Silk Protein Composite Bioinks and Their 3D Scaffolds and In Vitro Characterization
Abstract
:1. Background
2. Results
2.1. Bioink Synthesis
2.1.1. Synthesis of Biological Ink
2.1.2. Three-Dimensional Biological Printing
2.2. Mechanical Properties
2.2.1. Effect of GMA Content on Mechanical Properties of SF-Alone Scaffold
2.2.2. Effect of Silk Degumming Agent Concentration on Mechanical Properties of Pure SF Scaffold
2.2.3. Effect of Silk Degumming Time on Mechanical Properties of Pure SF Scaffold
2.2.4. Effect of Incorporating Grafted Sericin on the Mechanical Properties of SP Scaffolds
2.3. Microstructure
2.4. Infrared Spectrum
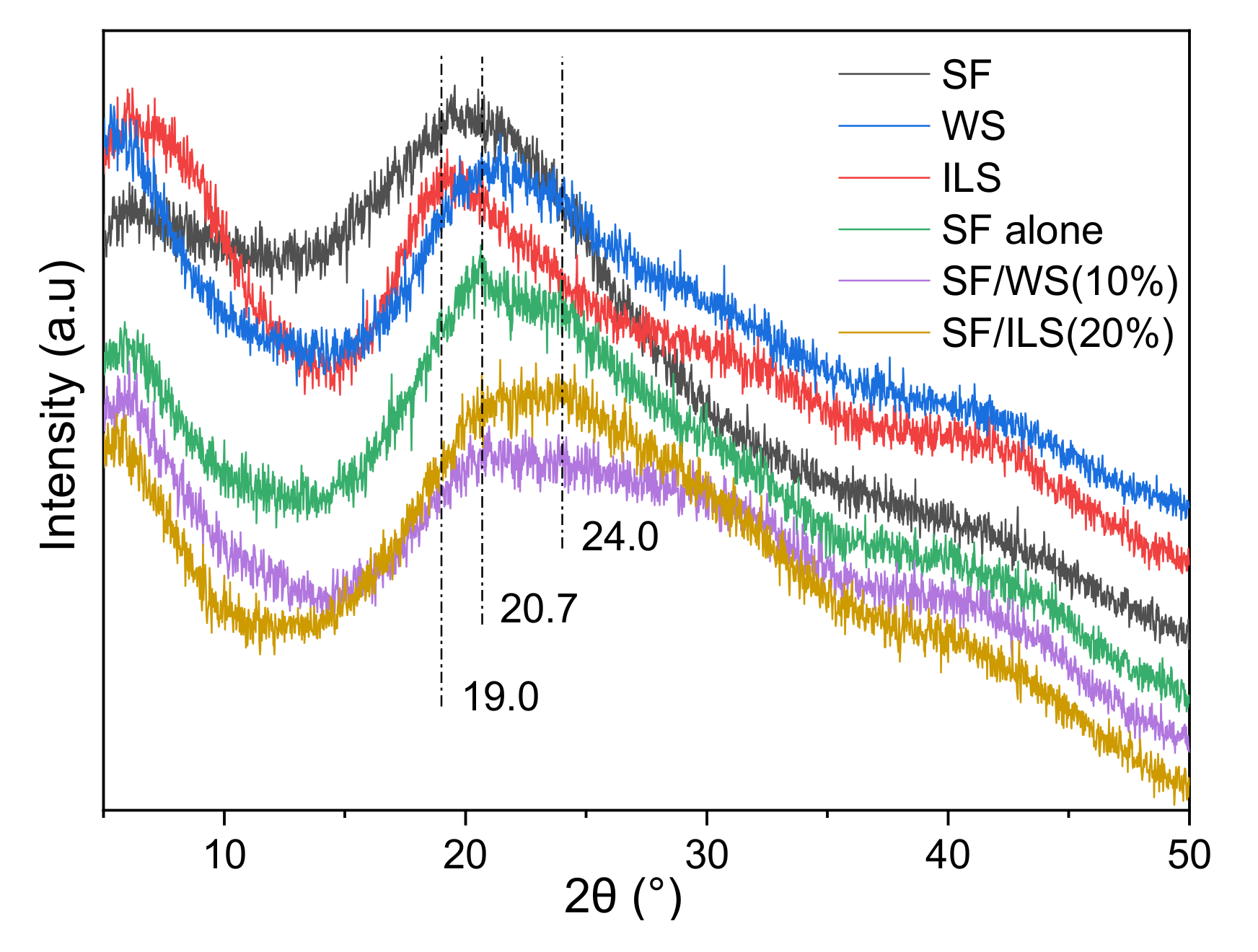
2.5. X-ray Diffraction
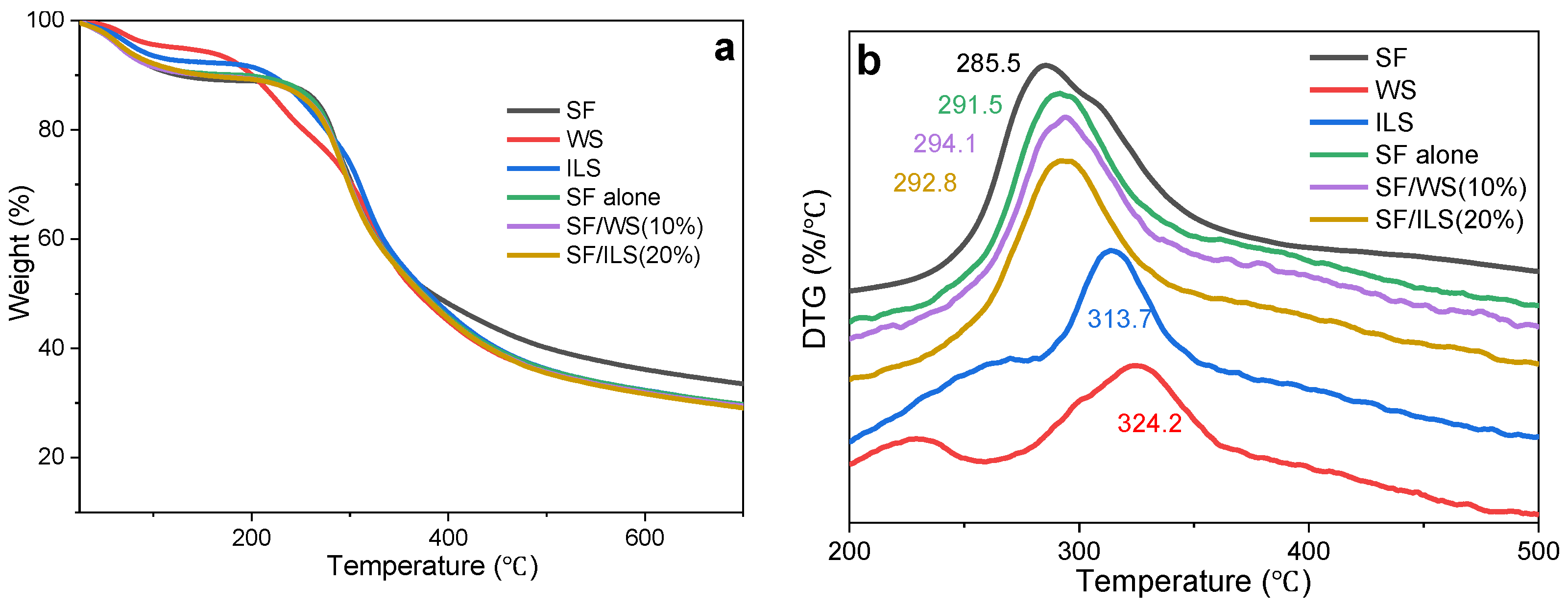
2.6. Thermal Analysis
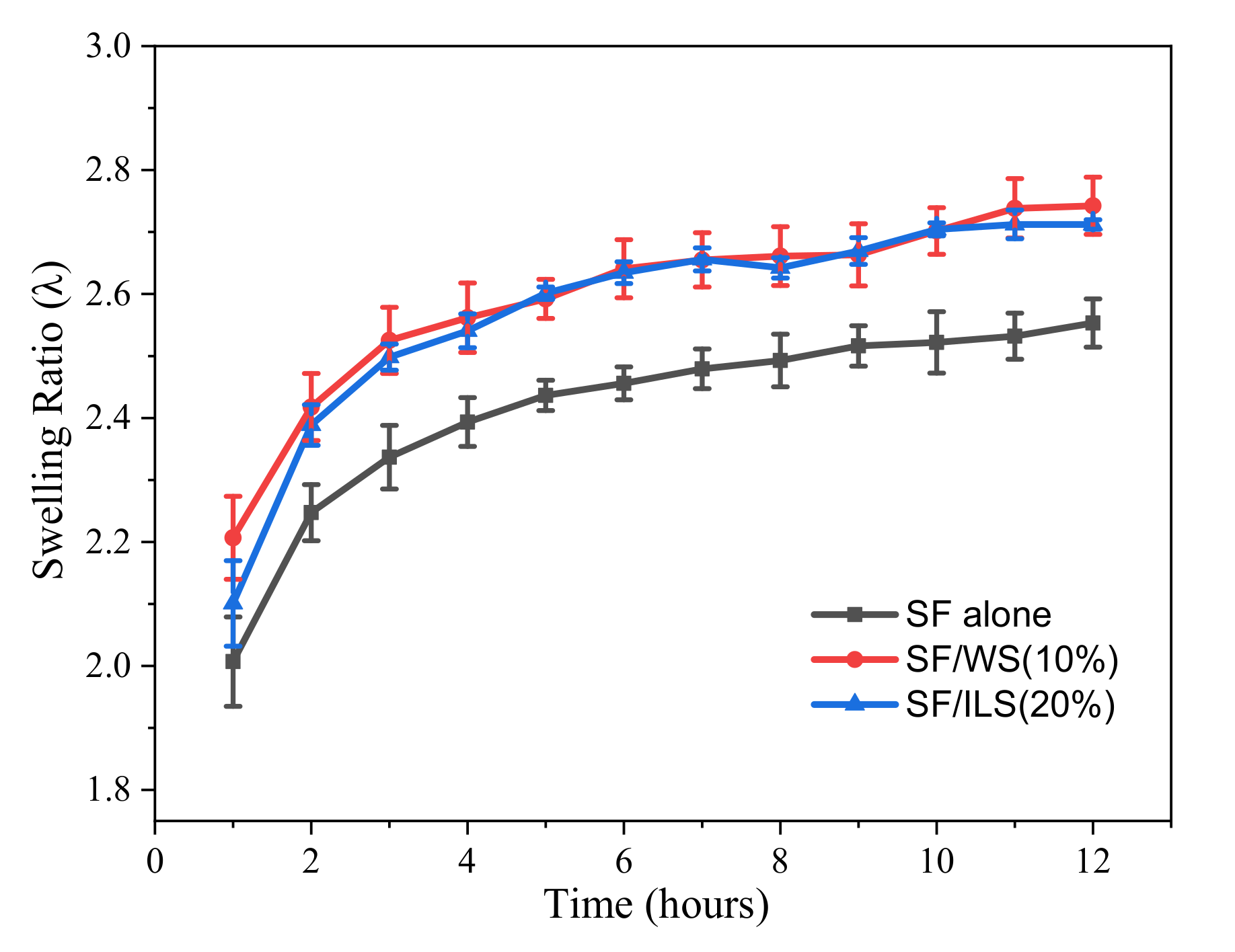
2.7. Swelling Rate
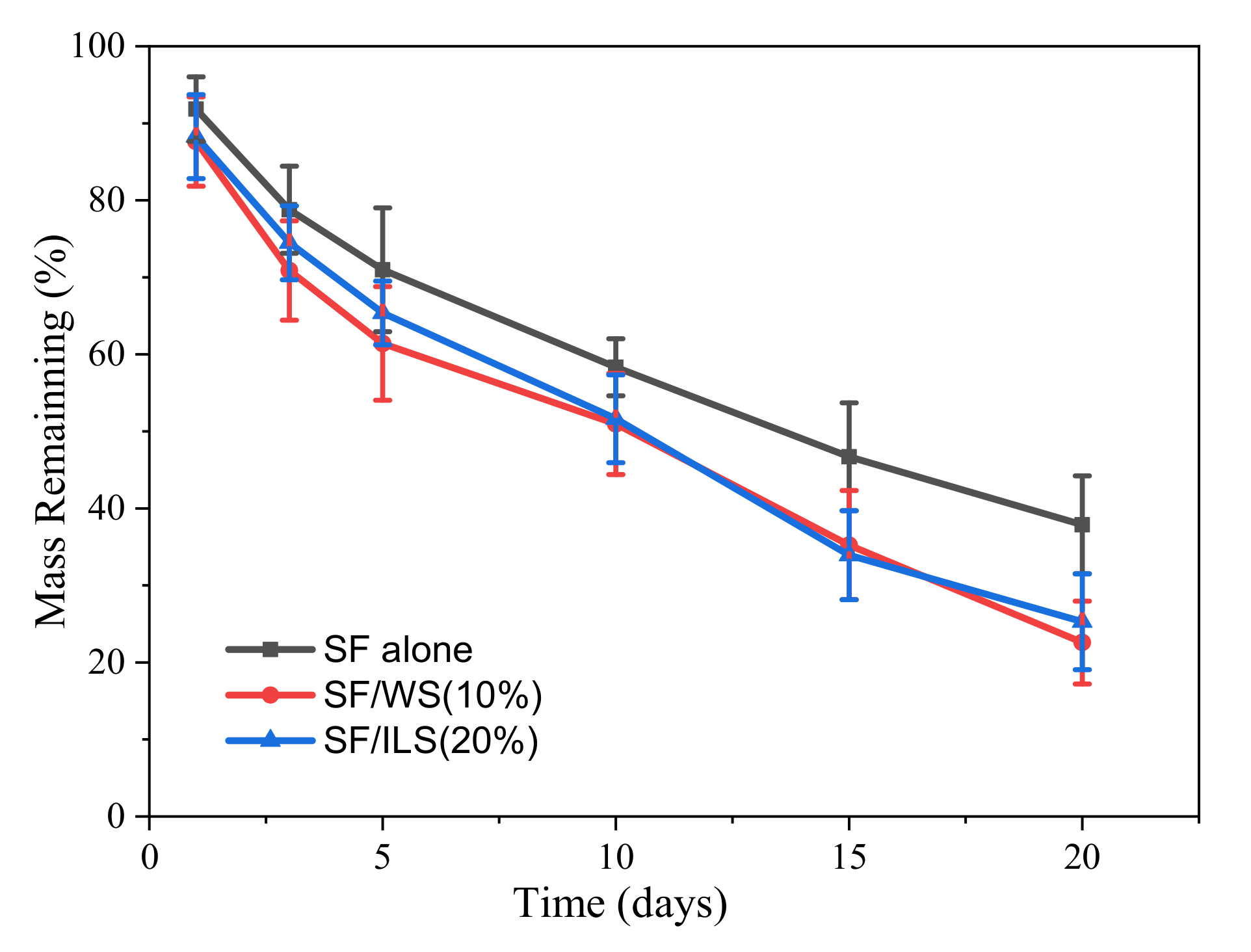
2.8. Enzyme Degradation
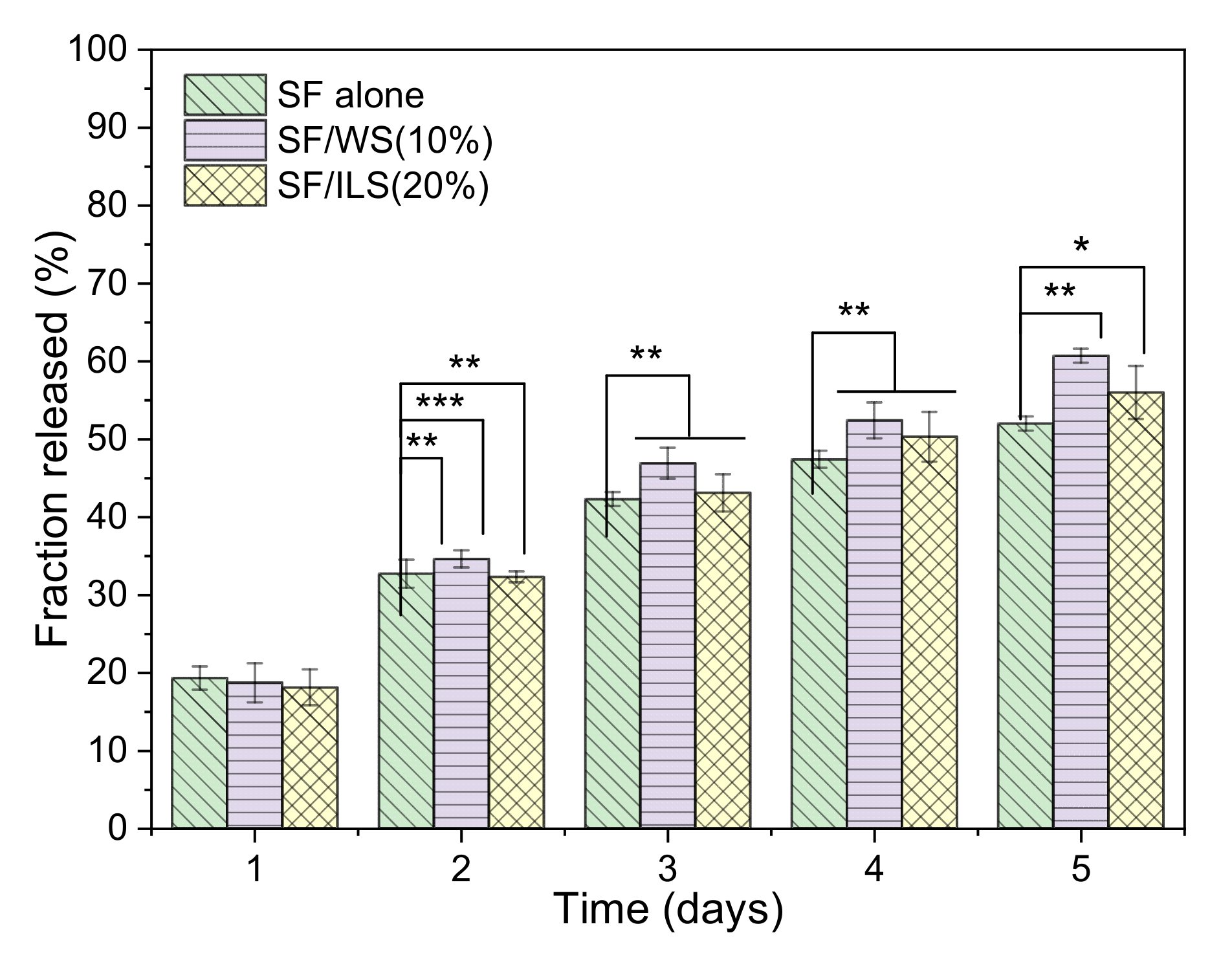
2.9. Drug Delivery Capacity
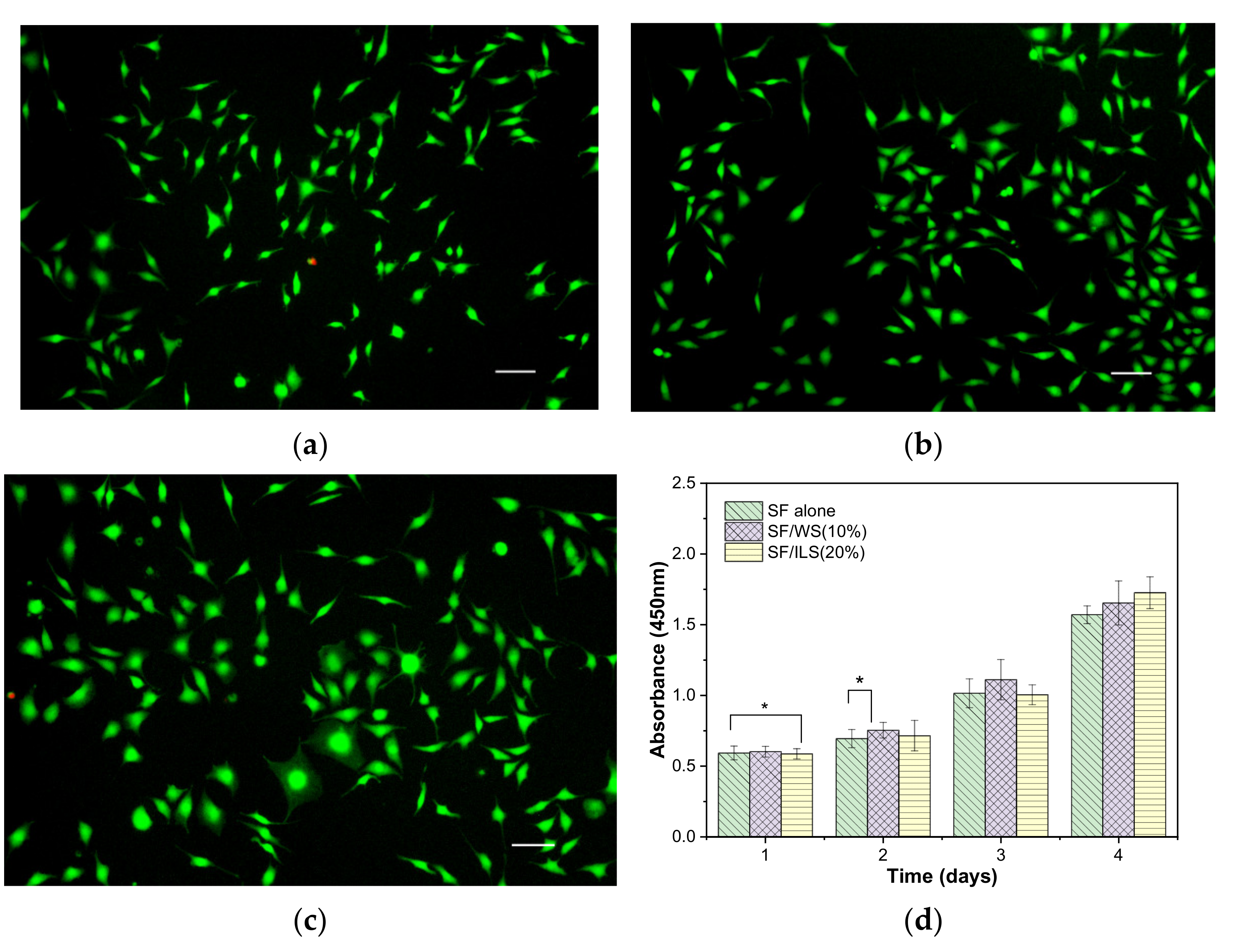
2.10. Cytocompatibility
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Separation and Purification
4.3. Grafting Methods
- (1)
- The dried SF fiber obtained from the Ca(OH)2-degumming process described above was dissolved in a water bath (60 °C) with a 9.3 M solution of LiBr solution and agitated to obtain the SF salt solution (10%, w/v). Next, the SF salt solution was placed on a magnetic stirrer, and the following concentrations of GMA were added dropwise to the 100 mL SF salt solution (10%, w/v), respectively, at a speed of approximately 0.5 mL/min: 1.0 mL, 2.5 mL, 5.0 mL, and 7.5 mL. The grafting conditions were as follows: rotating speed, 300 rpm; temperature, 60 °C; time, 3 h. The grafted solution was transferred to a dialysis bag (MW: 14,000) and dialyzed for 3 days, during which the deionized water was changed every 6 h. Finally, the grafted silk fibroin (GMA-SF, abbreviated to G-SF) was frozen at –80 °C, and then freeze-dried into a porous foam structure and stored in the dark. The four types of grafted silk fibroin were designated as G1.0-SF, G2.5-SF, G5.0-SF, and G7.5-SF, respectively.
- (2)
- The powdered WS and ILS obtained above were dissolved in deionized water to give a 10% (w/v) sericin solution. Next, the solution was placed on a magnetic stirrer, and the same amount of G-SF described above was added dropwise at a speed of about 0.5 mL/min. A dialysis bag (MW: 3500) was used for the dialysis. The remaining steps were the same as those for making G-SF. Two types of grafted sericin were obtained, namely grafted whole sericin (GMA-WS, abbreviated as G-WS) and grafted inner sericin (GMA-ILS, abbreviated as G-ILS), and the samples were kept away from light.
4.4. Biological Ink Formulation
4.5. Testing of Mechanical Properties
4.6. Swelling Termination
4.7. Determination of Pore Size and Porosity
4.8. Structure Analysis Methods
4.9. SEM
4.10. Enzymatic Hydrolysis
4.11. Drug Release Rate Measurement
4.12. Cell Culture and Viability Determination
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Gillispie, G.J.; Copus, J.S. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Freeman, F.E.; Kelly, D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2003, 20, 91–100. [Google Scholar] [CrossRef]
- Zhou, C.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucl. Acid Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. J. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Aldo, L.E.; Scheibel, T. Silk-based materials for biomedical applications. Biotechnol. Appl. Biochem. 2010, 55, 155–167. [Google Scholar]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Osman, R. Nanostructure formation through β-sheet self-assembly in silk-based materials. Macromolecules 2001, 34, 1477–1486. [Google Scholar]
- Salama, A. Cellulose/Silk Fibroin Assisted Calcium Phosphate Growth: Novel Bio composite for Dye Adsorption. Int. J. Biol. Macromol. 2020, 165, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Han, X.; Lu, Q.; Qi, X.; Guo, C.; Wu, X. Rhein Incorporated Silk Fibroin Hydrogels with Antibacterial and Anti-Inflammatory Efficacy to Promote Healing of Bacteria-Infected Burn Wounds. Int. J. Biol. Macromol. 2022, 201, 14–19. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mishra, N.S.; Saravanan, P. Polydopamine Modified Silk Fibroin 3-D Anode for Enhanced Microbial Fuel Cell Operation. Sustain. Energy Technol. Assess. 2022, 49, 101696. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, L.; Ma, Y.; Han, Q.; Li, X.; Li, J.; Wang, A.; Bai, S.; Yin, J. Preparation of Transient Electronic Devices with Silk Fibroin Film as a Flexible Substrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124896. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, D.; Engqvist, H.; Luo, J.; Persson, C. Silk Fibroin Hydrogels Induced and Reinforced by Acidic Calcium Phosphate–A Simple Way of Producing Bioactive and Drug-Loadable Composites for Biomedical Applications. Int. J. Biol. Macromol. 2021, 193, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 371–387. [Google Scholar] [PubMed]
- Ghosh, S.; Parker, S.T.; Wang, X.; Kaplan, D.L.; Lewis, J.A. Direct-write assembly of microperiodic silk fibroin scaffolds for tissue engineering applications. Adv. Funct. Mater. 2008, 18, 1883–1889. [Google Scholar] [CrossRef]
- Amanda, R.M.; David, L.K. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet biop rinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng. 2016, 3, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym 2020, 227, 115335. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, I.S.; Oh, J.E.; Jeong, L.; Lee, T.S.; Lee, S.J.; Park, W.H.; Min, B.M. Collagen-based biomimetic nanofibrous scaffolds preparation and characterization of collagen/silk fibroin bicomponent nanofibrous structures. Biomacromolecules 2008, 9, 1106–1116. [Google Scholar] [CrossRef]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Cilli, E.M.; Lima Ribeiro, S.J.; Scarel-Caminaga, R.M.; de Oliveira Capote, T.S. Silk fibroin/hydroxyapatite composite membranes: Production, characterization and toxicity evaluation. Toxicol. Vitr. 2020, 62, 104670. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D Printing of Mesoporous Bioactive Glass/Silk Fibroin Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Taylor, C.S.; Zhang, Y.; Gregory, D.A.; Tomeh, M.A.; Haycock, J.W.; Smith, P.J.; Wang, F.; Xia, Q.; Zhao, X. Cell Guidance on Peptide Micropatterned Silk Fibroin Scaffolds. J. Colloid Interface Sci. 2021, 603, 380–390. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from Ultrasonication-Induced Silk Fibroin−Hyaluronic Acid Hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Highly elastomeric photocurable silk hydrogels. Int. J. Biol. Macromol. 2019, 134, 838–845. [Google Scholar]
- Wen, Z.; Jiang, F.; Wu, F.; Yin, Z.; Kaur, K.; Chakraborty, J.; Ghosh, S.; Lu, S. Photocurable silk fibroin polyvinylpyrrolidone hydrogel. Materials 2020, 9, 100525. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; Park, C.H. Digital Light Processing 3D Printed Silk Fibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Bae SBin Kim, M.H.; Park, W.H. Electrospinning and Dual Crosslinking of Water-Soluble Silk Fibroin Modified with Glycidyl Methacrylate. Polym. Degrad. Stab. 2020, 179, 109304. [Google Scholar]
- Sinna, J.; Numpaisal, P.O.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Extraction of Silk Fibroin and Glycidyl Methacrylate Grafting on Silk Fibroin Optimization of SF-g-GMA for Meniscus Tissue Engineering. Mater. Today Proc. 2021, 47, 3476–3479. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.; Zhang, Q.; Liang, C.; Qin, H.; Liu, P.; Wang, K.; Zhang, X.; Chen, L.; Wei, Y. Shape changes and interaction mechanism of Escherichia coli cells treated with sericin and use of a sericin-based hydrogel for wound healing. Appl. Environ. Microbiol. 2016, 82, 4663–4672. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Tong, X.; Huang, Y.; Zhou, X.; Yang, C.; Chen, J.; Dai, F.; Xiao, B. Oral administration of hydrogel-embedding silk sericin alleviates ulcerative colitis through wound healing, anti-inflammation, and anti-oxidation. ACS Biomater. Sci. Eng. 2019, 5, 6231–6242. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 4, 513–521. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, S.-X.; Yin, X.-L.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Silk sericin has significantly hypoglycaemic effect in type 2diabetic mice via anti-oxidation and anti-inflammation. Int J Biol Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef]
- Ohnishi, K.; Murakami, M.; Morikawa, M.; Yamaguchi, A. Effect of the silk protein sericin on cryopreserved rat islets. J. Hepatobiliary Pancreat. Sci. 2012, 19, 354–360. [Google Scholar] [CrossRef]
- Verdanova, M.; Pytlik, R.; Kalbacova, M.H. Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells. Biopreserv. Biobank. 2014, 12, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Sahu, N.; Pal, S.; Sapru, S.; Kundu, J.; Talukdar, S.; Singh, N.I.; Yao, J.; Kundu, S.C. Non-mulberry and mulberry silk protein sericins as potential media supplement for animal cell culture. Biomed. Res. Int. 2016, 7461041, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.-T.; Zhang, Y.-Q. Viability and proliferation of L929,tumour and hybridoma cells in the culture media containing sericin protein as a supplement or serum substitute. Appl. Microbiol. Biotechnol. 2015, 99, 7219–7228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, T.-T.; Wei, Z.-G.; Zhang, Y.-Q. Silk Sericin Hydrolysate is a Potential Candidate as a Serum-Substitute in the Culture of Chinese Hamster Ovary and Henrietta Lacks Cells. J. Insect Sci. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akasu, Y.T.; Amada, H.Y.; Subouchi, K.T. Isolation of Three Main Sericin Components from the Cocoon of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar]
- Takasu, Y.; Hata, T.; Uchino, K.; Zhang, Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 339–344. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.Q. Three-Layered sericins around the silk fibroin fiber from bombyx mori cocoon and their amino acid composition. Adv. Mater. Res. 2011, 175, 158–163. [Google Scholar] [CrossRef]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-Crosslinkable, Injectable Sericin Hydrogel as 3D Biomimetic Extracellular Matrix for Minimally Invasive Repairing Cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Chen, C.S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.L.; Tan, R.W.; Liu, W.Q.; Zhang, Y.S.; She, Z.D.; Li, S.J. Three-Dimensionally Printed Silk-Sericin-Based Hydrogel Scaffold: A Promising Visualized Dressing Material for Real-Time Monitoring of Wounds. ACS Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Li, W.W.; Wang, F.; Zhang, Y.Q. Using of hydrated lime water as a novel degumming agent of silk and sericin recycling from wastewater. J. Clean. Prod. 2018, 172, 2090–2096. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Zhang, Y.Q. Greener degumming production of layered sericin peptides from a silkworm cocoon and their physicochemical characteristics and bioactivities in vitro. J. Clean. Prod. 2020, 261, 121080. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Chov, N.-J. Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2015, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Weng, Y.J.; Zhang, Y.Q. Accelerated desalting and purification of silk fibroin in a CaCl2-EtOH-H2O ternary system by excess isopropanol extraction. J. Chem. Technol. Biotechnol. 2021, 96, 1176–1186. [Google Scholar] [CrossRef]
- Asakura, T. Structure of Silk I (Bombyx mori Silk Fibroin before Spinning)-Type II β-Turn, Not α-Helix-. Molecules 2021, 26, 3706. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Zhang, Y.-Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C. 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Zhang, Y.-Q. The potential of silk sericin protein as a serum substitute or an additive in cell culture and cryopreservation. Amino Acids. 2017, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhang, Y.Q.; Wei, Z.G. Characterization of undegraded and degraded silk fibroin and its significant impact on the properties of the resulting silk biomaterials. Int. J. Biol. Macromol. 2021, 176, 578–588. [Google Scholar] [CrossRef]

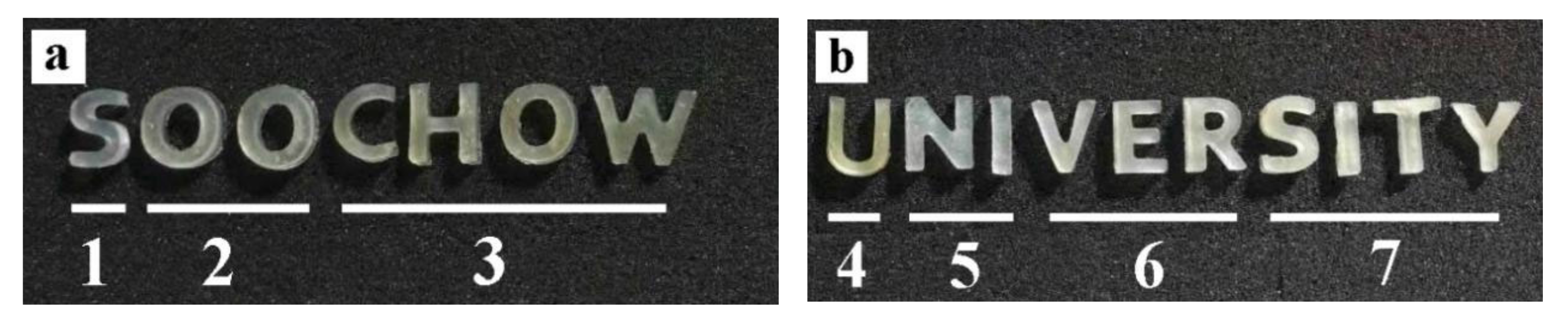
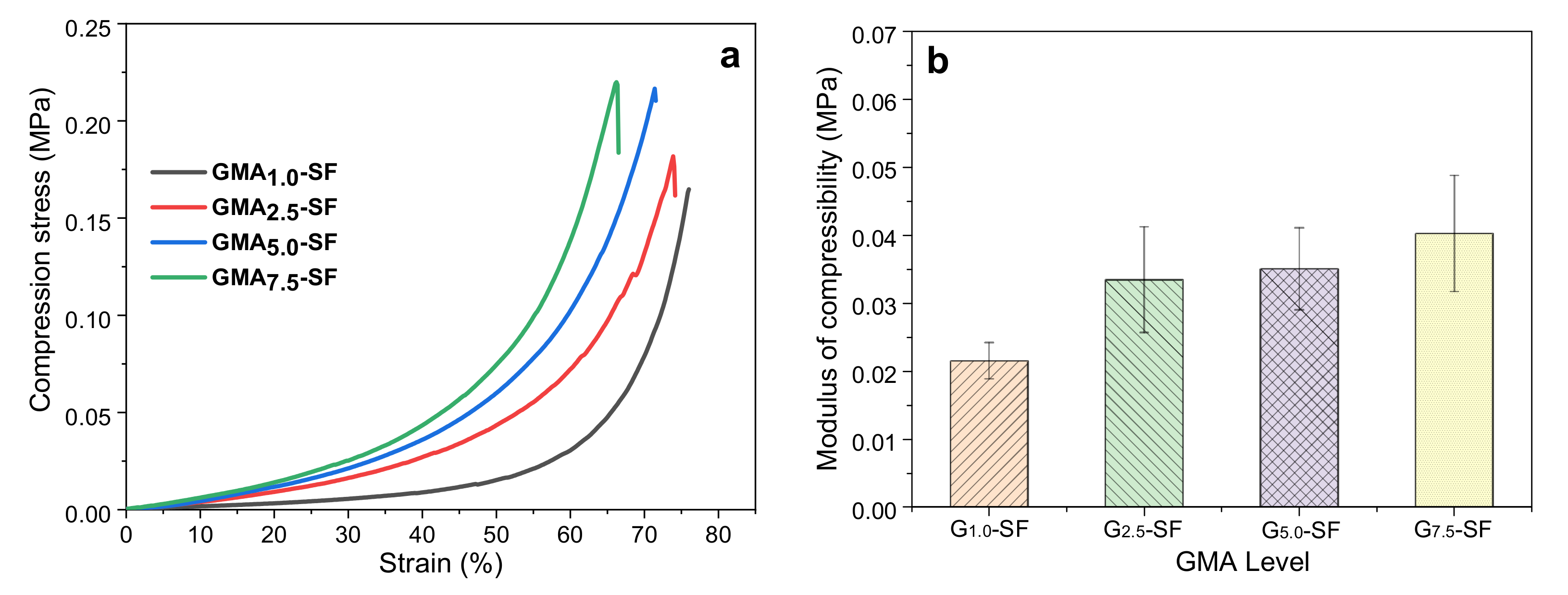

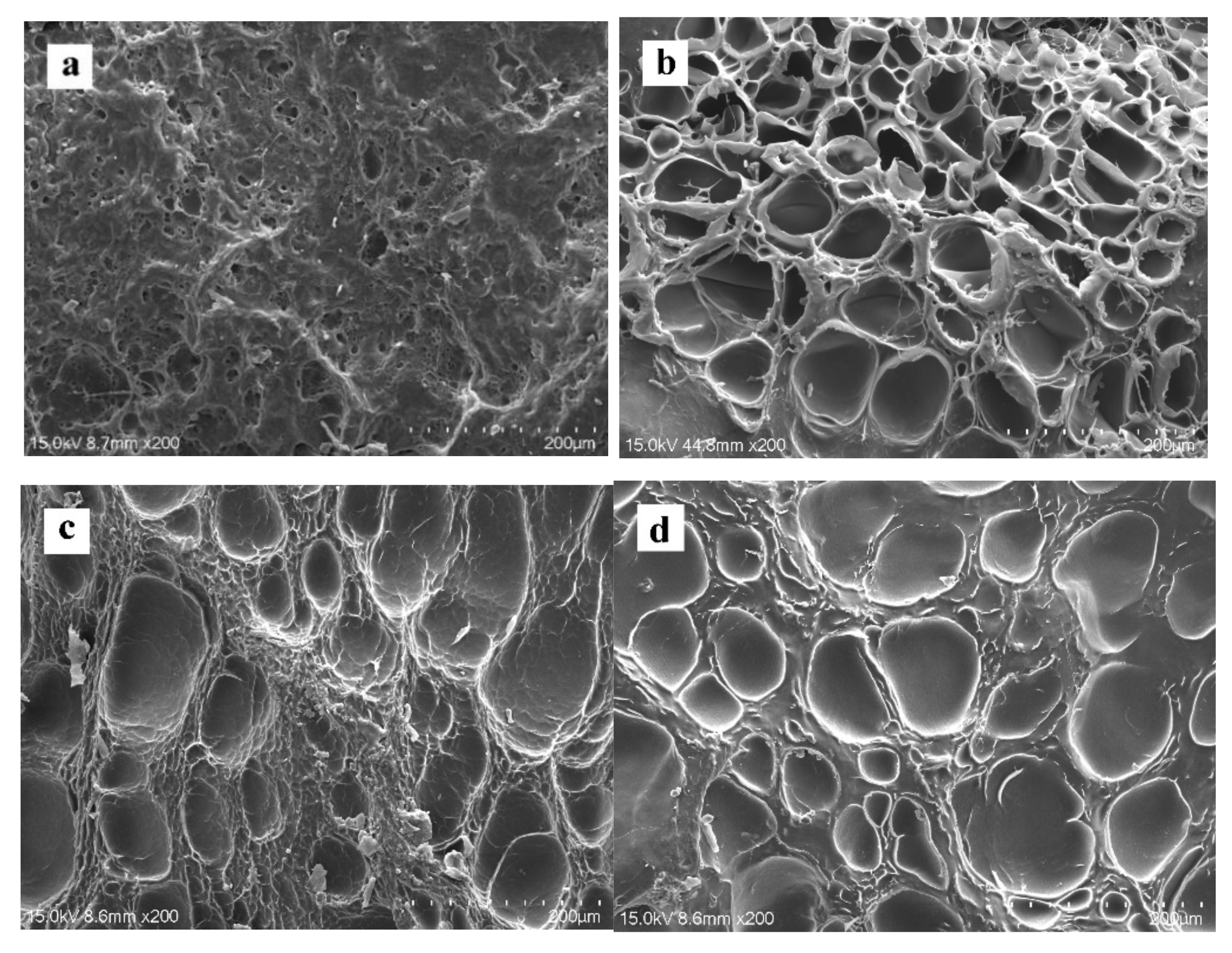
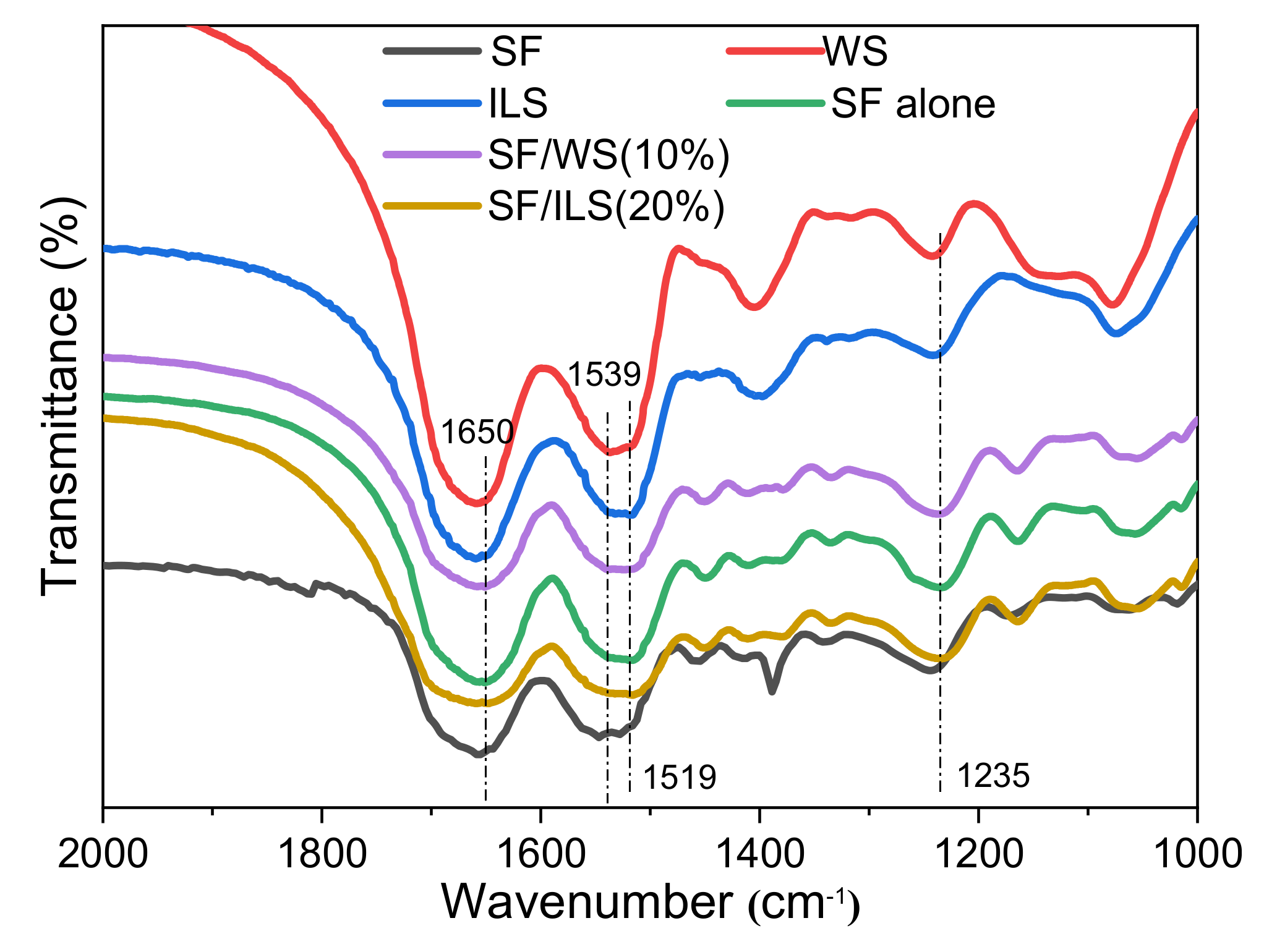
| No. | Composite Bioinks | G-SF (g) | G-WS (g) | G-ILS (g) | I2959 (g) |
|---|---|---|---|---|---|
| 1 | G-SF | 5.00 | - | - | 0.10 |
| 2 | G-SF/WS (10%) | 4.50 | 0.50 | - | 0.10 |
| 3 | G-SF/WS (20%) | 4.00 | 1.00 | - | 0.10 |
| 4 | G-SF/WS (30%) | 3.50 | 1.50 | - | 0.10 |
| 5 | G-SF/ILS (10%) | 4.50 | - | 0.50 | 0.10 |
| 6 | G-SF/ILS (20%) | 4.00 | - | 1.00 | 0.10 |
| 7 | G-SF/ILS (30%) | 3.50 | - | 1.50 | 0.10 |
| Degumming Time (min) | Compression Strength (MPa) | Maximum Compression Strain (%) | Compression Modulus (MPa) |
|---|---|---|---|
| 10 | 0.20 ± 0.01 | 70.27 ± 1.96 | 0.055 ± 0.003 |
| 20 | 0.20 ± 0.02 | 73.48 ± 5.10 | 0.039 ± 0.011 |
| 30 | 0.22 ± 0.06 | 73.25 ± 8.38 | 0.030 ± 0.011 |
| 40 | 0.21 ± 0.02 | 74.47 ± 1.16 | 0.037 ± 0.001 |
| 50 | 0.17 ± 0.01 | 71.01 ± 1.49 | 0.036 ± 0.002 |
| 3D Scaffolds | Compression Strength (MPa) | Maximum Compression Strain (%) | Compression Modulus (MPa) |
|---|---|---|---|
| SF alone | 0.20 ± 0.01 | 70.27 ± 1.96 | 0.055 ± 0.003 |
| SF/WS (10%) | 0.24 ± 0.04 | 78.37 ± 1.00 | 0.043 ± 0.003 *** |
| SF/WS (20%) | 0.21 ± 0.06 | 77.80 ± 1.59 | 0.029 ± 0.010 ** |
| SF/WS (30%) | 0.11 ± 0.05 | 76.28 ± 0.66 | 0.028 ± 0.003 *** |
| SF/ILS (10%) | 0.23 ± 0.05 | 78.20 ± 1.12 | 0.034 ± 0.001 *** |
| SF/ILS (20%) | 0.29 ± 0.03 | 75.11 ± 6.28 | 0.042 ± 0.007 ** |
| SF/ILS (30%) | 0.25 ± 0.06 | 73.37 ± 2.07 | 0.046 ± 0.005 * |
| 3D Scaffolds | Average Aperture (µm) | (%) Porosity |
|---|---|---|
| SF alone | 70.7 ± 18.2 | 79.7 ± 4.6 |
| SF/WS (10%) | 71.2 ± 22.4 | 83.0 ± 3.4 |
| SF/ILS (20%) | 74.9 ± 19.3 | 82.6 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-X.; Zhao, S.-X.; Zhang, Y.-Q. Silk Protein Composite Bioinks and Their 3D Scaffolds and In Vitro Characterization. Int. J. Mol. Sci. 2022, 23, 910. https://doi.org/10.3390/ijms23020910
Li J-X, Zhao S-X, Zhang Y-Q. Silk Protein Composite Bioinks and Their 3D Scaffolds and In Vitro Characterization. International Journal of Molecular Sciences. 2022; 23(2):910. https://doi.org/10.3390/ijms23020910
Chicago/Turabian StyleLi, Ji-Xin, Shu-Xiang Zhao, and Yu-Qing Zhang. 2022. "Silk Protein Composite Bioinks and Their 3D Scaffolds and In Vitro Characterization" International Journal of Molecular Sciences 23, no. 2: 910. https://doi.org/10.3390/ijms23020910






