEM) | Size, shape, surface properties, purity of sample | [14] |
| Transmission electron microscopy (TEM) | Size distribution, shape, dispersity, purity of sample | [15] |
| Fourier transformed infrared spectroscopy (FT-IR) | Identification of surface residues, chemical species or functional groups | [16] |
| Powder X-ray diffraction (XRD) | Morphology, crystal structure, phase identification and crystallite size, purity of sample | [17] |
| Energy dispersive spectroscopy (EDS) | Structure and purity by determining the elemental composition | [18] |
| Atomic force microscopy (AFM) | Size, shape, surface properties, purity of sample | [19] |
| Dynamic light scattering (DLS) | Size distribution, average hydrodynamic diameter and stability | [20] |
| Zeta-potential measurement (ZP) | Stability and surface charge determination | [20,21] |
| Thermogravimetric analysis (TGA) | Chemical composition and the amount of coating on the surface of nanoparticles, thermal stability of nanoparticles | [22] |
| Inductively coupled plasma mass spectrometry (ICP-MS) | Surface chemical structure and chemical composition | [23] |
| Raman spectroscopy | Identification of surface residues, chemical species and functional groups | [24] |
| X-ray photoelectron spectroscopy (XPS) | Surface chemical composition, determination of chemical bonds | [25] |
| Nanoparticle Applied | Feature | Model | Effect | Role of AgNPs | Ref. |
|---|---|---|---|---|---|
| AgNP-TAT | Cell penetrating peptide-functionalized NP | B16 melanoma xenograft | Reduced tumor growth | Ag as active compound | [33] |
| Ag/AuNP | Gold-silver alloy particles | Diethylnitrosamine-induced hepatocarcinogenesis | Reduced tumor growth | Ag as active compound | [171] |
| AgNP | PVP-coated particles | C6-glioma bearing rat | Increased life span, enhanced efficacy of radiation therapy | Ag as active compound | [156] |
| AgNP | PVP-coated particles | MDA-MB-231 TNBC xenograft in mice | Reduced tumor growth | Ag as active compound | [172] |
| Ag@AuNP | Au shell on AgNPs | PC-3 prostate carcinoma xengraft in mice | Increased tumor growth inhibition by photothermal therapy | Ag as active compound | [173] |
| Ag/Ali@PNPs–Cltx | Silver/alisertib@polymeric nanoparticles conjugated with chlorotoxin | U87MG glioblastoma Xenograft in mice | Decreased tumor size | AgNP for delivery | [49] |
| QagNP | Quinacrine-based hybrid silver NP | SCC-9 head and neck cancer cells xenograft in mice | Decreased tumor size | AgNP for delivery | [174] |
| Tat-FeAgNP-Dox | Dextrin-coated silver nanoparticles attached with iron oxide nanoparticles, cell penetrating peptide and loaded with doxorubicin | MCF-7 xenograft in mice | Reduced tumor growth | AgNP for delivery | [175] |
| rTL/ABZ@BSA/Ag NP | Albendazole encapsulated in albumin-coated AgNPs and modified with cell penetrating peptide | Xenograft of drug resistant A549/T cells, and metastasis to lung in mice | Reduced tumor growth and metastasis | AgNP for delivery | [176] |
| AsNP | Aptamer As1411-functionalized AgNP | C6-glioma bearing mice | Increased efficacy of radiation therapy and life span | Ag as active compound | [70] |
| Ag@TiO2NP | AgNPs in a TiO2 shell layer | B16-F10 mleanoma cell xenograft in mice | Inhibit tumor growth as a high-performance photothermal therapy agent | Ag as active compound | [170] |
| AgNP | PVP-coated particles | B16-F10 melanoma cell xenograft in mice | Reduced tumor growth and increased survival | Ag as active compound | [84] |
| pGAgNPs | PEGylated, graphene-decorated silver nanoprisms | HCT116 colorectal cancer cell xenograft-bearing mice | Decreased tumour growth and increased life span by enhancing radiotherapy | Ag as active compound | [177] |
| AgNP-MSA | Mouse serum albumin-coated AgNPs | 3-methylcholanthrene and 12-O-tetradecanoyl-phorbol-13-acetate-induced mice fibrosarcoma | Reduced tumor growth and decreased incidence | Ag as active compound | [178] |
| CNT/AgNPs | Carbon nanotube-decorated AgNPs | B16-F10 melanoma cell xenograft in mice | Decreased tumor size as a photothermal therapy agent | Ag as active compound | [179] |
| Au@Ag | Au core Ag shell nanoparticles | 4T1 mice tumor metastasis model | Inhibition of lung metastasis | Ag as active compound | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. https://doi.org/10.3390/ijms23020839
Kovács D, Igaz N, Gopisetty MK, Kiricsi M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? International Journal of Molecular Sciences. 2022; 23(2):839. https://doi.org/10.3390/ijms23020839
Chicago/Turabian StyleKovács, Dávid, Nóra Igaz, Mohana K. Gopisetty, and Mónika Kiricsi. 2022. "Cancer Therapy by Silver Nanoparticles: Fiction or Reality?" International Journal of Molecular Sciences 23, no. 2: 839. https://doi.org/10.3390/ijms23020839
APA StyleKovács, D., Igaz, N., Gopisetty, M. K., & Kiricsi, M. (2022). Cancer Therapy by Silver Nanoparticles: Fiction or Reality? International Journal of Molecular Sciences, 23(2), 839. https://doi.org/10.3390/ijms23020839






