Cu(II) Binding Increases the Soluble Toxicity of Amyloidogenic Light Chains
Abstract
:1. Introduction
2. Results
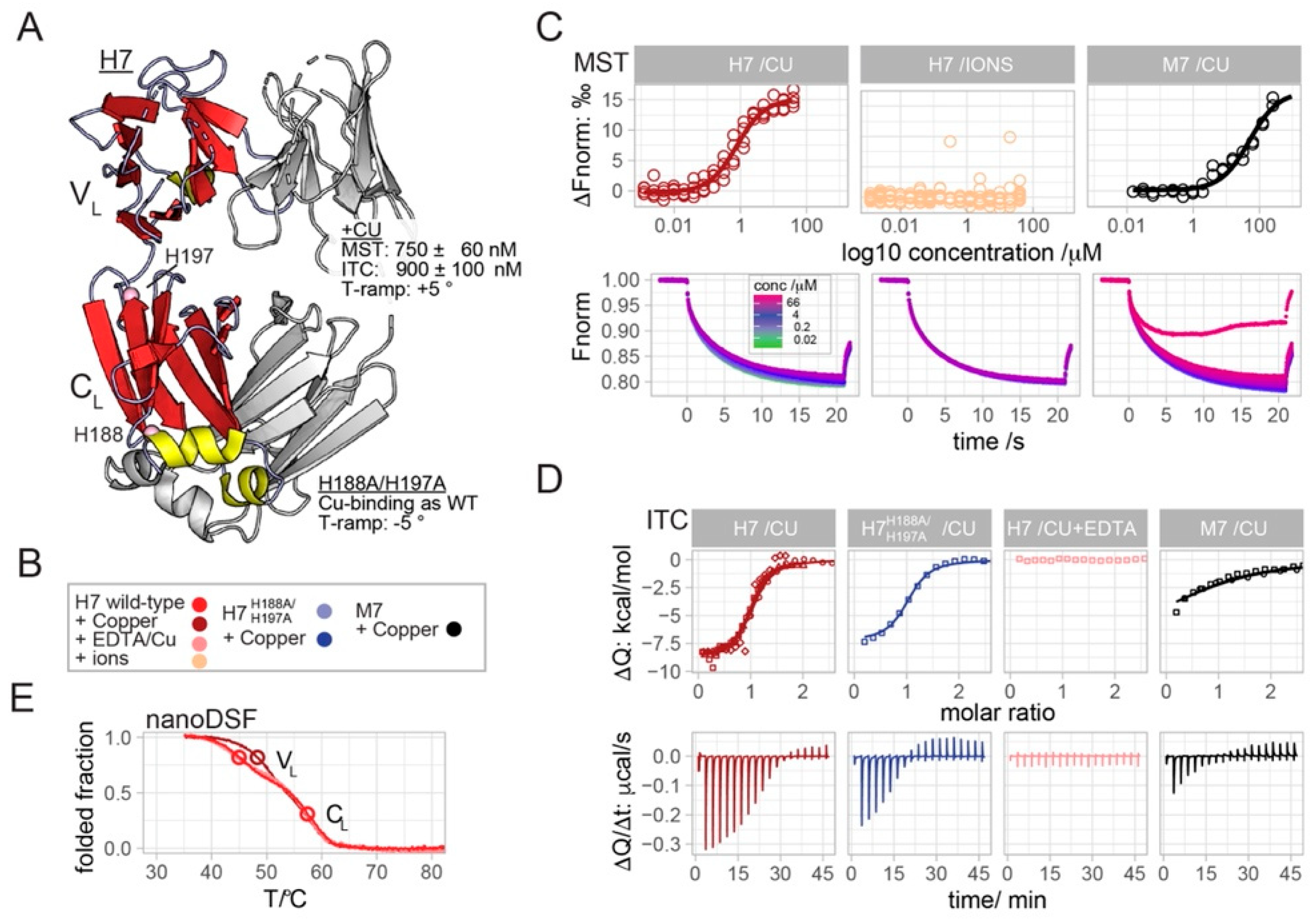
2.1. Copper Binds to H7 with Sub-Micromolar Affinity
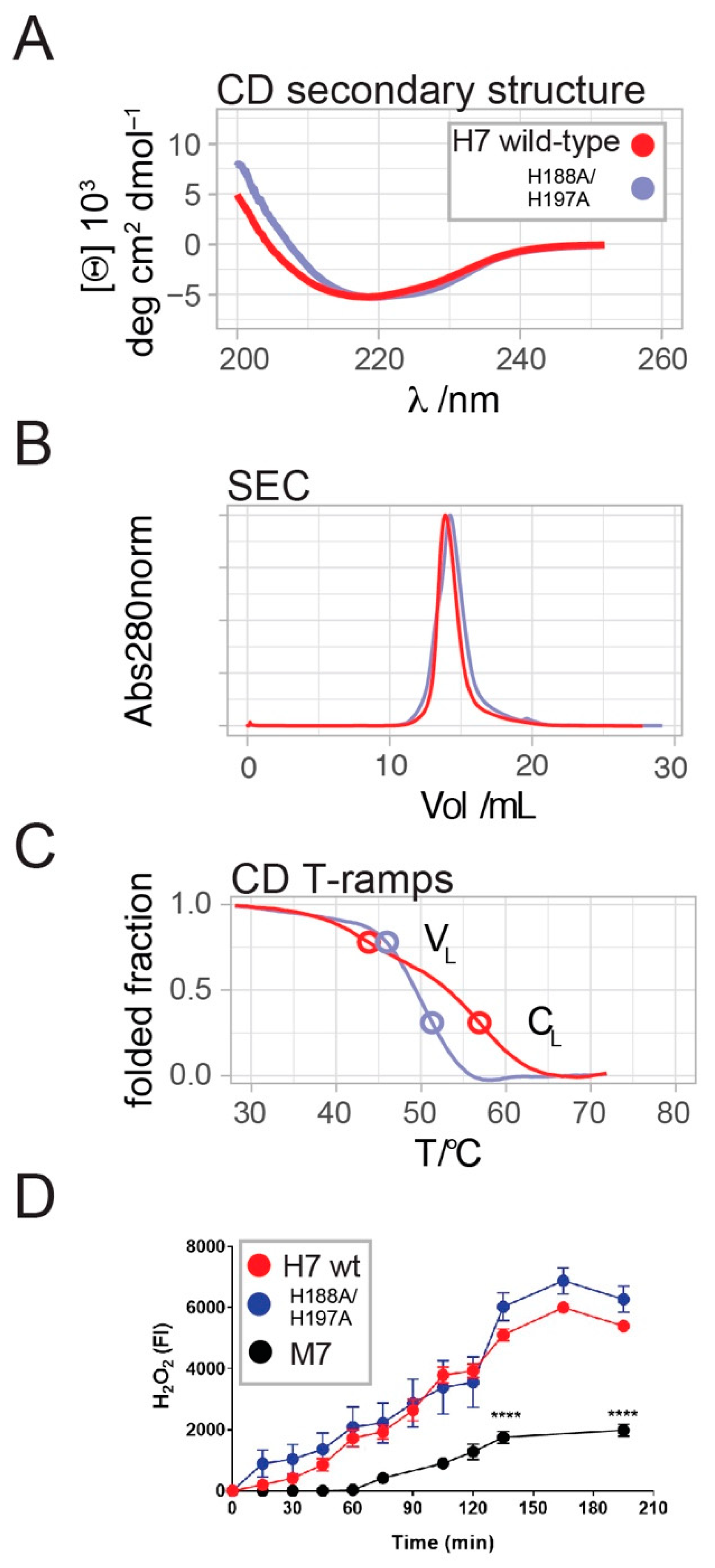
2.2. H7-H188A/H197A Mutant Binds Cu2+ and Produces H2O2 Similar to Wild-Type H7
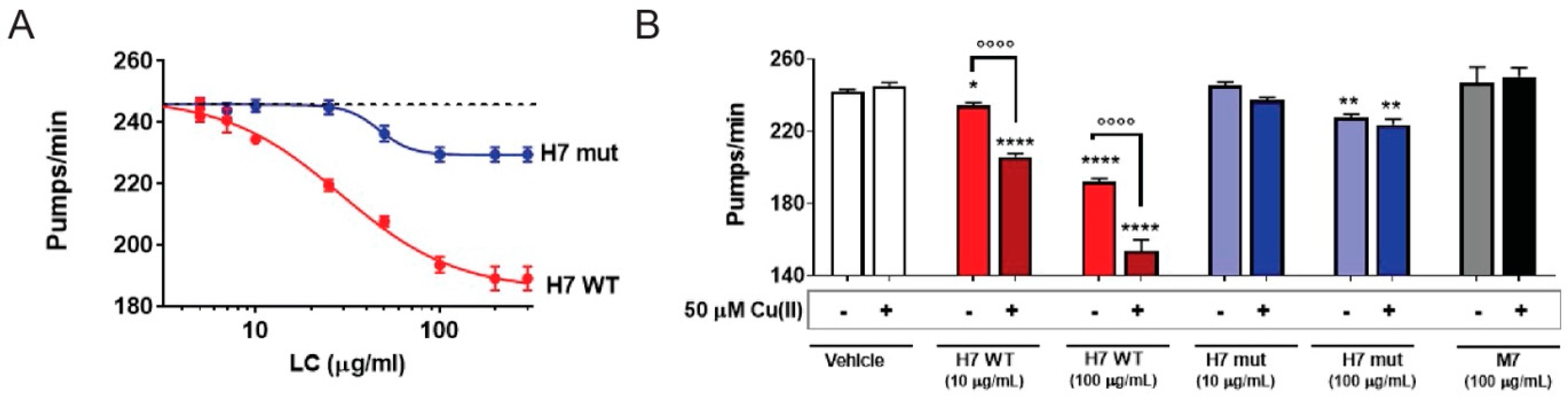
2.3. H7-H188A/H197A Mutant Is Less Toxic In Vivo
3. Discussion
4. Materials and Methods
4.1. Recombinant LC Production and Purification
4.2. Microscale Thermophoresis (MST)
4.3. Isothermal Titration Calorimetry (ITC)
4.4. Circular Dichroism and Tycho Measurements
4.5. Data Integration and Figure Preparation
4.6. Effect of LCs on C. elegans’ Pharyngeal Behaviour
4.7. Hydrogen Peroxide Determination
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2018: Recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2018, 25, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuvolone, M.; Merlini, G. Systemic amyloidosis: Novel therapies and role of biomarkers. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2017, 32, 770–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisele, Y.S.; Janssen, M.E.; MA, D.; Nguyen, B.; Plate, L.; Morgan, G.J.; Reixach, N.; Buxbaum, J.N.; Wiseman, R.L.; Lander, G.C.; et al. Formation and toxicity of transthyretin (ttr) oligomers in vitro and in patients with familial amyloid polyneuropathy. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 1123–1124. [Google Scholar] [CrossRef]
- Madhivanan, K.; Greiner, E.R.; Alves-Ferreira, M.; Soriano-Castell, D.; Rouzbeh, N.; Aguirre, C.A.; Paulsson, J.F.; Chapman, J.; Jiang, X.; Ooi, F.K.; et al. Cellular clearance of circulating transthyretin decreases cell-nonautonomous proteotoxicity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2018, 115, E7710–E7719. [Google Scholar] [CrossRef] [Green Version]
- Palladini, G.; Lavatelli, F.; Russo, P.; Perlini, S.; Perfetti, V.; Bosoni, T.; Obici, L.; Bradwell, A.R.; D’Eril, G.M.; Fogari, R.; et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 2006, 107, 3854–3858. [Google Scholar] [CrossRef]
- Reixach, N.; Deechongkit, S.; Jiang, X.; Kelly, J.W.; Buxbaum, J.N. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. USA 2004, 101, 2817–2822. [Google Scholar] [CrossRef] [Green Version]
- Sapp, V.; Jain, M.; Liao, R. Viewing extrinsic proteotoxic stress through the lens of amyloid cardiomyopathy. Physiology 2016, 31, 294–299. [Google Scholar] [CrossRef]
- Schonhoft, J.D.; Monteiro, C.; Plate, L.; Eisele, Y.S.; Kelly, J.M.; Boland, D.; Parker, C.G.; Cravatt, B.F.; Teruya, S.; Helmke, S.; et al. Peptide probes detect misfolded transthyretin oligomers in plasma of hereditary amyloidosis patients. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Diomede, L.; Rognoni, P.; Lavatelli, F.; Romeo, M.; Del Favero, E.; Cantu, L.; Ghibaudi, E.; di Fonzo, A.; Corbelli, A.; Fiordaliso, F.; et al. A Caenorhabditis elegans-based assay recognizes immunoglobulin light chains causing heart amyloidosis. Blood 2014, 123, 3543–3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diomede, L.; Romeo, M.; Rognoni, P.; Beeg, M.; Foray, C.; Ghibaudi, E.; Palladini, G.; Cherny, R.A.; Verga, L.; Capello, G.L.; et al. Cardiac light chain amyloidosis: The role of metal ions in oxidative stress and mitochondrial damage. Antioxid. Redox Signal. 2017, 27, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Imperlini, E.; Gnecchi, M.; Rognoni, P.; Sabido, E.; Ciuffreda, M.C.; Palladini, G.; Espadas, G.; Mancuso, F.M.; Bozzola, M.; Malpasso, G.; et al. Proteotoxicity in cardiac amyloidosis: Amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci. Rep. 2017, 7, 15661. [Google Scholar] [CrossRef]
- Lavatelli, F.; Imperlini, E.; Orru, S.; Rognoni, P.; Sarnataro, D.; Palladini, G.; Malpasso, G.; Soriano, M.E.; Di Fonzo, A.; Valentini, V.; et al. Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 4614–4628. [Google Scholar] [CrossRef]
- Liao, R.; Jain, M.; Teller, P.; Connors, L.H.; Ngoy, S.; Skinner, M.; Falk, R.H.; Apstein, C.S. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 2001, 104, 1594–1597. [Google Scholar] [CrossRef]
- Marin-Argany, M.; Guell-Bosch, J.; Blancas-Mejia, L.M.; Villegas, S.; Ramirez-Alvarado, M. Mutations can cause light chains to be too stable or too unstable to form amyloid fibrils. Protein Sci. 2015, 24, 1829–1840. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; Del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; Macgillivray, T.E.; et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef] [Green Version]
- Sikkink, L.A.; Ramirez-Alvarado, M. Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture. Cell Death Dis. 2010, 1, e98. [Google Scholar] [CrossRef]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schonland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Primers 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Palladini, G. Light chain amyloidosis: The heart of the problem. Haematologica 2013, 98, 1492–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlini, G.; Wechalekar, A.D.; Palladini, G. Systemic light chain amyloidosis: An update for treating physicians. Blood 2013, 121, 5124–5130. [Google Scholar] [CrossRef] [Green Version]
- Palladini, G.; Dispenzieri, A.; Gertz, M.A.; Kumar, S.; Wechalekar, A.; Hawkins, P.N.; Schonland, S.; Hegenbart, U.; Comenzo, R.; Kastritis, E.; et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J. Clin. Oncol. 2012, 30, 4541–4549. [Google Scholar] [CrossRef]
- Vaxman, I.; Gertz, M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019, 141, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kazman, P.; Vielberg, M.T.; Pulido Cendales, M.D.; Hunziger, L.; Weber, B.; Hegenbart, U.; Zacharias, M.; Kohler, R.; Schonland, S.; Groll, M.; et al. Fatal amyloid formation in a patient’s antibody light chain is caused by a single point mutation. eLife 2020, 9, e52300. [Google Scholar] [CrossRef]
- Klimtchuk, E.S.; Gursky, O.; Patel, R.S.; Laporte, K.L.; Connors, L.H.; Skinner, M.; Seldin, D.C. The critical role of the constant region in thermal stability and aggregation of amyloidogenic immunoglobulin light chain. Biochemistry 2010, 49, 9848–9857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maritan, M.; Romeo, M.; Oberti, L.; Sormanni, P.; Tasaki, M.; Russo, R.; Ambrosetti, A.; Motta, P.; Rognoni, P.; Mazzini, G.; et al. Inherent biophysical properties modulate the toxicity of soluble amyloidogenic light chains. J. Mol. Biol. 2020, 432, 845–860. [Google Scholar] [CrossRef]
- Morgan, G.J.; Kelly, J.W. The kinetic stability of a full-length antibody light chain dimer determines whether endoproteolysis can release amyloidogenic variable domains. J. Mol. Biol. 2016, 428, 4280–4297. [Google Scholar] [CrossRef] [Green Version]
- Morgan, G.J.; Yan, N.L.; Mortenson, D.E.; Rennella, E.; Blundon, J.M.; Gwin, R.M.; Lin, C.Y.; Stanfield, R.L.; Brown, S.J.; Rosen, H.; et al. Stabilization of amyloidogenic immunoglobulin light chains by small molecules. Proc. Natl. Acad. Sci. USA 2019, 116, 8360–8369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberti, L.; Rognoni, P.; Barbiroli, A.; Lavatelli, F.; Russo, R.; Maritan, M.; Palladini, G.; Bolognesi, M.; Merlini, G.; Ricagno, S. Concurrent structural and biophysical traits link with immunoglobulin light chains amyloid propensity. Sci. Rep. 2017, 7, 16809. [Google Scholar] [CrossRef] [Green Version]
- Rennella, E.; Morgan, G.J.; Yan, N.; Kelly, J.W.; Kay, L.E. The role of protein thermodynamics and primary structure in fibrillogenesis of variable domains from immunoglobulin light chains. J. Am. Chem. Soc. 2019, 141, 13562–13571. [Google Scholar] [CrossRef]
- Weber, B.; Hora, M.; Kazman, P.; Gobl, C.; Camilloni, C.; Reif, B.; Buchner, J. The antibody light-chain linker regulates domain orientation and amyloidogenicity. J. Mol. Biol. 2018, 430, 4925–4940. [Google Scholar] [CrossRef] [Green Version]
- Weber, B.; Hora, M.; Kazman, P.; Pradhan, T.; Ruhrnossl, F.; Reif, B.; Buchner, J. Domain interactions determine the amyloidogenicity of antibody light chain mutants. J. Mol. Biol. 2020, 432, 6187–6199. [Google Scholar] [CrossRef]
- Blancas-Mejia, L.M.; Horn, T.J.; Marin-Argany, M.; Auton, M.; Tischer, A.; Ramirez-Alvarado, M. Thermodynamic and fibril formation studies of full length immunoglobulin light chain AL-09 and its germline protein using scan rate dependent thermal unfolding. Biophys. Chem. 2015, 207, 13–20. [Google Scholar] [CrossRef] [Green Version]
- del Pozo-Yauner, L.; Wall, J.S.; Gonzalez Andrade, M.; Sanchez-Lopez, R.; Rodriguez-Ambriz, S.L.; Perez Carreon, J.I.; Ochoa-Leyva, A.; Fernandez-Velasco, D.A. The N-terminal strand modulates immunoglobulin light chain fibrillogenesis. Biochem. Biophys. Res. Commun. 2014, 443, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Andrade, M.; Becerril-Lujan, B.; Sanchez-Lopez, R.; Cecena-Alvarez, H.; Perez-Carreon, J.I.; Ortiz, E.; Fernandez-Velasco, D.A.; del Pozo-Yauner, L. Mutational and genetic determinants of lambda6 light chain amyloidogenesis. FEBS J. 2013, 280, 6173–6183. [Google Scholar] [CrossRef]
- Sternke-Hoffmann, R.; Boquoi, A.; Lopez, Y.N.D.; Platten, F.; Fenk, R.; Haas, R.; Buell, A.K. Biochemical and biophysical characterisation of immunoglobulin free light chains derived from an initially unbiased population of patients with light chain disease. PeerJ 2020, 8, e8771. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Piccoli, L.; Romeo, M.; Barzago, M.M.; Ravasio, S.; Foglierini, M.; Matkovic, M.; Sgrignani, J.; De Gasparo, R.; Prunotto, M.; et al. Machine learning analyses of antibody somatic mutations predict immunoglobulin light chain toxicity. Nat. Commun. 2021, 12, 3532. [Google Scholar] [CrossRef]
- Radamaker, L.; Baur, J.; Huhn, S.; Haupt, C.; Hegenbart, U.; Schonland, S.; Bansal, A.; Schmidt, M.; Fandrich, M. Cryo-EM reveals structural breaks in a patient-derived amyloid fibril from systemic AL amyloidosis. Nat. Commun. 2021, 12, 875. [Google Scholar] [CrossRef]
- Radamaker, L.; Karimi-Farsijani, S.; Andreotti, G.; Baur, J.; Neumann, M.; Schreiner, S.; Berghaus, N.; Motika, R.; Haupt, C.; Walther, P.; et al. Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM. Nat. Commun. 2021, 12, 6434. [Google Scholar] [CrossRef]
- Radamaker, L.; Lin, Y.H.; Annamalai, K.; Huhn, S.; Hegenbart, U.; Schonland, S.O.; Fritz, G.; Schmidt, M.; Fandrich, M. Cryo-EM structure of a light chain-derived amyloid fibril from a patient with systemic AL amyloidosis. Nat. Commun. 2019, 10, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swuec, P.; Lavatelli, F.; Tasaki, M.; Paissoni, C.; Rognoni, P.; Maritan, M.; Brambilla, F.; Milani, P.; Mauri, P.; Camilloni, C.; et al. Cryo-EM structure of cardiac amyloid fibrils from an immunoglobulin light chain AL amyloidosis patient. Nat. Commun. 2019, 10, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavatelli, F.; Mazzini, G.; Ricagno, S.; Iavarone, F.; Rognoni, P.; Milani, P.; Nuvolone, M.; Swuec, P.; Caminito, S.; Tasaki, M.; et al. Mass spectrometry characterization of light chain fragmentation sites in cardiac AL amyloidosis: Insights into the timing of proteolysis. J. Biol. Chem. 2020, 295, 16572–16584. [Google Scholar] [CrossRef]
- Lavatelli, F.; Perlman, D.H.; Spencer, B.; Prokaeva, T.; McComb, M.E.; Theberge, R.; Connors, L.H.; Bellotti, V.; Seldin, D.C.; Merlini, G.; et al. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Mol. Cell. Proteom. 2008, 7, 1570–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzini, G.; Ricagno, S.; Caminito, S.; Rognoni, P.; Milani, P.; Nuvolone, M.; Basset, M.; Foli, A.; Russo, R.; Merlini, G.; et al. Protease-sensitive regions in amyloid light chains: What a common pattern of fragmentation across organs suggests about aggregation. FEBS J. 2021. [Google Scholar] [CrossRef]
- Schonfelder, J.; Pfeiffer, P.B.; Pradhan, T.; Bijzet, J.; Hazenberg, B.P.C.; Schonland, S.O.; Hegenbart, U.; Reif, B.; Haupt, C.; Fandrich, M. Protease resistance of ex vivo amyloid fibrils implies the proteolytic selection of disease-associated fibril morphologies. Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2021, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marin-Argany, M.; Lin, Y.; Misra, P.; Williams, A.; Wall, J.S.; Howell, K.G.; Elsbernd, L.R.; McClure, M.; Ramirez-Alvarado, M. Cell damage in light chain amyloidosis: Fibril internalization, toxicity and cell-mediated seeding. J. Biol. Chem. 2016, 291, 19813–19825. [Google Scholar] [CrossRef] [Green Version]
- Rubino, J.T.; Franz, K.J. Coordination chemistry of copper proteins: How nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012, 107, 129–143. [Google Scholar] [CrossRef]
- Pelaez-Aguilar, A.E.; Valdes-Garcia, G.; French-Pacheco, L.; Pastor, N.; Amero, C.; Rivillas-Acevedo, L. Site-specific interactions with copper promote amyloid fibril formation for lambda6aJL2-R24G. ACS Omega 2020, 5, 7085–7095. [Google Scholar] [CrossRef]
- Mishra, S.; Guan, J.; Plovie, E.; Seldin, D.C.; Connors, L.H.; Merlini, G.; Falk, R.H.; MacRae, C.A.; Liao, R. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H95–H103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognoni, P.; Lavatelli, F.; Casarini, S.; Palladini, G.; Verga, L.; Pedrazzoli, P.; Valentini, G.; Merlini, G.; Perfetti, V. A strategy for synthesis of pathogenic human immunoglobulin free light chains in E. coli. PLoS ONE 2013, 8, e76022. [Google Scholar] [CrossRef]
- Duhr, S.; Braun, D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA 2006, 103, 19678–19682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brautigam, C.A.; Zhao, H.; Vargas, C.; Keller, S.; Schuck, P. Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions. Nat. Protoc. 2016, 11, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, T.H.; Padrick, S.B.; Gardner, K.H.; Brautigam, C.A. On the acquisition and analysis of microscale thermophoresis data. Anal. Biochem. 2016, 496, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Wickham, H. tidyverse: Easily Install and Load the ‘Tidyverse. 2017.
- Wickham, H.; Grolemund, G. R for Data Science; O’Reilly Media: Newton, MA, USA, 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, R.; Romeo, M.; Schulte, T.; Maritan, M.; Oberti, L.; Barzago, M.M.; Barbiroli, A.; Pappone, C.; Anastasia, L.; Palladini, G.; et al. Cu(II) Binding Increases the Soluble Toxicity of Amyloidogenic Light Chains. Int. J. Mol. Sci. 2022, 23, 950. https://doi.org/10.3390/ijms23020950
Russo R, Romeo M, Schulte T, Maritan M, Oberti L, Barzago MM, Barbiroli A, Pappone C, Anastasia L, Palladini G, et al. Cu(II) Binding Increases the Soluble Toxicity of Amyloidogenic Light Chains. International Journal of Molecular Sciences. 2022; 23(2):950. https://doi.org/10.3390/ijms23020950
Chicago/Turabian StyleRusso, Rosaria, Margherita Romeo, Tim Schulte, Martina Maritan, Luca Oberti, Maria Monica Barzago, Alberto Barbiroli, Carlo Pappone, Luigi Anastasia, Giovanni Palladini, and et al. 2022. "Cu(II) Binding Increases the Soluble Toxicity of Amyloidogenic Light Chains" International Journal of Molecular Sciences 23, no. 2: 950. https://doi.org/10.3390/ijms23020950
APA StyleRusso, R., Romeo, M., Schulte, T., Maritan, M., Oberti, L., Barzago, M. M., Barbiroli, A., Pappone, C., Anastasia, L., Palladini, G., Diomede, L., & Ricagno, S. (2022). Cu(II) Binding Increases the Soluble Toxicity of Amyloidogenic Light Chains. International Journal of Molecular Sciences, 23(2), 950. https://doi.org/10.3390/ijms23020950








