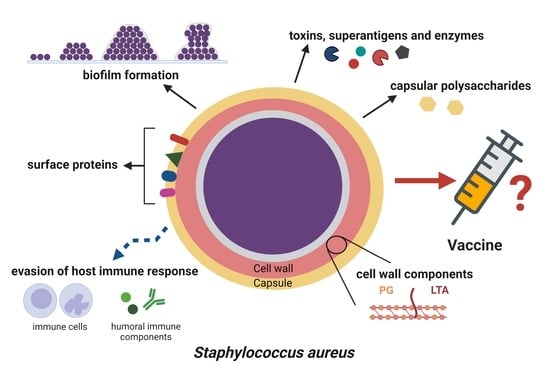
Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win?
Abstract
:1. Introduction
2. S. aureus Infections as a Challenge to Vaccinology: Why Is It Important?
S. aureus Immune Evasion Strategies
3. Past and Present in Active and Passive Immunotherapy of Staphylococcal Infections
4. The Prospects of Immune Prophylaxis Trends against S. aureus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rosini, R.; Nicch, S.; Pizza, M.; Rappuoli, R. Vaccines against antimicrobial resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.-L.; Privor-Dumm, L.; Kim, J.H. Supply and delivery of vaccines for global health. Curr. Opin. Immunol. 2021, 71, 13–20. [Google Scholar] [CrossRef]
- Castro, S.A.; Dorfmueller, H.C. A brief review on Group A Streptococcus pathogenesis and vaccine development. R. Soc. Open Sci. 2021, 8, 201991. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Caulfield, A.; Dewan, K.K.; Harvill, E.T. Pertactin-deficient Bordetella pertussis, vaccine-driven evolution, and reemergence of pertussis. Emerg. Infect. Dis. 2021, 27, 1561–1566. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Stuart, J.M. Editorial for the Special Issue: Bacterial meningitis—Epidemiology and vaccination. Microorganisms 2021, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Proctor, R.A. Where does a Staphylococcus aureus vaccine stand? Clin. Microbiol. Infect. 2014, 20, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Teymournejad, O.; Montgomery, C.P. Evasion of immunological memory by S. aureus infection: Implications for vaccine design. Front. Immunol. 2021, 12, 633672. [Google Scholar] [CrossRef]
- Flouchi, R.; Elmniai, A.; Hibatallah, A.; Fahsi, K.; Touzani, I.; Fikri-Benbrahim, K. The relationship between nasal carriage of Staphylococcus aureus and surgical site infections in a Hospital Center in Morocco. Int. J. Microbiol. 2021, 2021, 5585588. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Y.; Xu, P.; Zhang, T.; Bai, C.; Lin, D.; Ou, Q.; Yao, Z. Methicillin-resistant Staphylococcus aureus nasal colonization in Chinese children: A prevalence meta-analysis and review of influencing factors. PLoS ONE 2016, 11, e0159728. [Google Scholar] [CrossRef]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, P.O.; Gagnaire, J.; Botelho-Nevers, E.; Grattard, F.; Carricajo, A.; Lucht, F.; Pozzetto, B.; Berthelot, P. Detection and clinical relevance of Staphylococcus aureus nasal carriage: An update. Expert Rev. Anti. Infect. Ther. 2014, 12, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Mohamed, N.; Scully, I.L.; Cooper, D.; Begier, E.; Eiden, J.; Jansen, K.U.; Gurtman, A.; Anderson, A.S. Staphylococcus aureus: The current state of disease, pathophysiology and strategies for prevention. Expert Rev. Vaccines 2016, 15, 1373–1392. [Google Scholar] [CrossRef]
- Hoerr, V.; Franz, M.; Pletz, M.W.; Diab, M.; Niemann, S.; Faber, C.; Doenst, T.; Schulze, P.C.; Deinhardt-Emmer, S.; Löffler, B. S. aureus endocarditis: Clinical aspects and experimental approaches. Int. J. Med. Microbiol. 2018, 308, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [Green Version]
- Bello-Chavolla, O.Y.; Bahena-Lopez, J.P.; Garciadiego-Fosass, P.; Volkow, P.; Garcia-Horton, A.; Velazquez-Acosta, C.; Vilar-Compte, D. Bloodstream infection caused by S. aureus in patients with cancer: A 10-year longitudinal single-center study. Support. Care Cancer 2018, 26, 4057–4065. [Google Scholar] [CrossRef]
- Lu, H.Y.; Turvey, S.E. Human MALT1 deficiency and predisposition to infections. Curr. Opin. Immunol. 2021, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14, S7–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Yamauchi, Y.; Jo, T.; Michihata, N.; Hasegawa, W.; Takeshima, H.; Matsui, H.; Fushimi, K.; Yasunaga, H.; Nagase, T. In-hospital mortality associated with community-acquired pneumonia due to methicillin-resistant Staphylococcus aureus: A matched-pair cohort study. BMC Pulm. Med. 2021, 21, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Lundborg, C.S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: A multicentre study in China. Sci. Rep. 2020, 10, 3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Staphylococcus aureus resistant to vancomycin--United States, 2002. MMWR Morb. Mortal Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Resch, A.; Rosenstein, R.; Nerz, C.; Götz, F. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 2005, 71, 2663–2676. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, B.; Więckowska-Szakiel, M.; Paszkiewicz, M.; Różalska, B. The immunomodulatory activity of Staphylococcus aureus products derived from biofilm and planktonic cultures. Arch. Immunol. Ther. Exp. (Warsz) 2013, 61, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- de Jong, N.W.M.; van Kessel, K.P.M.; van Strijp, J.A.G. Immune evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 1–27. [Google Scholar] [CrossRef]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front. Cell Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shettigar, K.; Murali, T.S. Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- de Vor, L.; Rooijakkers, S.H.M.; van Strijp, J.A.G. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett. 2020, 594, 2556–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooijakkers, S.H.; Ruyken, M.; Roos, A.; Daha, M.R.; Presanis, J.S.; Sim, R.B.; van Wamel, W.J.; van Kessel, K.P.; van Strijp, J.A. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 2005, 6, 920–927. [Google Scholar] [CrossRef]
- Ko, Y.P.; Kuipers, A.; Freitag, C.M.; Jongerius, I.; Medina, E.; van Rooijen, W.J.; Spaan, A.N.; van Kessel, K.P.; Höök, M.; Rooijakkers, S.H. Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS Pathog. 2013, 9, e1003816. [Google Scholar] [CrossRef]
- Kang, M.; Ko, Y.P.; Liang, X.; Ross, C.L.; Liu, Q.; Murray, B.E.; Höök, M. Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J. Biol. Chem. 2013, 288, 20520–20531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, M.; Hang, T.; Wang, C.; Yang, Y.; Pan, W.; Zang, J.; Zhang, M.; Zhang, X. Staphylococcus aureus SdrE captures complement factor H’s C-terminus via a novel ‘close, dock, lock and latch’ mechanism for complement evasion. Biochem. J. 2017, 474, 1619–1631. [Google Scholar] [CrossRef] [Green Version]
- Jusko, M.; Potempa, J.; Kantyka, T.; Bielecka, E.; Miller, H.K.; Kalinska, M.; Dubin, G.; Garred, P.; Shaw, L.N.; Blom, A.M. Staphylococcal proteases aid in evasion of the human complement system. J. Innate Immun. 2014, 6, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Phukan, U.J. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med. Microbiol. Immunol. 2019, 208, 585–607. [Google Scholar] [CrossRef]
- Guerra, F.E.; Borgogna, T.R.; Patel, D.M.; Sward, E.W.; Voyich, J.M. Epic immune battles of history: Neutrophils vs. Staphylococcus aureus. Front. Cell Infect. Microbiol. 2017, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The role of macrophages in Staphylococcus aureus infection. Front. Immunol. 2021, 11, 620339. [Google Scholar] [CrossRef]
- Yamada, K.J.; Heim, C.E.; Xi, X.; Attri, K.S.; Wang, D.; Zhang, W.; Singh, P.K.; Bronich, T.K.; Kielian, T. Monocyte metabolic reprogramming promotes pro-inflammatory activity and Staphylococcus aureus biofilm clearance. PLoS Pathog. 2020, 16, e1008354. [Google Scholar] [CrossRef]
- Mohamed, N.; Timofeyeva, Y.; Jamrozy, D.; Rojas, E.; Hao, L.; Silmon de Monerri, N.C.; Hawkins, J.; Singh, G.; Cai, B.; Liberator, P.; et al. Molecular epidemiology and expression of capsular polysaccharides in Staphylococcus aureus clinical isolates in the United States. PLoS ONE 2019, 14, e0208356. [Google Scholar] [CrossRef] [Green Version]
- Nanra, J.S.; Buitrago, S.M.; Crawford, S.; Ng, J.; Fink, P.S.; Hawkins, J.; Scully, I.L.; McNeil, L.K.; Aste-Amézaga, J.M.; Cooper, D.; et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum. Vaccin. Immunother. 2013, 9, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.G.; McAdow, M.; Kim, H.K.; Bae, T.; Missiakas, D.M.; Schneewind, O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010, 6, e1001036. [Google Scholar] [CrossRef] [Green Version]
- Herman-Bausier, P.; Labate, C.; Towell, A.M.; Derclaye, S.; Geoghegan, J.A.; Dufrêne, Y.F. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, 5564–5569. [Google Scholar] [CrossRef] [Green Version]
- Tam, K.; Torres, V.J. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Clauditz, A.; Resch, A.; Wieland, K.P.; Peschel, A.; Götz, F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef] [Green Version]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, K.; Coutts, G.; Jonsson, I.M.; Tarkowski, A.; Kokai-Kun, J.F.; Mond, J.J.; Foster, S.J. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandell, G.L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J. Clin. Investig. 1975, 55, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.S.; Otto, M. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim. Biophys. Acta. 2015, 1848, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol. 2016, 18, 514–535. [Google Scholar] [CrossRef] [Green Version]
- Jubrail, J.; Morris, P.; Bewley, M.A.; Stoneham, S.; Johnston, S.A.; Foster, S.J.; Peden, A.A.; Read, R.C.; Marriott, H.M.; Dockrell, D.H. Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell Microbiol. 2016, 18, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, R.A.; Kriegeskorte, A.; Kahl, B.C.; Becker, K.; Löffler, B.; Peters, G. Staphylococcus aureus small colony variants (SCVs): A road map for the metabolic pathways involved in persistent infections. Front. Cell. Infect. Microbiol. 2014, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Pietrocola, G. Staphylococcus aureus induces neutrophil extracellular traps (NETs) and neutralizes their bactericidal potential. Comput. Struct. Biotechnol. J. 2021, 19, 3451–3457. [Google Scholar] [CrossRef]
- Missiakas, D.; Winstel, V. Selective host cell death by Staphylococcus aureus: A strategy for bacterial persistence. Front. Immunol. 2021, 11, 621733. [Google Scholar] [CrossRef]
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [Green Version]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab. Investig. 2007, 87, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Darboe, S.; Dobreniecki, S.; Jarju, S.; Jallow, M.; Mohammed, N.I.; Wathuo, M.; Ceesay, B.; Tweed, S.; Basu Roy, R.; Okomo, U.; et al. Prevalence of Panton-Valentine leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an urban Gambian hospital: A 11-year period retrospective pilot study. Front. Cell Infect. Microbiol. 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Graves, S.F.; Kobayashi, S.D.; DeLeo, F.R. Community-associated methicillin-resistant Staphylococcus aureus immune evasion and virulence. J. Mol. Med. 2010, 88, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Cheung, G.Y.C.; Joo, H.S.; Chatterjee, S.S.; Otto, M. Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 2014, 38, 698–719. [Google Scholar] [CrossRef]
- Surewaard, B.G.; de Haas, C.J.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.; Nijland, R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Berlon, N.R.; Qi, R.; Sharma-Kuinkel, B.K.; Joo, H.S.; Park, L.P.; George, D.; Thaden, J.T.; Messina, J.A.; Maskarinec, S.A.; Mueller-Premru, M.; et al. Clinical MRSA isolates from skin and soft tissue infections show increased in vitro production of phenol soluble modulins. J. Infect. 2015, 71, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Cruz, A.R.; Boer, M.A.D.; Strasser, J.; Zwarthoff, S.A.; Beurskens, F.J.; de Haas, C.J.C.; Aerts, P.C.; Wang, G.; de Jong, R.N.; Bagnoli, F.; et al. Staphylococcal protein A inhibits complement activation by interfering with IgG hexamer formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2016772118. [Google Scholar] [CrossRef] [PubMed]
- Deis, L.N.; Pemble, C.W., 4th; Qi, Y.; Hagarman, A.; Richardson, D.C.; Richardson, J.S.; Oas, T.G. Multiscale conformational heterogeneity in staphylococcal protein A: Possible determinant of functional plasticity. Structure 2014, 22, 1467–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauli, N.T.; Kim, H.K.; Falugi, F.; Huang, M.; Dulac, J.; Henry Dunand, C.; Zheng, N.Y.; Kaur, K.; Andrews, S.F.; Huang, Y.; et al. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J. Exp. Med. 2014, 211, 2331–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodyear, C.S.; Silverman, G.J. Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 2003, 197, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Staphylococcal superantigens: Pyrogenic toxins induce toxic shock. Toxins 2019, 11, 178. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, J.; Mehl, A.; Askim, Å.; Solligård, E.; Åsvold, B.O.; Damås, J.K. Epidemiology and outcome of Staphylococcus aureus bloodstream infection and sepsis in a Norwegian county 1996-2011: An observational study. BMC Infect. Dis. 2015, 15, 116. [Google Scholar] [CrossRef] [Green Version]
- Tuffs, S.W.; Haeryfar, S.M.M.; McCormick, J.K. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik-Bojek, U.; Rywaniak, J.; Bernat, P.; Podsędek, A.; Kajszczak, D.; Sadowska, B. An in vitro study of the effect of Viburnum opulus extracts on key processes in the development of staphylococcal infections. Molecules 2021, 26, 1758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Marichannegowda, M.H.; Rakesh, K.P.; Qin, H.L. Master mechanisms of Staphylococcus aureus: Consider its excellent protective mechanisms hindering vaccine development! Microbiol. Res. 2018, 212-213, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, K.; Ishikawa, M.; Morita, Y.; Yokogawa, N.; Xie, C.; de Mesy Bentley, K.L.; Ito, H.; Kates, S.L.; Daiss, J.L.; Schwarz, E.M. IsdB antibody–mediated sepsis following S. aureus surgical site infection. JCI Insight. 2020, 5, e141164. [Google Scholar] [CrossRef] [PubMed]
- Gurtman, A.; Begier, E.; Mohamed, N.; Baber, J.; Sabharwal, C.; Haupt, R.M.; Edwards, H.; Cooper, D.; Jansen, K.U.; Anderson, A.S. The development of a Staphylococcus aureus four antigen vaccine for use prior to elective orthopedic surgery. Hum. Vaccin. Immunother. 2019, 15, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, S.A.; Bozio, C.H.; Duffy, J.; Rubin, L.G.; Hariri, S.; Stephens, D.S.; MacNeil, J.R. Meningococcal vaccination: Recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Prevention of community-acquired pneumonia with available pneumococcal vaccines. Int. J. Mol. Sci. 2016, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Frenck, R.W., Jr.; Creech, C.B.; Sheldon, E.A.; Seiden, D.J.; Kankam, M.K.; Baber, J.; Zito, E.; Hubler, R.; Eiden, J.; Severs, J.M.; et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 2017, 35, 375–384. [Google Scholar] [CrossRef]
- Nissen, M.; Marshall, H.; Richmond, P.; Shakib, S.; Jiang, Q.; Cooper, D.; Rill, D.; Baber, J.; Eiden, J.; Gruber, W.; et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 2015, 33, 1846–1854. [Google Scholar] [CrossRef] [Green Version]
- Creech, C.B.; Frenck, R.W.; Fiquet, A.; Feldman, R.; Kankam, M.K.; Pathirana, S.; Baber, J.; Radley, D.; Cooper, D.; Eiden, J.; et al. Persistence of immune responses through 36 months in healthy adults after vaccination with a novel Staphylococcus aureus 4-Antigen Vaccine (SA4Ag). Open Forum Inf. Dis. 2020, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Handke, L.D.; Gribenko, A.V.; Timofeyeva, Y.; Scully, I.L.; Anderson, A.S. MntC-dependent manganese transport is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 2018, 3, e00336-18. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.S.; Fowler, V.G., Jr.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatasubramaniam, A.; Liao, G.; Cho, E.; Adhikari, R.P.; Kort, T.; Holtsberg, F.W.; Elsass, K.E.; Kobs, D.J.; Rudge, T.L., Jr.; Kauffman, K.D.; et al. Safety and immunogenicity of a 4-component toxoid-based Staphylococcus aureus vaccine in rhesus macaques. Front. Immunol. 2021, 12, 621754. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Otto, M.; Reppschläger, K.; Iqbal, J.; Holtfreter, S. Fighting Staphylococcus aureus biofilms with monoclonal antibodies. Trends Microbiol. 2019, 27, 303–322. [Google Scholar] [CrossRef]
- Rupp, M.E.; Holley, H.P., Jr.; Lutz, J.; Dicpinigaitis, P.V.; Woods, C.W.; Levine, D.P.; Veney, N.; Fowler, V.G., Jr. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2007, 51, 4249–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.Q.; Robbie, G.J.; Wu, Y.; Esser, M.T.; Jensen, K.; Schwartz, H.I.; Bellamy, T.; Hernandez-Illas, M.; Jafri, H.S. Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-Staphylococcus aureus alpha-toxin human monoclonal antibody, in healthy adults. Antimicrob. Agents Chemother. 2016, 61, e01020-16. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.C.; Chaili, S.; Filler, S.G.; Miller, L.S.; Solis, N.V.; Wang, H.; Johnson, C.W.; Lee, H.K.; Diaz, L.F.; Yeaman, M.R. Innate immune memory contributes to host defense against recurrent skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus. Infect. Immun. 2017, 85, e00876-16. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.C.; Rossetti, M.; Miller, L.S.; Filler, S.G.; Johnson, C.W.; Lee, H.K.; Wang, H.; Gjertson, D.; Fowler, V.G., Jr.; Reed, E.F.; et al. Protective immunity in recurrent Staphylococcus aureus infection reflects localized immune signatures and macrophage-conferred memory. Proc. Natl. Acad. Sci. USA 2018, 115, E11111–E11119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leentjens, J.; Bekkering, S.; Joosten, L.A.B.; Netea, M.G.; Burgner, D.P.; Riksen, N.P. Trained innate immunity as a novel mechanism linking infection and the development of atherosclerosis. Circ. Res. 2018, 122, 664–669. [Google Scholar] [CrossRef]
- Maher, B.M.; Mulcahy, M.E.; Murphy, A.G.; Wilk, M.; O’Keeffe, K.M.; Geoghegan, J.A.; Lavelle, E.C.; McLoughlin, R.M. Nlrp-3-driven interleukin 17 production by γδT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect. Immun. 2013, 81, 4478–4489. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.C.; Chaili, S.; Filler, S.G.; Barr, K.; Wang, H.; Kupferwasser, D.; Edwards, J.E., Jr.; Xiong, Y.Q.; Ibrahim, A.S.; Miller, L.S.; et al. Nonredundant roles of interleukin-17A (IL-17A) and IL-22 in murine host defense against cutaneous and hematogenous infection due to methicillin-resistant Staphylococcus aureus. Infect. Immun. 2015, 83, 4427–4437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillen, C.A.; Pinsker, B.L.; Marusina, A.I.; Merleev, A.A.; Farber, O.N.; Liu, H.; Archer, N.K.; Lee, D.B.; Wang, Y.; Ortines, R.V.; et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Investig. 2018, 128, 1026–1042. [Google Scholar] [CrossRef]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus vaccine research and development: The past, present and future, including novel therapeutic strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; De Gregorio, E.; Del Giudice, G.; Phogat, S.; Pecetta, S.; Pizza, M.; Hanon, E. Vaccinology in the post−COVID-19 era. Proc. Natl. Acad. Sci. USA 2021, 118, e2020368118. [Google Scholar] [CrossRef] [PubMed]
- Gavi, The Vaccine Alliance. Available online: https://www.gavi.org/our-alliance/about (accessed on 1 December 2021).
- World Health Organization. Implementing the Immunization Agenda 2030. Available online: https://www.who.int/publications/m/item/implementing-the-immunization-agenda-2030 (accessed on 1 December 2021).
- Medical Countermeasures.gov. Partnering on Vaccines to Counter AMR Threats. Available online: https://www.medicalcountermeasures.gov/barda/cbrn/mdrvaccines/ (accessed on 1 December 2021).
- World Health Organization. Call to Action on Antimicrobial Resistance. 2021. Available online: https://www.who.int/news/item/30-07-2021-call-to-action-on-antimicrobial-resistance-2021 (accessed on 1 December 2021).
- CIDRAP (Center for Infectious Disease Research and Policy). Stewardship/Resistance Scan for Nov 10, 2021. Available online: https://www.cidrap.umn.edu/news-perspective/2021/11/stewardship-resistance-scan-nov-10-2021 (accessed on 1 December 2021).
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025; FAO: Rome, Italy, 2021; pp. 1–46. ISBN 978-92-5-134673-0. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence strategies for the treatment of Staphylococcus aureus infections: A mini review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef] [PubMed]
- Klimka, A.; Mertins, S.; Nicolai, A.K.; Rummler, L.M.; Higgins, P.G.; Günther, S.D.; Tosetti, B.; Krut, O.; Krönke, M. Epitope-specific immunity against Staphylococcus aureus coproporphyrinogen III oxidase. Vaccines 2021, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Bidmos, F.A.; Siris, S.; Gladstone, C.A.; Langford, P.R. Bacterial vaccine antigen discovery in the reverse vaccinology 2.0 era: Progress and challenges. Front. Immunol. 2018, 9, 2315. [Google Scholar] [CrossRef] [Green Version]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P.; Molle, V. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, F.; Serruto, D. The role of vaccines in combating antimicrobial resistance. Nature Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Vekemans, J.; Hasso-Agopsowicz, M.; Kang, G.; Hausdorff, W.P.; Fiore, A.; Tayler, E.; Klemm, E.J.; Laxminarayan, R.; Srikantiah, P.; Friede, M.; et al. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: A World Health Organization action framework. Clin. Infect. Dis. 2021, 73, e1011–e1017. [Google Scholar] [CrossRef] [PubMed]
- Medaglini, D.; De Azero, M.R.; Leroy, O.; Bietrix, F.; Denoel, P. Innovation partnership for a roadmap on vaccines in Europe (IPROVE): A vision for the vaccines of tomorrow. Vaccine 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Park, Y. All d-Lysine analogues of the antimicrobial Peptide HPA3NT3-A2 increased serum stability and without drug resistance. Int. J. Mol. Sci. 2020, 21, 5632. [Google Scholar] [CrossRef]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speziale, P.; Pietrocola, G. Monoclonal antibodies targeting surface-exposed and secreted proteins from staphylococci. Vaccines 2021, 9, 459. [Google Scholar] [CrossRef]
- Swolana, D.; Kępa, M.; Kabała-Dzik, A.; Dzik, R.; Wojtyczka, R.D. Sensitivity of staphylococcal biofilm to selected compounds of plant origin. Antibiotics 2021, 10, 607. [Google Scholar] [CrossRef]
- Walsh, L.; Johnson, C.N.; Hill, C.; Ross, R.P. Efficacy of phage- and bacteriocin-based therapies in combating nosocomial MRSA infections. Front. Mol. Biosci. 2021, 8, 654038. [Google Scholar] [CrossRef]
| Type of Virulence Factor | Name | Target | Effect |
|---|---|---|---|
| Cell wall-associated factors | Cell wall components—peptidoglycan, teichoic acid, lipoteichoic acid | Immune cells, other tissues | Stimulate immune cell activation and inflammatory response; participate in adhesion and biofilm formation |
| Staphylococcal protein A (SpA) | IgG, IgM, complement | Binds Fc region of IgG and IgM, thus inhibiting opsonization and phagocytosis; activates B cells | |
| Fibronectin-binding proteins (FnBPA, FnBPB) | Fibronectin, fibrinogen, elastin, plasminogen, keratin, complement | Binding to extracellular matrix proteins (ECM), enable adhesion to host tissues and biomaterials; limit phagocytosis and complement activation | |
| Collagen-binding protein (Cna) | Cartilage and collagen-rich tissues, complement | Binding cartilage and collagen, enables adhesion to host tissues; inhibits complement activation | |
| Clumping factors (ClfA, ClfB) | Fibrinogen, blood platelets, complement (ClfA), cytokeratin 10 (ClfB) | Binding to fibrinogen, enables adhesion to host tissues; inhibit complement preventing opsonization and phagocytosis; activate platelets | |
| Serine-aspartate repeat protein E (SdrE) | Complement | Inhibits complement preventing opsonization and phagocytosis | |
| Iron-regulated surface determinant proteins (IsdA, IsdB) | Heme-iron | Heme uptake and iron acquisition contribute to increased pathogenesis, tissue invasion and abscess formation | |
| Polysaccharide intercellular adhesion/polymeric N-acetyl-glucosamine (PIA/PNAG) | Staphylococcal cells, mucous membranes, other tissues, abiotic surfaces | Participates in bacterial aggregation, adhesion and biofilm formation (major component of biofilm matrix); reduces phagocytosis | |
| Capsular polysaccharides | Mucous membranes, other tissues, abiotic surfaces | Reduce phagocytosis; increase the efficiency of colonization and durability on the surface of mucous membranes or biomaterials | |
| Enzymes | Catalase | Hydrogen peroxide | Catalyzes breakdown of hydrogen peroxide into water and oxygen, preventing oxidative stress |
| Coagulase | Prothrombin | Reacts with prothrombin, allowing fibrinogen polymerization and clot formation, thus reducing phagocytosis | |
| Staphylokinase (SAK) | Plasminogen | Converts plasminogen to active serine protease plasmin, which promotes degradation of ECM, complement and IgG | |
| Lipases | Lipids of cell membranes and components of sebum | Decompose lipids, which allows spreading of staphylococci | |
| Nucleases | Nucleic acids | Degrade nucleic acids, thereby releasing them from extracellular traps (ETs) | |
| Proteases, e.g., serine protease V8 (SspA), staphopain A (Scp A) and B (SspB), aureolysin (Aur) | ECM proteins, complement, mucins, pulmonary surfactant | Degrade ECM proteins, mucins and pulmonary surfactant, which allow staphylococcal spread in the host tissues; inhibit chemotaxis and phagocytosis by proteolysis of immune cell receptors; degrade complement preventing opsonization and lysis of bacteria; degrade antimicrobial peptides | |
| Superoxide dismutases | Superoxide | Convert superoxide to hydrogen peroxide and oxygen, thereby preventing oxidative stress | |
| Toxins | Hemolysins (alpha, beta, gamma, delta) | Erythrocytes, platelets, leukocytes | Cause lysis of red blood cells, platelets, leukocytes—evading of host immune response; bacterial spreading |
| Enterotoxins | Enterocytes, lymphocytes T | Cause diarrhea; after translocation into blood, activate lymphocytes T leading to cytokine storm | |
| Exfoliative toxins | Desmosomes between keratinocytes | Cleave the granular layer of the epidermis by damaging desmosomes (staphylococcal scalded skin syndrome) | |
| Panton-–Valentine leukocidin (PVL) | Neutrophils, monocytes, macrophages | Causes lysis of neutrophils, monocytes, macrophages—avoiding innate immune response; development of necrotic changes | |
| Toxic shock syndrome toxin 1 (TSST-1) | Lymphocytes T | Activates lymphocytes T, which causes massive production of cytokines and leads to toxic shock syndrome | |
| Other secreted proteins | Chemotaxis inhibitory protein of Staphylococcus (CHIPS) | Neutrophils | Binds to cell receptors (FPR1 and C5aR) inhibiting neutrophils chemotaxis, thereby preventing phagocytosis |
| Staphylococcal complement inhibitor (SCIN) | Complement (C4, C3b) | Inhibits complement activation, thus preventing bacterial lysis, opsonization and phagocytosis | |
| SSL-5 | Neutrophils, platelets | Binds to cell receptors (PSGL-1 and GPCRs) inhibiting neutrophil diapedesis and activation; activates platelets (aggregate formation) | |
| SSL-7 | IgA, complement (C5) | Binds Fc region of IgA and complement protein C5, thus blocking antibodies and inhibiting complement activation | |
| Extracellular fibrinogen-binding protein (Efb) | Fibrinogen, blood platelets, complement | Binds fibrinogen enabling adhesion and aggregation: interferes with platelet aggregation; inhibits complement activation | |
| Extracellular adherence protein (Eap) | ICAM-1 | Binds ICAM-1 inhibiting neutrophil rolling and migration (diapedesis) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Bojek, U.; Różalska, B.; Sadowska, B. Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win? Int. J. Mol. Sci. 2022, 23, 948. https://doi.org/10.3390/ijms23020948
Wójcik-Bojek U, Różalska B, Sadowska B. Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win? International Journal of Molecular Sciences. 2022; 23(2):948. https://doi.org/10.3390/ijms23020948
Chicago/Turabian StyleWójcik-Bojek, Urszula, Barbara Różalska, and Beata Sadowska. 2022. "Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win?" International Journal of Molecular Sciences 23, no. 2: 948. https://doi.org/10.3390/ijms23020948
APA StyleWójcik-Bojek, U., Różalska, B., & Sadowska, B. (2022). Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win? International Journal of Molecular Sciences, 23(2), 948. https://doi.org/10.3390/ijms23020948