Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products
Abstract
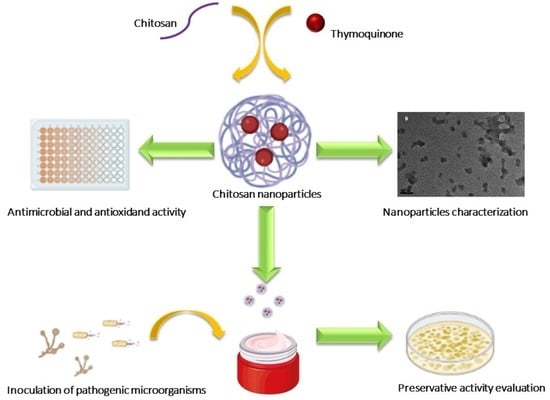
:1. Introduction
2. Results
2.1. Particle Size and Z Potential of NPCH and NPCH-TQ
2.2. Successful Encapsulation of Thymoquinone in Chitosan Nanoparticles
2.3. Morphology Characterization of Nanoparticles
2.4. Chemical Structure of NPCH-TQ
2.5. Thermal Properties of NPCH-TQ
2.6. In Vitro Drug Release of NPCH-TQ
2.7. DPPH Scavenging Activity of Free TQ and Nano-Formulations
2.8. Antimicrobial Evaluation of Free Terpene and Its Nano-Formulation
2.9. Evaluation of NPCH-TQ as a New Preservative Agent in Cosmetic Products
3. Discussion
4. Materials and Methods
4.1. Preparation of Loaded and Unloaded Thymoquinone-Chitosan Nanoparticles (NPCH-TQ)
4.1.1. Formulation of NPCH
4.1.2. Formulation of NPCH-TQ
4.2. Determination of Encapsulation Efficiency and Loading Efficiency of Thymoquinone-Chitosan Nanoparticles (NPCH-TQ)
Determination of Encapsulated TQ in Nanoparticles
4.3. Instrumental Characterization of Nanoparticles
4.3.1. Particle Size Analysis
4.3.2. Chemical Analysis of Nanoparticles
4.3.3. Thermal Properties of Nanoparticles
4.3.4. Morphology Studies of Nanoparticles
4.4. In Vitro Release Studies of NPCH-TQ
4.5. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
4.6. Antimicrobial Assay
4.7. Moisturizing Cream Formulation
4.8. Preservative Activity of AgNPs in Formulated Cream
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Michalek, I.; John, S.; Dos Santos, F.C. Microbiological contamination of cosmetic products—Observations from Europe, 2005–2018. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2151–2157. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [Green Version]
- Palacios, S.; Shaman, F.; Garcá, J.A. Prevalence of cosmetic sensitivity among beauticians. Allergol. Immunopathol. 1995, 23, 148–152. [Google Scholar]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; López-Jiménez, A.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Kam, W.R.; Li, Y.; Sullivan, D.A. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp. Eye Res. 2020, 196, 108057. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; De Lima, Á.A.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Aslam, H.; Shahzad, M.; Shabbir, A.; Irshad, S. Immunomodulatory effect of thymoquinone on atopic dermatitis. Mol. Immunol. 2018, 101, 276–283. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M.-A.; Hanan, E.-A.; Nabila, E.-M. Thymoquinone: Novel gastroprotective mechanisms. Eur. J. Pharmacol. 2012, 697, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.-F.; Samarghandian, S.; Farkhondeh, T. An Overview on Renoprotective Effects of Thymoquinone. Kidney Dis. 2018, 4, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Neuroprotective Effects of Thymoquinone in Acrylamide-Induced Peripheral Nervous System Toxicity Through MAPKinase and Apoptosis Pathways in Rat. Neurochem. Res. 2019, 44, 1101–1112. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Ela, F.I.A.; Alshahrani, F.K.; Bin-Jumah, M.; Al-Zharani, M.; Almutairi, B.; Alyousif, M.S.; Bungau, S.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ. Sci. Pollut. Res. 2020, 27, 37709–37717. [Google Scholar] [CrossRef]
- Fan, Q.; Yuan, Y.; Jia, H.; Zeng, X.; Wang, Z.; Hu, Z.; Gao, Z.; Yue, T. Antimicrobial and anti-biofilm activity of thymoquinone against Shigella flexneri. Appl. Microbiol. Biotechnol. 2021, 105, 4709–4718. [Google Scholar] [CrossRef]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Sánchez, A.L.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol. Food Chem. 2020, 328, 127131. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2020, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Woś-Latosi, K.; Jacyna, J.; Dąbrowska, M.; Potaś, J.; Markuszewski, M.J.; Winnicka, K. The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles. Pharmaceutics 2020, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Ince, I.; Yıldırım, Y.; Güler, G.; Medine, E.I.; Ballıca, G.; Kusdemir, B.C.; Göker, E. Synthesis and characterization of folic acid-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J. Radioanal. Nucl. Chem. 2020, 324, 71–85. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Khadrawy, Y.A.; Daim, T.M.A.-E.; Elfeky, A.S.; Rabo, A.A.A.; Mustafa, A.B.; Mostafa, I.T. Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol. Behav. 2020, 222, 112934. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Estupiñán, Ó.; Niza, E.; Bravo, I.; Rey, V.; Tornín, J.; Gallego, B.; Clemente-Casares, P.; Moris, F.; Ocaña, A.; Blanco-Lorenzo, V.; et al. Mithramycin delivery systems to develop effective therapies in sarcomas. J. Nanobiotechnol. 2021, 19, 267. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Rubio-Moraga, A.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym. 2021, 277, 118815. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.; Cuffini, A.M.; Alonzo, V.; Carlone, N. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Dehghani, H.; Hashemi, M.; Entezari, M.; Mohsenifar, A. The Comparison of Anticancer Activity of Thymoquinone and Nanothymoquinone on Human Breast Adenocarcinoma. Iran. J. Pharm. Res 2015, 14, 539–546. [Google Scholar] [PubMed]
- Alam, S.; Mustafa, G.; Khan, Z.I.; Islam, F.; Bhatnagar, A.; Ahmad, F.J.; Kumar, M. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Ahmad, R.; Kaus, N.H.M.; Hamid, S. Synthesis and Characterization of PLGA-PEG Thymoquinone Nanoparticles and Its Cytotoxicity Effects in Tamoxifen- Resistant Breast Cancer Cells. In Cancer Biology and Advances in Treatment. Advances in Experimental Medicine and Biology; Pham, P.V., Ed.; Springer: Cham, Switzerland, 2018; Volume 1292. [Google Scholar]
- Bhatta, A.; Krishnamoorthy, G.; Marimuthu, N.; Dihingia, A.; Manna, P.; Biswal, H.T.; Das, M.; Krishnamoorthy, G. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int. J. Biol. Macromol. 2019, 136, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Sarker, S.; Ghosh, A.; Gupta, P.; Das, S.; Ahir, M.; Bhattacharya, S.; Chattopadhyay, S.; Ghosh, S.; Adhikary, A. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater. Sci. 2019, 7, 4325–4344. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Anbukkarasi, M.; Thomas, P.A.; Geraldine, P. A comparison between plain eugenol and eugenol-loaded chitosan nanoparticles for prevention of in vitro selenite-induced cataractogenesis. J. Drug Deliv. Sci. Technol. 2021, 65, 102696. [Google Scholar] [CrossRef]
- Chen, F.; Shi, Z.; Neoh, K.; Kang, E. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Gondal, M.A.; Al-Zahrani, A.-H.J.; Rashid, S.G.; Ali, A. Synthesis, morphology and antifungal activity of nano-particulated amphotericin-B, ketoconazole and thymoquinone against Candida albicans yeasts and Candida biofilm. J. Environ. Sci. Health Part A 2015, 50, 119–124. [Google Scholar] [CrossRef]
- Xiao, X.-Y.; Zhu, Y.-X.; Bu, J.-Y.; Li, G.-W.; Zhou, J.-H.; Zhou, S.-P. Evaluation of Neuroprotective Effect of Thymoquinone Nanoformulation in the Rodent Cerebral Ischemia-Reperfusion Model. BioMed Res. Int. 2016, 2016, 2571060. [Google Scholar] [CrossRef] [Green Version]
- Qidwai, A.; Kumar, R.; Dikshit, A. Green synthesis of silver nanoparticles by seed of Phoenix sylvestris L. and their role in the management of cosmetics embarrassment. Green Chem. Lett. Rev. 2018, 11, 176–188. [Google Scholar] [CrossRef] [Green Version]




| Formulation | Average Size (nm) | PDI | Z-Value (mV) | EE% | EL% |
|---|---|---|---|---|---|
| NPCH | 48.6 ± 3.40 | 0.4 ± 0.02 | +49.8 ± 0.75 | - | - |
| NPCH-TQ 1:0.25 | 65.0 ± 1.40 | 0.4 ± 0.01 | +35.8 ± 3.23 | 88.2 ± 6.39 | 44.8 ± 0.70 |
| NPCH-TQ 1:0.5 | 57.5 ± 0.36 | 0.3 ± 0.01 | +27.8 ± 1.13 | 93.2 ± 2.05 | 48.4 ± 1.14 |
| NPCH-TQ 1:0.75 | 57.5 ± 0.33 | 0.2 ± 0.01 | +25.3 ± 0.76 | 94.5 ± 1.94 | 48.8 ± 1.24 |
| NPCH-TQ 1:1 | 63.4 ± 0.65 | 0.2 ± 0.01 | +23.9 ± 0.58 | 90.6 ± 10.4 | 50.7 ± 8.70 |
| Microorganism | Control MIC (µg/mL) | TQ (µg/mL) | NPCH (µg/mL) | NPCH-TQ (µg/mL) |
|---|---|---|---|---|
| E. coli | * 1000 | 1000 | 1000 | 292 |
| P. aeruginosa | * 1000 | >1000 | 1000 | 417 |
| S. aureus | * 1000 | >1000 | >1000 | 333 |
| C. albicans | ** 250 | 333 | >1000 | 250 |
| A. brasiliensis | ** 250 | 250 | >1000 | 500 |
| Ingredient | Control Cream (%) | NPCH-TQ Cream (%) |
|---|---|---|
| Water (A) | 47 | 46.5 |
| Vegetable glycerin (A) | 10 | 10 |
| Urea (A) | 3 | 3 |
| Glyceryl monostearate (B) | 8 | 8 |
| Argania spinosa kernel oil (B) | 28 | 28 |
| Allantoin (C) | 0.4 | 0.4 |
| Avena sativa extract (C) | 3 | 3 |
| Vitamin E (C) | 0.5 | 0.5 |
| Parfum (C) | 0.1 | 0.1 |
| NPCH-TQ (C) | - | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondéjar-López, M.; López-Jiménez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. Int. J. Mol. Sci. 2022, 23, 898. https://doi.org/10.3390/ijms23020898
Mondéjar-López M, López-Jiménez AJ, Martínez JCG, Ahrazem O, Gómez-Gómez L, Niza E. Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. International Journal of Molecular Sciences. 2022; 23(2):898. https://doi.org/10.3390/ijms23020898
Chicago/Turabian StyleMondéjar-López, María, Alberto José López-Jiménez, Joaquín C. García Martínez, Oussama Ahrazem, Lourdes Gómez-Gómez, and Enrique Niza. 2022. "Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products" International Journal of Molecular Sciences 23, no. 2: 898. https://doi.org/10.3390/ijms23020898
APA StyleMondéjar-López, M., López-Jiménez, A. J., Martínez, J. C. G., Ahrazem, O., Gómez-Gómez, L., & Niza, E. (2022). Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. International Journal of Molecular Sciences, 23(2), 898. https://doi.org/10.3390/ijms23020898