Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis
Abstract
:1. Introduction
2. Results
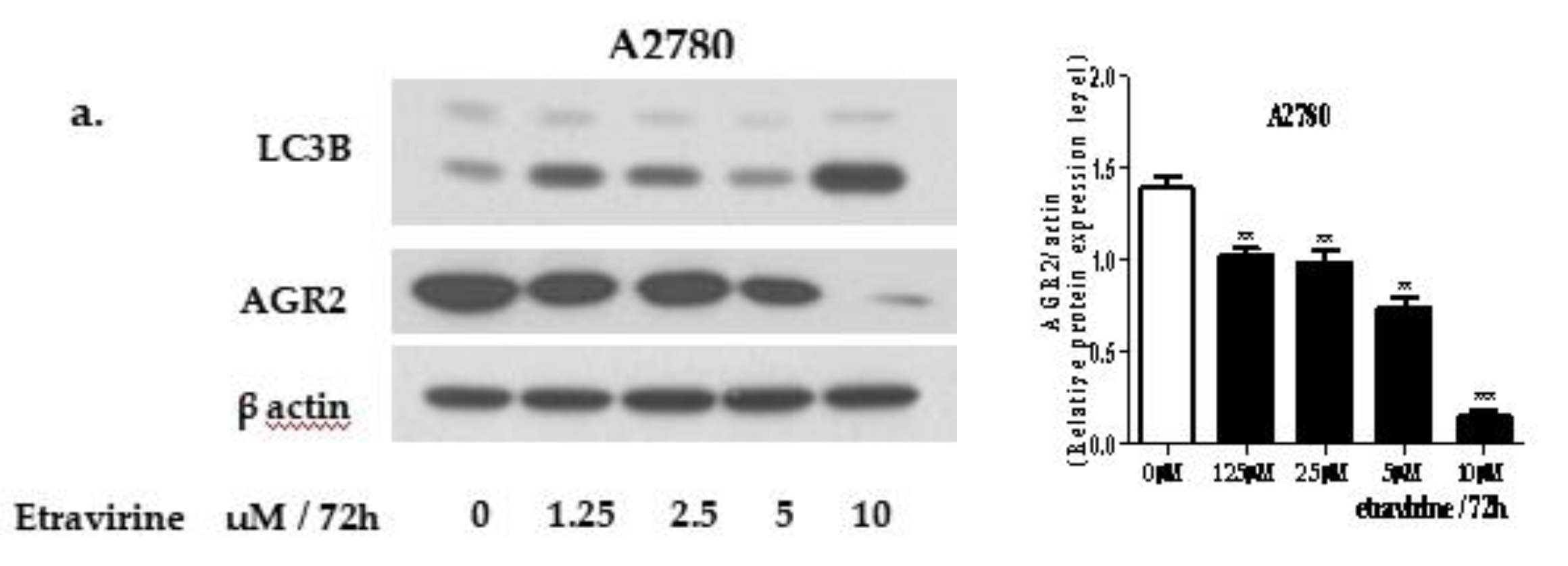
2.1. Etravirine Induces AGR2 Degradation via the Induction of Autophagy
2.2. Etravirine Reduces Cell Proliferation in Ovarian Cancer
2.3. Etravirine Suppresses Spheroid Formation
2.4. Etravirine Inhibits HUVEC Tube Formation
2.5. Etravirine Inhibits Ovarian Cell Migration and Invasion
2.6. Etravirine Suppresses Tumor Progression in an Orthotopic Ovarian Cancer Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell lines and Culture Conditions
4.2. Drugs and Reagents
4.3. Quantitative Real-Time PCR (RT-PCR)
4.4. Western Blot Analysis
4.5. Quantification of AGR2 Secretion
4.6. Intracellular Staining
4.7. Immunofluorescence Assay
4.8. Cell Viability and Colony Formation Assay
4.9. Cell Cycle Assay
4.10. RNA Interference Assay
4.11. Spheroid Culture Conditions
4.12. Effect of Etravirine on Spheroid Growth
4.13. Transwell Assays
4.14. Wound-Healing Assays
4.15. Tube Formation Assays
4.16. Animal Studies
4.17. Immunohistochemical (IHC) Staining and Hematoxylin and Eosin (H&E) Staining
4.18. Cyto-Id Autophagy Detection Assay
4.19. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochum, F.; Vermel, M.; Faller, E.; Boisrame, T.; Lecointre, L.; Akladios, C. Three and Five-Year Mortality in Ovarian Cancer after Minimally Invasive Compared to Open Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2507. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Rottapel, R.; Oza, A.M. Advances in ovarian cancer, from biology to treatment. Nat. Cancer 2021, 2, 6–8. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy resistance in advanced ovarian cancer patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Wimberger, P.; Wehling, M.; Lehmann, N.; Kimmig, R.; Schmalfeldt, B.; Burges, A.; Harter, P.; Pfisterer, J.; du Bois, A. Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease. Ann. Surg. Oncol. 2010, 17, 1642–1648. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef]
- Hong, X.; Li, Z.-X.; Hou, J.; Zhang, H.-Y.; Zhang, C.-Y.; Zhang, J.; Sun, H.; Pang, L.-H.; Wang, T.; Deng, Z.-H. Effects of ER-resident and secreted AGR2 on cell proliferation, migration, invasion, and survival in PANC-1 pancreatic cancer cells. BMC Cancer 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Delom, F.; Mohtar, M.A.; Hupp, T.; Fessart, D. The anterior gradient-2 interactome. Am. J. Physiol. Cell Physiol. 2020, 318, C40–C47. [Google Scholar] [CrossRef]
- Fritzsche, F.R.; Dahl, E.; Pahl, S.; Burkhardt, M.; Luo, J.; Mayordomo, E.; Gansukh, T.; Dankof, A.; Knuechel, R.; Denkert, C. Prognostic relevance of AGR2 expression in breast cancer. Clin. Cancer Res. 2006, 12, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.-B.; Tao, K.-X.; Hu, J.; Liu, Z.-B.; Ding, X.-L.; Chu, Y.-N.; Cui, J.-Y.; Shuai, X.-M.; Gao, J.-B.; Cai, K.-L. The prognostic value of AGR2 expression in solid tumours: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jin, Y.; Xu, S.; Zheng, J.; Zhang, Q.; Wang, Y.; Chen, J.; Huang, Y.; He, X.; Zhao, Z. AGR2 is associated with gastric cancer progression and poor survival. Oncol. Lett. 2016, 11, 2075–2083. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.S.; Gong, A.; Cheville, J.C.; Smith, D.I.; Young, C.Y. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer 2005, 43, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Maresh, E.L.; Mah, V.; Alavi, M.; Horvath, S.; Bagryanova, L.; Liebeskind, E.S.; Knutzen, L.A.; Zhou, Y.; Chia, D.; Liu, A.Y. Differential expression of anterior gradient gene AGR2 in prostate cancer. BMC Cancer 2010, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, M.; Mah, V.; Maresh, E.L.; Bagryanova, L.; Horvath, S.; Chia, D.; Goodglick, L.; Liu, A.Y. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Fei, X.; Hou, W.; Zhang, Y.; Liu, L.; Hu, R. miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett. 2018, 412, 170–178. [Google Scholar] [CrossRef]
- Sung, H.Y.; Choi, E.N.; Lyu, D.; Park, A.K.; Ju, W.; Ahn, J.-H. Aberrant hypomethylation-mediated AGR2 overexpression induces an aggressive phenotype in ovarian cancer cells. Oncol. Rep. 2014, 32, 815–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darb-Esfahani, S.; Fritzsche, F.; Kristiansen, G.; Weichert, W.; Sehouli, J.; Braicu, I.; Dietel, M.; Denkert, C. Anterior gradient protein 2 (AGR2) is an independent prognostic factor in ovarian high-grade serous carcinoma. Virchows Arch. 2012, 461, 109–116. [Google Scholar] [CrossRef]
- Lepreux, S.; Bioulac-Sage, P.; Chevet, E. Differential expression of the anterior gradient protein-2 is a conserved feature during morphogenesis and carcinogenesis of the biliary tree. Liver Int. 2011, 31, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Deng, D.; Bover, L.; Wang, H.; Logsdon, C.D.; Ramachandran, V. New blocking antibodies against novel AGR2–C4. 4A pathway reduce growth and metastasis of pancreatic tumors and increase survival in mice. Mol. Cancer Ther. 2015, 14, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Delom, F.; Nazaraliyev, A.; Fessart, D. The role of protein disulphide isomerase AGR2 in the tumour niche. Biol. Cell 2018, 110, 271–282. [Google Scholar] [CrossRef]
- Ondrouskova, E.; Sommerova, L.; Nenutil, R.; Coufal, O.; Bouchal, P.; Vojtesek, B.; Hrstka, R. AGR2 associates with HER2 expression predicting poor outcome in subset of estrogen receptor negative breast cancer patients. Exp. Mol. Pathol. 2017, 102, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Liu, Z.; Jiang, Y.; Lu, J.; Xie, H.; Tang, F. AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin. Chim. Acta 2018, 484, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Q.; Yu, X.; Merugu, S.; Mangukiya, H.; Smith, N.; Li, Z.; Zhang, B.; Negi, H.; Rong, R. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene 2017, 36, 5098–5109. [Google Scholar] [CrossRef]
- Armes, J.E.; Davies, C.M.; Wallace, S.; Taheri, T.; Perrin, L.C.; Autelitano, D.J. AGR2 expression in ovarian tumours: A potential biomarker for endometrioid and mucinous differentiation. Pathology 2013, 45, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qian, M.; Zhou, T.; Cui, P. TP53 mediated miR-3647-5p prevents progression of cervical carcinoma by targeting AGR2. Cancer Med. 2019, 8, 6095–6105. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Yang, J.; Wang, X.; Xu, K.; Ikezoe, T. miR-217 sensitizes chronic myelogenous leukemia cells to tyrosine kinase inhibitors by targeting pro-oncogenic anterior gradient 2. Exp. Hematol. 2018, 68, 80–88. [Google Scholar] [CrossRef]
- Franceschi, S.; Dal Maso, L.; Rickenbach, M.; Polesel, J.; Hirschel, B.; Cavassini, M.; Bordoni, A.; Elzi, L.; Ess, S.; Jundt, G. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br. J. Cancer 2008, 99, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Maskew, M.; Fox, M.P.; van Cutsem, G.; Chu, K.; MacPhail, P.; Boulle, A.; Egger, M. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: A cohort study. PLoS ONE 2013, 8, e64392. [Google Scholar] [CrossRef] [Green Version]
- Dalu, D.; Fasola, C.; De Francesco, D.; Bombonati, G.; Ammoni, L.; La Verde, N. Non-Hodgkin lymphoma in HIV-positive patients treated with antiretroviral therapy and chemotherapy: A single institution retrospective study. Ann. Oncol. 2019, 30, v436. [Google Scholar] [CrossRef]
- Re, A.; Gini, G.; Rupolo, M.; Levis, A.; Bandera, A.; Liberati, A.; Tozzi, P.; Cattaneo, C.; Casari, S.; Skert, C. Early consolidation with high-dose therapy and autologous stem cell transplantation is a feasible and effective treatment option in HIV-associated non-Hodgkin lymphoma at high risk. Bone Marrow Transplant. 2018, 53, 228–230. [Google Scholar] [CrossRef] [Green Version]
- de Vries, H.J.; Steenbergen, R.D. The effect of ART on cervical cancer precursor lesions. Lancet HIV 2018, 5, e6–e8. [Google Scholar] [CrossRef]
- Kelly, H.; Weiss, H.A.; Benavente, Y.; de Sanjose, S.; Mayaud, P.; Qiao, Y.-l.; Feng, R.-M.; DeVuyst, H.; Tenet, V.; Jaquet, A. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV 2018, 5, e45–e58. [Google Scholar] [CrossRef] [Green Version]
- Tanida, I. Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 2011, 14, 2201–2214. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, B. Autophagy inhibitior autophagy-related 7 small interfering RNA and doxorubicin dual-loaded nanostructured lipid carrier to combat multidrug resistance. J. Mater. Res. 2020, 35, 3136–3143. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Coakley, F.V.; Choi, P.H.; Gougoutas, C.A.; Pothuri, B.; Venkatraman, E.; Chi, D.; Bergman, A.; Hricak, H. Peritoneal metastases: Detection with spiral CT in patients with ovarian cancer. Radiology 2002, 223, 495–499. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimada, Y.; Nishino, K.; Yoshihara, K.; Nakano, M.; Kameyama, H.; Enomoto, T.; Wakai, T. Clinical significance of mesenteric lymph node involvement in the pattern of liver metastasis in patients with ovarian cancer. Ann. Surg. Oncol. 2021, 28, 1–8. [Google Scholar] [CrossRef]
- Otrock, Z.; Seoud, M.; Khalifeh, M.; Makarem, J.; Shamseddine, A. Laparoscopic splenectomy for isolated parenchymal splenic metastasis of ovarian cancer. Int. J. Gynecol. Cancer 2006, 16, 1933–1935. [Google Scholar] [CrossRef]
- Kerr, V.E.; Cadman, E. Pulmonary metastases in ovarian cancer analysis of 357 patients. Cancer 1985, 56, 1209–1213. [Google Scholar] [CrossRef]
- Kanzaki, R.; Okami, J.; Takami, K.; Iwasaki, T.; Ikeda, N.; Funakoshi, Y.; Sakamaki, Y.; Kodama, K.; Yokouchi, H.; Kadota, Y. Outcomes of surgical resection for pulmonary metastasis from ovarian cancer. J. Cardiothorac. Surg. 2020, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kolomainen, D.F.; Larkin, J.M.; Badran, M.; A’Hern, R.P.; King, D.M.; Fisher, C.; Bridges, J.E.; Blake, P.R.; Barton, D.P.; Shepherd, J.H. Epithelial ovarian cancer metastasizing to the brain: A late manifestation of the disease with an increasing incidence. J. Clin. Oncol. 2002, 20, 982–986. [Google Scholar] [CrossRef] [PubMed]
- CHEN, Y.L.; CHENG, W.F.; HSIEH, C.Y.; CHEN, C.A. Brain metastasis as a late manifestation of ovarian carcinoma. Eur. J. Cancer Care 2011, 20, 44–49. [Google Scholar] [CrossRef]
- Kleppe, M.; Wang, T.; Van Gorp, T.; Slangen, B.; Kruse, A.J.; Kruitwagen, R. Lymph node metastasis in stages I and II ovarian cancer: A review. Gynecol. Oncol. 2011, 123, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered models to parse apart the metastatic cascade. NPJ Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Seminari, E.; Castagna, A.; Lazzarin, A. Etravirine for the treatment of HIV infection. Expert Rev. Anti-Infect. Ther. 2008, 6, 427–433. [Google Scholar] [CrossRef]
- Perna, A.; Lucariello, A.; Sellitto, C.; Agliata, I.; Carleo, M.A.; Sangiovanni, V.; Esposito, V.; Guerra, G.; Cobellis, L.; De Luca, A. Different cell cycle modulation in SKOV-3 ovarian cancer cell line by anti-HIV drugs. Oncol. Res. 2017, 25, 1617. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Y.; Lowe, A.W. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008, 68, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Chung, Y.J.; So, H.; Kim, K.; Park, J.; Oh, M.; Jo, M.; Choi, K.; Lee, E.-J.; Choi, Y.-L. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp. Mol. Med. 2011, 43, 91–100. [Google Scholar] [CrossRef]
- Kani, K.; Malihi, P.D.; Jiang, Y.; Wang, H.; Wang, Y.; Ruderman, D.L.; Agus, D.B.; Mallick, P.; Gross, M.E. Anterior gradient 2 (AGR2): Blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate 2013, 73, 306–315. [Google Scholar] [CrossRef]
- Tohti, M.; Li, J.; Tang, C.; Wen, G.; Abdujilil, A.; Yizim, P.; Ma, C. Serum AGR2 as a useful biomarker for pituitary adenomas. Clin. Neurol. Neurosurg. 2017, 154, 19–22. [Google Scholar] [CrossRef]
- Salmans, M.L.; Zhao, F.; Andersen, B. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: A potential drug target and biomarker. Breast Cancer Res. 2013, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hrstka, R.; Brychtova, V.; Fabian, P.; Vojtesek, B.; Svoboda, M. AGR2 predicts tamoxifen resistance in postmenopausal breast cancer patients. Dis. Markers 2013, 35, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Chuang, H.-N.; Hsiao, T.-H.; Kumar, V.B.; Hsu, C.-H.; Huang, C.-Y.; Lee, L.-W.; Mao, C.-L.; Ko, J.-L.; Hsu, C.-P. AGR2–a Novel Predictor of Neoadjuvant Chemoradiotherapy Response in Esophageal Squamous Cell Carcinoma. Res. Sq. 2021. [Google Scholar]
- Nakatogawa, H. Autophagic degradation of the endoplasmic reticulum. Proc. Jpn. Acad. Ser. B 2020, 96, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-K.; Lee, J.-A. Role of the mammalian ATG8/LC3 family in autophagy: Differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep. 2016, 49, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xu, Q.; Yuan, Q.; Jia, M.; Niu, H.; Liu, X.; Zhang, J.; Young, C.Y.; Yuan, H. Proteasome inhibition boosts autophagic degradation of ubiquitinated-AGR2 and enhances the antitumor efficiency of bevacizumab. Oncogene 2019, 38, 3458–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, J.; Li, K.; Deng, L.; Wang, H. Combination of an autophagy inducer and an autophagy inhibitor: A smarter strategy emerging in cancer therapy. Front. Pharmacol. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, H.-S.; Shin, M.J.; Kwon, S.G.; Lee, H.; Kim, D.K.; Choi, K.U.; Kim, J.-H.; Kim, J.H. Yolk–Shell-Type Gold Nanoaggregates for Chemo-and Photothermal Combination Therapy for Drug-Resistant Cancers. ACS Appl. Mater. Interfaces 2021, 13, 53519–53529. [Google Scholar] [CrossRef]
- Lamichane, B.D.; Jung, S.Y.; Yun, J.; Kang, S.; Lamichane, S.; Kim, Y.J.; Park, J.H.; Jang, W.B.; Ji, S.T.; Dehua, L. AGR2 is a target of canonical Wnt/β-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 515, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Kwon, Y.W.; Jang, I.H.; Kim, D.K.; Lee, S.I.; Choi, E.J.; Kim, K.H.; Suh, D.S.; Lee, J.H.; Choi, K.U. Autotaxin regulates maintenance of ovarian cancer stem cells through lysophosphatidic acid-mediated autocrine mechanism. Stem Cells 2016, 34, 551–564. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, T.T.G.; Yun, J.; Ha, J.-S.; Kim, Y.-J.; Jang, W.-B.; Van Le, T.H.; Rethineswaran, V.K.; Choi, J.; Kim, J.-H.; Min, S.-H.; et al. Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 944. https://doi.org/10.3390/ijms23020944
Ly TTG, Yun J, Ha J-S, Kim Y-J, Jang W-B, Van Le TH, Rethineswaran VK, Choi J, Kim J-H, Min S-H, et al. Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis. International Journal of Molecular Sciences. 2022; 23(2):944. https://doi.org/10.3390/ijms23020944
Chicago/Turabian StyleLy, Thanh Truong Giang, Jisoo Yun, Jong-Seong Ha, Yeon-Ju Kim, Woong-Bi Jang, Thi Hong Van Le, Vinoth Kumar Rethineswaran, Jaewoo Choi, Jae-Ho Kim, Sang-Hyun Min, and et al. 2022. "Inhibitory Effect of Etravirine, a Non-Nucleoside Reverse Transcriptase Inhibitor, via Anterior Gradient Protein 2 Homolog Degradation against Ovarian Cancer Metastasis" International Journal of Molecular Sciences 23, no. 2: 944. https://doi.org/10.3390/ijms23020944





