Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues
Abstract
:1. Introduction
2. Results and Discussion
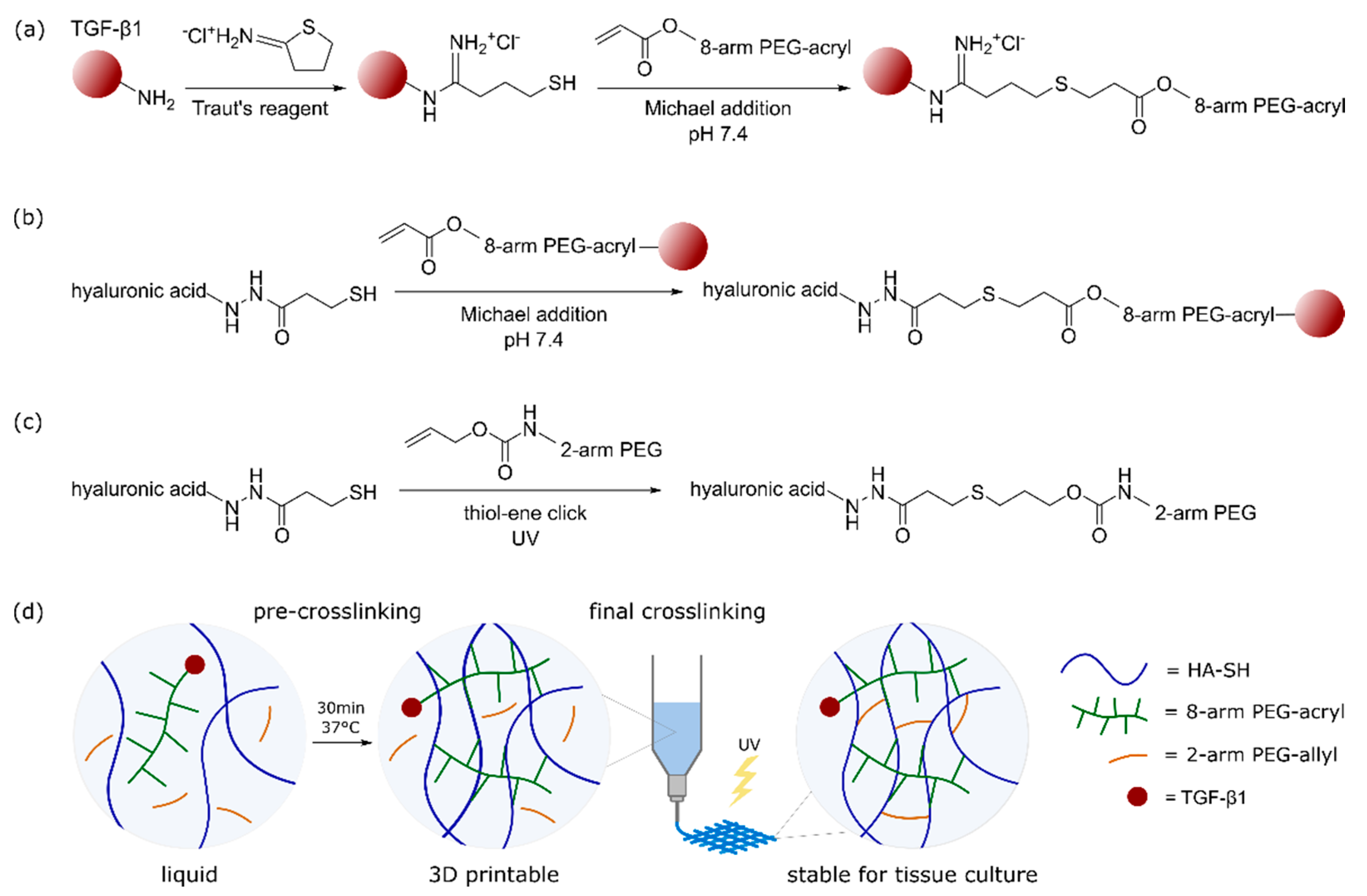
2.1. Bioink Composition and Dual-Stage Crosslinking
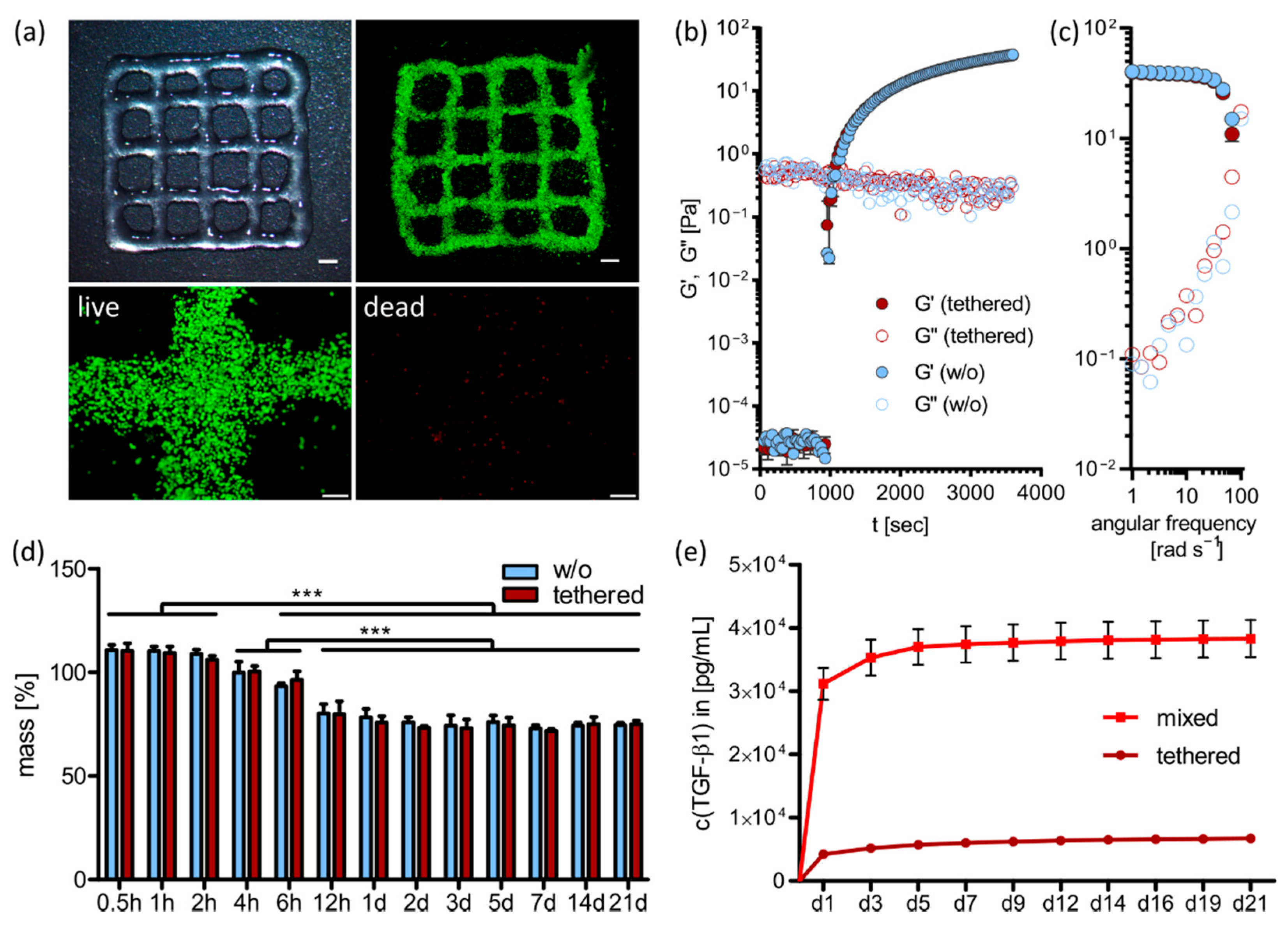
2.2. 3D Printing and Ink Characterization
2.3. Impact of TGF-β1 Concentration and Administration on MSC Differentiation
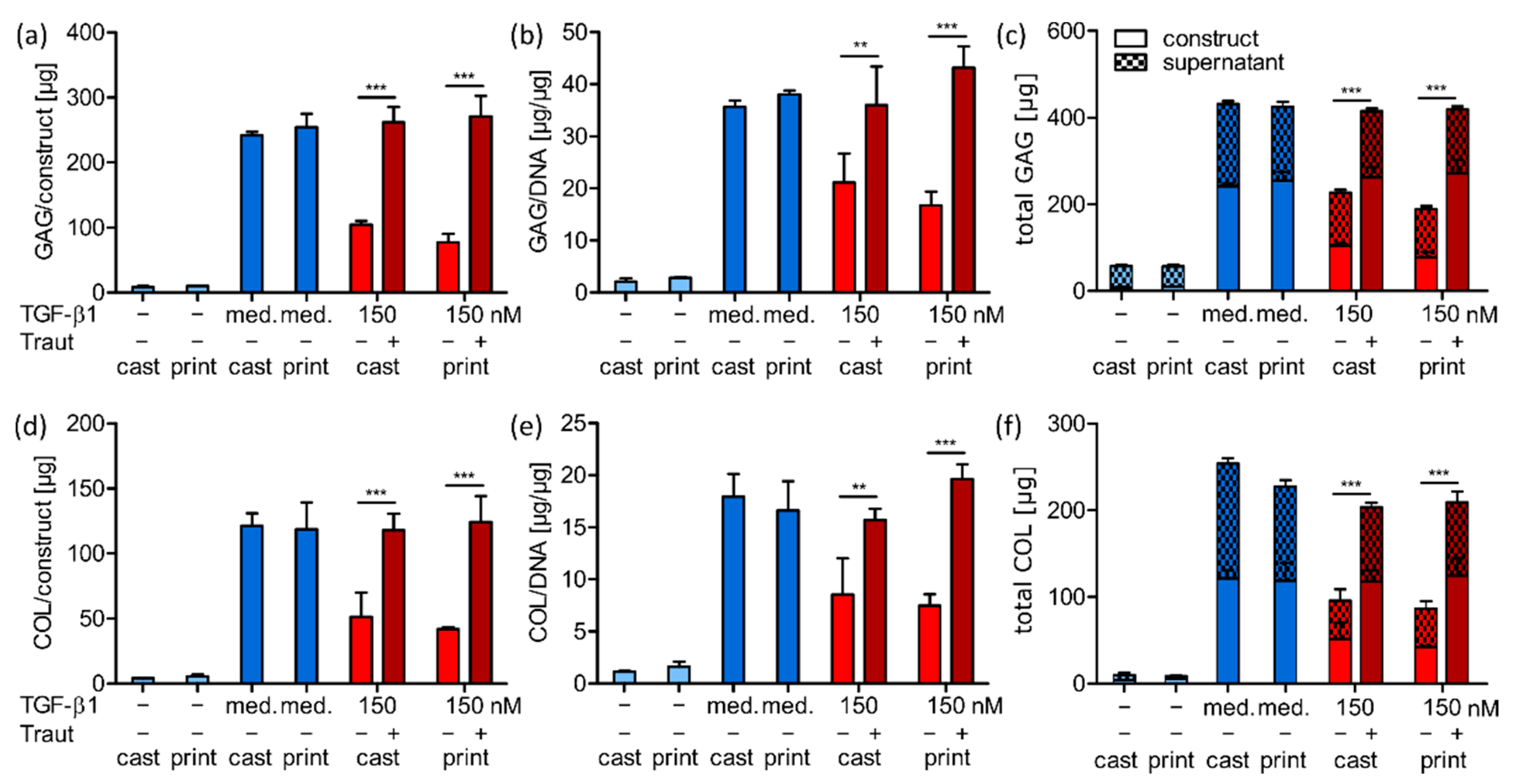
2.4. Investigation of TGF-β1 Functionality after Bioprinting
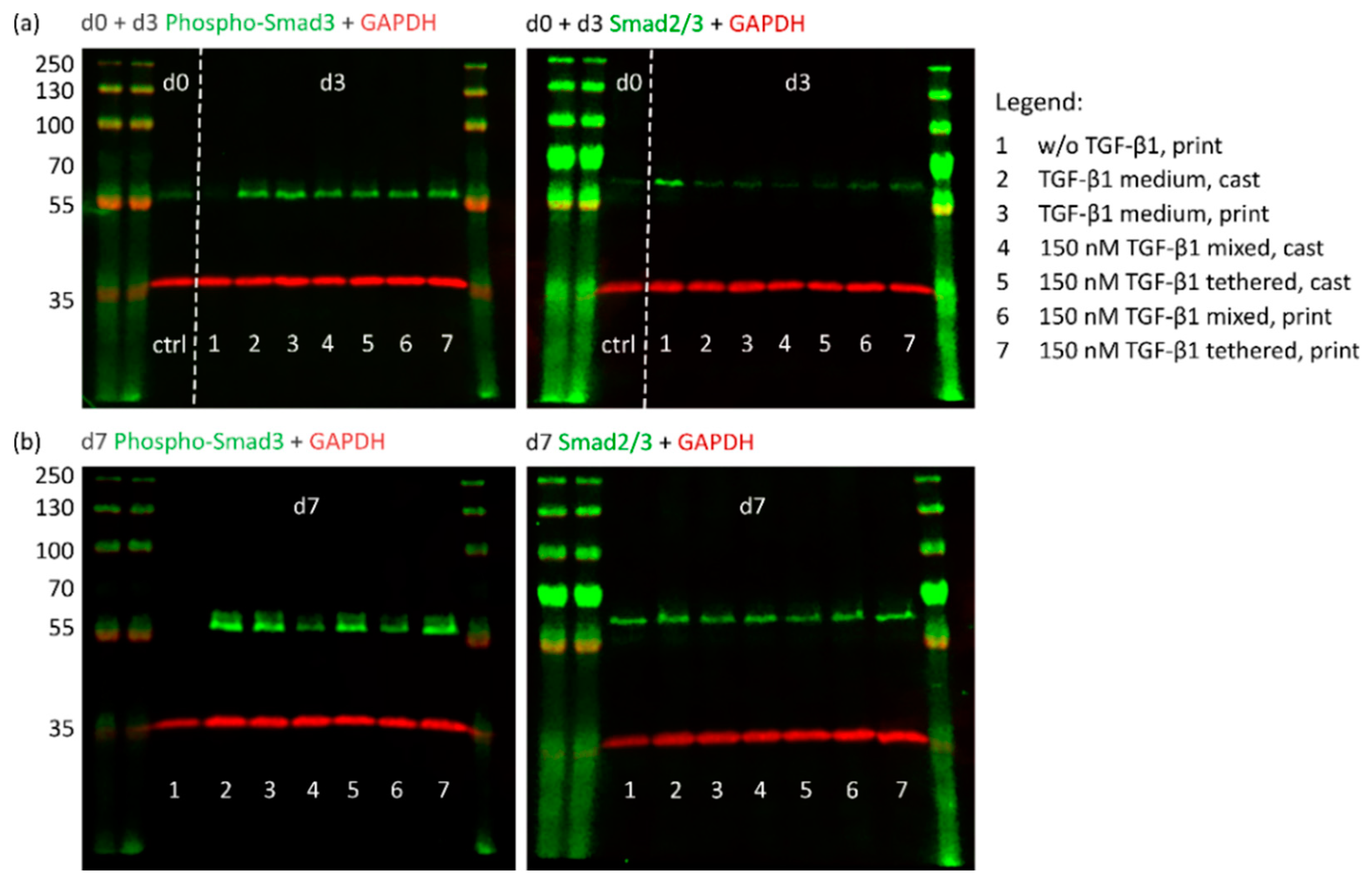
2.4.1. TGF-β1 Signaling and Chondrogenic Gene Expression
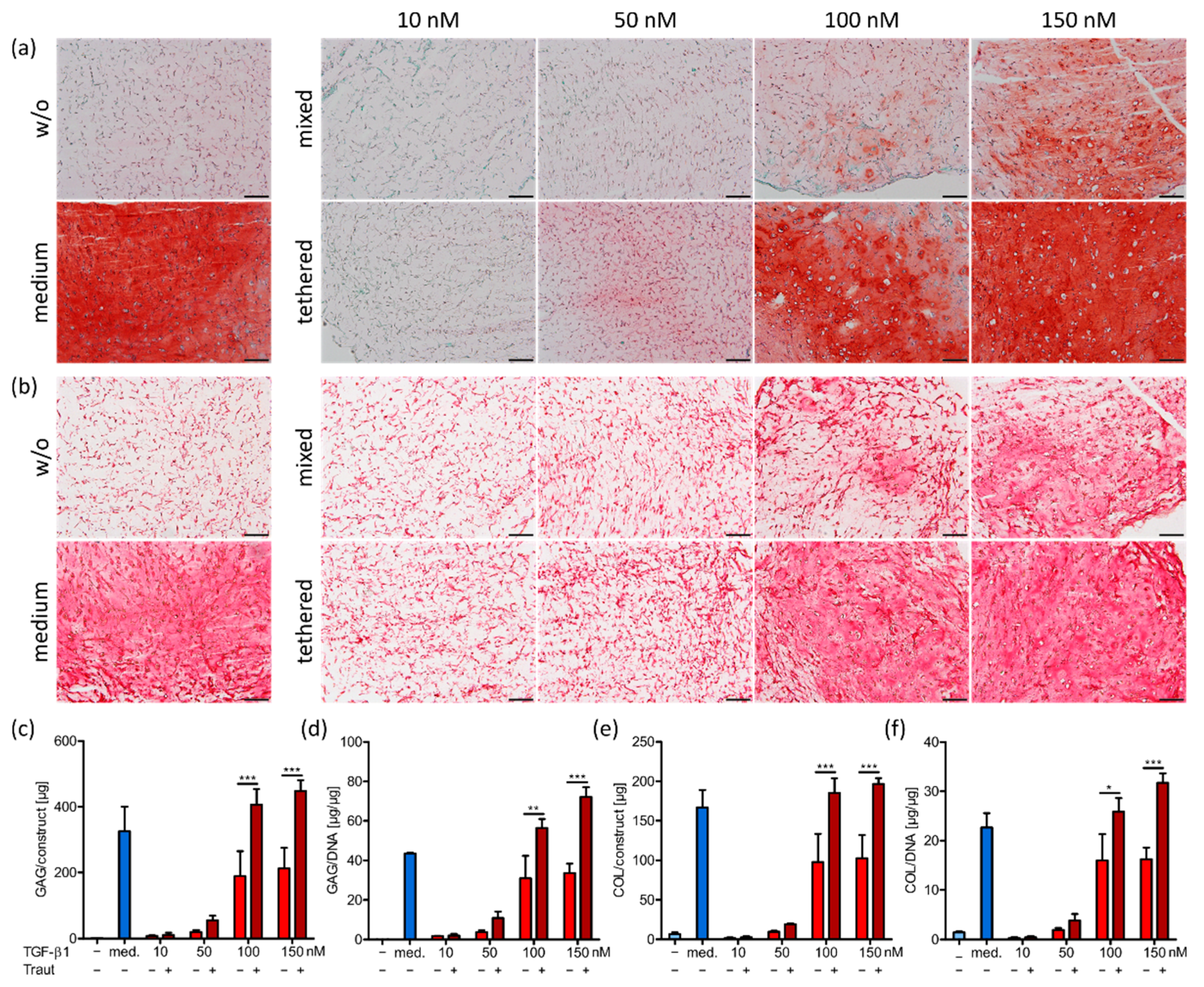
2.4.2. ECM Production and Distribution
2.4.3. Correlation of ECM Production and Construct Stiffness
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Different Bioink Components
3.3. Synthesis of Polyethylene Glycol Octaacrylate (8-Arm PEG-Acryl)
3.4. NMR Analysis
3.5. GPC Analysis
3.6. Ink Preparation, TGF-β1 Tethering and 3D Printing
3.7. Rheological Analysis
3.8. Swelling Analysis
3.9. TGF-β1 Release Analysis
3.10. MSC Isolation and Expansion
3.11. Cell Viability Analysis and Quantification
3.12. Histological and Immunohistochemical Analysis
3.13. Biochemical Analysis
3.14. RNA Isolation and Gene Expression Analysis
3.15. Protein Separation and Western Blot Analysis
3.16. Mechanical Analysis
3.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef] [Green Version]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, e1906423. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Levato, R.; Bonassar, L.J.; D’Lima, D.D.; Grande, D.A.; Klein, T.J.; Saris, D.B.F.; Zenobi-Wong, M.; Gawlitta, D.; Malda, J. Three-Dimensional Bioprinting and Its Potential in the Field of Articular Cartilage Regeneration. Cartilage 2017, 8, 327–340. [Google Scholar] [CrossRef]
- Wu, Y.; Kennedy, P.; Bonazza, N.; Yu, Y.; Dhawan, A.; Ozbolat, I. Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review. Cartilage 2021, 12, 76–92. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication 2018, 10, 045006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.M.; Chen, S.; Jang, T.-S.; Han, W.T.; Jung, H.-D.; Li, Y.; Song, J. Plant seed-inspired cell protection, dormancy, and growth for large-scale biofabrication. Biofabrication 2019, 11, 025008. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Grijpma, D.W.; Alini, M.; Eglin, D.; D’Este, M. Three-Dimensional Printing of a Tyramine Hyaluronan Derivative with Double Gelation Mechanism for Independent Tuning of Shear Thinning and Postprinting Curing. ACS Biomater. Sci. Eng. 2018, 4, 3088. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Locke, R.C.; Witherel, C.E.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, J.A. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 2021, 14, 014106. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Horder, H.; Stahlhut, P.; Groll, J.; Blunk, T.; Teßmar, J. Bioink Platform Utilizing Dual-stage Crosslinking of Hyaluronic Acid Tailored for Chondrogenic Differentiation of Mesenchymal Stromal Cells. Macromol. Biosci. 2021, e2100331. [Google Scholar] [CrossRef] [PubMed]
- Redini, F.; Daireaux, M.; Mauviel, A.; Galera, P.; Loyau, G.; Pujol, J.P. Characterization of proteoglycans synthesized by rabbit articular chondrocytes in response to transforming growth factor-beta (TGF-beta). Biochim. Biophys. Acta 1991, 1093, 196–206. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: Role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 2006, 65, 1414–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J. Cytokine delivery and tissue engineering. Yonsei Med. J. 2000, 41, 704–719. [Google Scholar] [CrossRef] [PubMed]
- van Beuningen, H.M.; van der Kraan, P.M.; Arntz, O.J.; van den Berg, W.B. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab. Invest. 1994, 71, 279–290. [Google Scholar] [PubMed]
- Blaney Davidson, E.N.; van der Kraan, P.M.; van den Berg, W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madry, H.; Rey-Rico, A.; Venkatesan, J.K.; Johnstone, B.; Cucchiarini, M. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng. Part B 2014, 20, 106–125. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Böck, T.; Schill, V.; Krähnke, M.; Steinert, A.F.; Tessmar, J.; Blunk, T.; Groll, J. TGF-β1-Modified Hyaluronic Acid/Poly(glycidol) Hydrogels for Chondrogenic Differentiation of Human Mesenchymal Stromal Cells. Macromol. Biosci. 2018, 18, 1700390. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Doyle, N.R.; Randolph, M.A.; Anseth, K.S. Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J. Biomed. Mater. Res. Part A 2014, 102, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.D.; Luoma, J.E.; Anseth, K.S. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv. Transl. Res. 2012, 2, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthcare Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lepowsky, E.; Muradoglu, M.; Tasoglu, S. Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory. Bioprinting 2018, 11, e00034. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Fischer, H. Shear Stress in 3D-Bioprinting strongly impacts Human MSC Survival and Proliferation Potential. BioNanoMaterials 2015, 16, 81. [Google Scholar]
- Müller, S.J.; Mirzahossein, E.; Iftekhar, E.N.; Bächer, C.; Schrüfer, S.; Schubert, D.W.; Fabry, B.; Gekle, S. Flow and hydrodynamic shear stress inside a printing needle during biofabrication. PLoS ONE 2020, 15, e0236371. [Google Scholar] [CrossRef]
- Bekard, I.B.; Asimakis, P.; Bertolini, J.; Dunstan, D.E. The effects of shear flow on protein structure and function. Biopolymers 2011, 95, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R.; Geer, D. Effects of shear on proteins in solution. Biotechnol. Lett. 2011, 33, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.C.; Chu, S.; Randolph, M.A.; Bryant, S.J. An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor β3. Acta Biomater. 2019, 93, 97–110. [Google Scholar] [CrossRef]
- Kopesky, P.W.; Vanderploeg, E.J.; Kisiday, J.D.; Frisbie, D.D.; Sandy, J.D.; Grodzinsky, A.J. Controlled delivery of transforming growth factor β1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng. Part A 2011, 17, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.; Kim, S.; Fan, J.; Kowalski, T.; Petrigliano, F.; Evseenko, D.; Lee, M. Covalently conjugated transforming growth factor-β1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater. Sci. 2015, 3, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Erickson, I.E.; Huang, A.H.; Sengupta, S.; Kestle, S.; Burdick, J.A.; Mauck, R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthr. Cartil. 2009, 17, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and Improving Quality of Deposited Cartilaginous Extracellular Matrix. Adv. Healthc. Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sun, A.X.; Overholt, K.J.; Yu, G.Z.; Fritch, M.R.; Alexander, P.G.; Shen, H.; Tuan, R.S.; Lin, H. Enhancing chondrogenesis and mechanical strength retention in physiologically relevant hydrogels with incorporation of hyaluronic acid and direct loading of TGF-β. Acta Biomater. 2019, 83, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.D.; Valentini, R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res. 2002, 59, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, Y.; Zheng Shu, X.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Chen, Y.-G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, L.; Aravind, A.; Emma, P.; Christophe, M.; Edwin-Joffrey, C. Rheology, simulation and data analysis toward bioprinting cell viability awareness. Bioprinting 2021, 21, e00119. [Google Scholar]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2020, 9, 535–573. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C 2016, 22, 221–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurtrey, R.J. Roles of Diffusion Dynamics in Stem Cell Signaling and Three-Dimensional Tissue Development. Stem Cells Dev. 2017, 26, 1293–1303. [Google Scholar] [CrossRef]
- Weiss, A.; Attisano, L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C 2014, 102, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C 2021, 118, 111388. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Wang, B.; Díaz-Payno, P.J.; Browe, D.C.; Freeman, F.E.; Nulty, J.; Burdis, R.; Kelly, D.J. Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues. Acta Biomater. 2021, 128, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, B.; Meng, Q.; Liu, S.; Zheng, X.; Zhang, C.; Wang, H.; Li, H.; Wang, N.; Dai, J. 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 2015, 7, 044104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Warwel, S.; Steinke, G.; Klaas, M.R.G. An efficient method for lipase-catalysed preparation of acrylic and methacrylic acid esters. Biotechnol. Tech. 1996, 10, 283–286. [Google Scholar] [CrossRef]
- Heeres, A.; Vanbroekhoven, K.; Van Hecke, W. Solvent-free lipase-catalyzed production of (meth)acrylate monomers: Experimental results and kinetic modeling. Biochem. Eng. J. 2019, 142, 162–169. [Google Scholar] [CrossRef]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S113–S116. [Google Scholar] [CrossRef] [Green Version]
- Martin, I.; Obradovic, B.; Freed, L.E.; Vunjak-Novakovic, G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann. Biomed. Eng. 1999, 27, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sah, R.L.; Doong, J.Y.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Hollander, A.P.; Heathfield, T.F.; Webber, C.; Iwata, Y.; Bourne, R.; Rorabeck, C.; Poole, A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J. Clin. Investig. 1994, 93, 1722–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, K.P.; Marecak, D.M.; Marecek, J.F.; Prestwich, G.D. Synthesis and in Vitro Degradation of New Polyvalent Hydrazide Cross-Linked Hydrogels of Hyaluronic Acid. Bioconjugate Chem. 1997, 8, 686–694. [Google Scholar] [CrossRef]
- Iijima, M.; Ulkoski, D.; Sakuma, S.; Matsukuma, D.; Nishiyama, N.; Otsuka, H.; Scholz, C. Synthesis of PEGylated poly(amino acid) pentablock copolymers and their self-assembly. Polym. Int. 2016, 65, 1132–1141. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauptstein, J.; Forster, L.; Nadernezhad, A.; Groll, J.; Teßmar, J.; Blunk, T. Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. Int. J. Mol. Sci. 2022, 23, 924. https://doi.org/10.3390/ijms23020924
Hauptstein J, Forster L, Nadernezhad A, Groll J, Teßmar J, Blunk T. Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. International Journal of Molecular Sciences. 2022; 23(2):924. https://doi.org/10.3390/ijms23020924
Chicago/Turabian StyleHauptstein, Julia, Leonard Forster, Ali Nadernezhad, Jürgen Groll, Jörg Teßmar, and Torsten Blunk. 2022. "Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues" International Journal of Molecular Sciences 23, no. 2: 924. https://doi.org/10.3390/ijms23020924
APA StyleHauptstein, J., Forster, L., Nadernezhad, A., Groll, J., Teßmar, J., & Blunk, T. (2022). Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. International Journal of Molecular Sciences, 23(2), 924. https://doi.org/10.3390/ijms23020924





