The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders?
Abstract
1. Introduction
2. Methodology and Literature Search
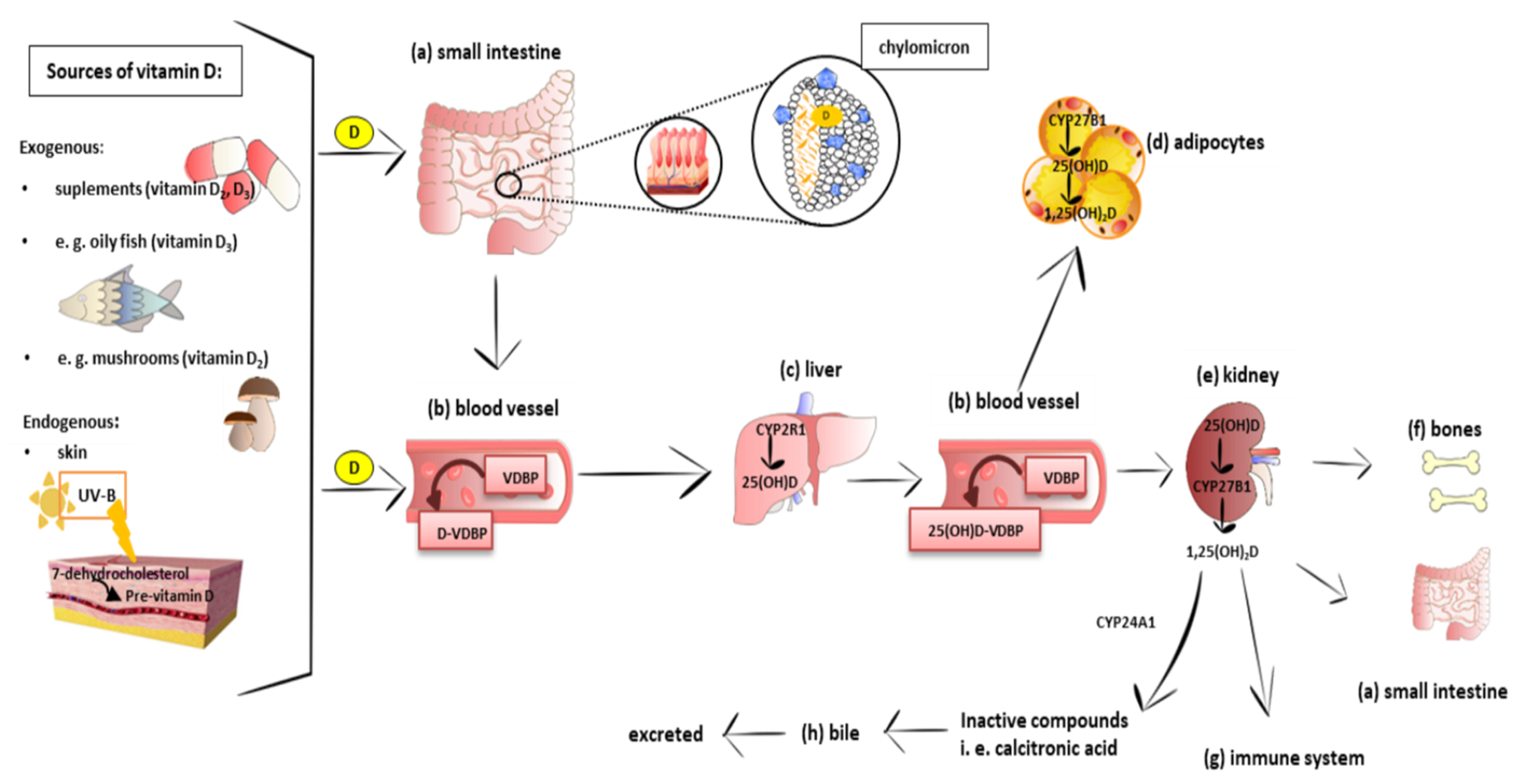
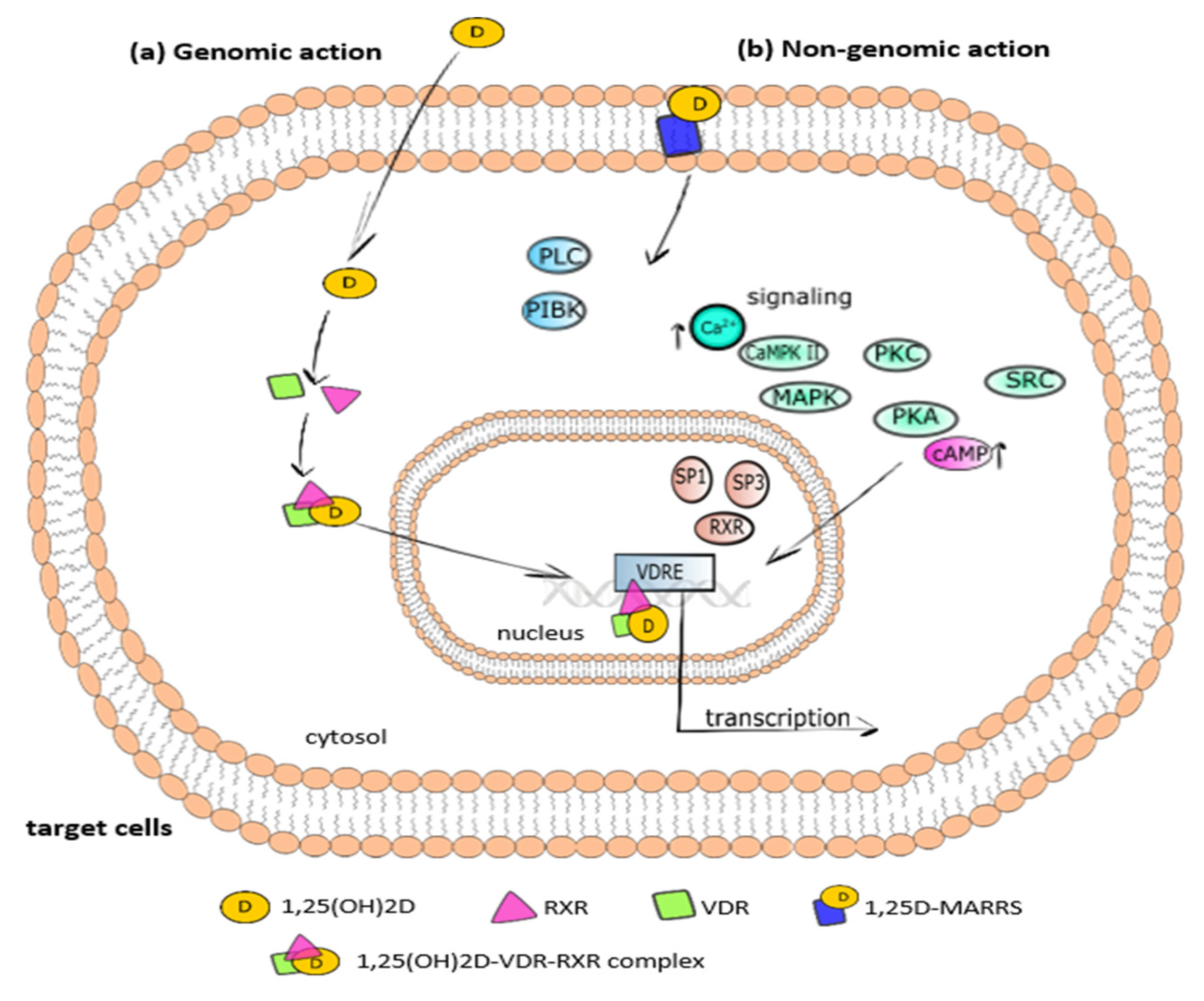
3. Vitamin D Metabolism, Mechanism of Action, and Tissue Distribution
3.1. Metabolism and Molecular Response to Vitamin D
3.2. Distribution of Vitamin D
4. Vitamin D Action in Adipose Tissue
4.1. A Brief Insight into the Adipogenesis Process
4.2. The Action of Vitamin D in the Process of Adipogenesis—In Vitro Studies
4.3. The Effect of Vitamin D in Adipogenesis—Animal Models
4.4. Vitamin D and Apoptosis of Adipocytes
4.5. Vitamin D as a Regulator of Metabolism and Adipocytokines Secretion in Adipose Tissue
4.5.1. Vitamin D as a Regulator of Lipid Metabolism in Adipose Tissue
4.5.2. The Effect of Vitamin D on Production of Adipocytokines
4.6. Effects of Vitamin D on Adipose Tissue Inflammation
4.7. Oxidative Stress in Adipose Tissue
Anti/Prooxidant Activity of Vitamin D
4.8. The Effect of Vitamin D on Thermogenesis
5. Is Vitamin D Level Related to Lipid Metabolism Disorders and Obesity? Results from Interventional Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Zaiou, M.; El Amri, H.; Bakillah, A. The clinical potential of adipogenesis and obesity-related microRNAs. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 91–111. [Google Scholar] [CrossRef]
- Gurmaches, J.S.; Hung, C.-M.; Guertin, D.A. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016, 26, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, D.; Xiang, J.; Zhou, J.; Cao, H.; Che, Q.; Bai, Y.; Guo, J.; Su, Z. Non-shivering Thermogenesis Signalling Regulation and Potential Therapeutic Applications of Brown Adipose Tissue. Int. J. Biol. Sci. 2021, 17, 2853–2870. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, J.; Cereijo, R.; Villarroya, F. An endocrine role for brown adipose tissue? Am. J. Physiol. Metab. 2013, 305, E567–E572. [Google Scholar] [CrossRef]
- Hansen, I.R.; Jansson, K.M.; Cannon, B.; Nedergaard, J. Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.; Petrovic, N.; de Jong, J.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in Brite/Beige Adipose Tissue Mitochondria Is Functionally Thermogenic. Cell Rep. 2013, 5, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.T.; Kiefer, F.W. Adipose tissue browning in mice and humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef] [PubMed]
- Finer, N. Medical consequences of obesity. Medicine 2015, 43, 88–93. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Joseph, F. Adipose Tissue and Adipokines: The Association with and Application of Adipokines in Obesity. Sci. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846. [Google Scholar] [PubMed]
- Gallagher, J.C.; Sai, A.J. Vitamin D Insufficiency, Deficiency, and Bone Health. J. Clin. Endocrinol. Metab. 2010, 95, 2630–2633. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Mutt, S.J.; Hyppönen, E.; Saarnio, J.; Jã¤Rvelin, M.-R.; Herzig, K.-H. Vitamin D and adipose tissue—More than storage. Front. Physiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Muralidhara, K.S.; Zimmerman, A. Vitamin D-3 intestinal absorption in vivo: Influence of fatty acids, bile salts, and perfusate pH on absorption. Gut 1978, 19, 267–272. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Investig. 1993, 91, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Kiourtzidis, M.; Kühn, J.; Brandsch, C.; Stangl, G.I. Vitamin D Status of Mice Deficient in Scavenger Receptor Class B Type 1, Cluster Determinant 36 and ATP-Binding Cassette Proteins G5/G8. Nutrients 2020, 12, 2169. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Compston, J.E.; Merrett, A.L.; Hammett, F.G.; Magill, P. Comparison of the Appearance of Radiolabelled Vitamin D3 and 25-Hydroxy-Vitamin D3 in the Chylomicron Fraction of Plasma after Oral Administration in Man. Clin. Sci. 1981, 60, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, R.; Forsgren, L.; Bak, T.A.; Holmberg, P.; Eriksson, G.; Blinc, R.; Pausak, S.; Ehrenberg, L.; Dumanović, J. Intestinal Absorption and Esterification of Vitamin D3-1,2-3H in Man. Acta Chem. Scand. 1967, 21, 1662–1663. [Google Scholar] [CrossRef] [PubMed]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Simultaneous Synthesis of Vitamins D2, D4, D5, D6, and D7 from Commercially Available Phytosterol, β-Sitosterol, and Identification of Each Vitamin D by HSQC NMR. Metab. 2019, 9, 107. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- A Houghton, L.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef]
- Hollis, B.W. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J. Steroid Biochem. 1984, 21, 81–86. [Google Scholar] [CrossRef]
- Horst, R.L.; Reinhardt, T.; Ramberg, C.F.; Koszewski, N.J.; Napoli, J.L. 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process. J. Biol. Chem. 1986, 261, 9250–9256. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Komba, S.; Hase, M. Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities. Nutrients 2021, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in Mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired Release of Vitamin D in Dysfunctional Adipose Tissue: New Cues on Vitamin D Supplementation in Obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef]
- Li, J.; Byrne, M.E.; Chang, E.; Jiang, Y.; Donkin, S.S.; Buhman, K.K.; Burgess, J.R.; Teegarden, D. 1α,25-Dihydroxyvitamin D hydroxylase in adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 112, 122–126. [Google Scholar] [CrossRef]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Kawada, T.; Kazuki, R.; Ono, T.; Kato, S.; Sugimoto, E. Vitamin D Receptor Gene Expression Is Up-Regulated by 1, 25-Dihydroxyvitamin D3 in 3T3-L1 Preadipocytes. Biochem. Biophys. Res. Commun. 1993, 193, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C.; Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; et al. Hepatocyte production of modulators of extracellular liver matrix in normal and cirrhotic rat liver. Br. J. Nutr. 2012, 108, 1915–1923. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pr. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular Mechanisms of Vitamin D Action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89-90, 281–285. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Simmons, K.M.; Brunton, J.; Salinero, A.; Chittur, S.V.; Welsh, J.E. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J. Cell. Physiol. 2013, 228, 2024–2036. [Google Scholar] [CrossRef]
- Mahajan, A.; Stahl, C.H. Dihydroxy-cholecalciferol stimulates adipocytic differentiation of porcine mesenchymal stem cells. J. Nutr. Biochem. 2009, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Landrier, J.-F. Vitamin D, adipose tissue, and obesity. Horm. Mol. Biol. Clin. Investig. 2013, 15, 123–128. [Google Scholar] [CrossRef]
- Bikle, D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Rosenstreich, S.J.; Rich, C.; Volwiler, W. Deposition in and release of vitamin D3 from body fat: Evidence for a storage site in the rat. J. Clin. Investig. 1971, 50, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Li, X.; Elli, E.F.; Ayloo, S.M.; Castellanos, K.J.; Fantuzzi, G.; Freels, S.; Braunschweig, C.L. Vitamin D, inflammation, and relations to insulin resistance in premenopausal women with morbid obesity. Obes. 2015, 23, 1591–1597. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Muñoz-Garach, A.; Serrano, M.; Garrido-Sánchez, L.; Bernal-López, M.R.; Fernández-García, D.; Moreno-Santos, I.; Garriga, N.; Castellano-Castillo, D.; Camargo, A.; et al. Serum 25-Hydroxyvitamin D and Adipose Tissue Vitamin D Receptor Gene Expression: Relationship With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2015, 100, E591–E595. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Holick, M.; Fried, S.K.; Lee, M.-J. 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 Promote the Differentiation of Human Subcutaneous Preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, B.D.; Dolnikowski, G.; Seyoum, E.; Thomas, A.P.; Gertz, E.R.; Souza, E.C.; Woodhouse, L.R.; Newman, J.W.; Keim, N.L.; Adams, S.H.; et al. Association between Subcutaneous White Adipose Tissue and Serum 25-Hydroxyvitamin D in Overweight and Obese Adults. Nutrients 2013, 5, 3352–3366. [Google Scholar] [CrossRef]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef]
- Malmberg, P.; Karlsson, T.; Svensson, H.; Lönn, M.; Carlsson, N.-G.; Sandberg, A.-S.; Jennische, E.; Osmancevic, A.; Holmäng, A. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: A pilot study. J. Photochem. Photobiol. B Biol. 2014, 138, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Backhouse, J.; Holman, C.A.; Lumb, G.A.; Stanbury, S.W. The Distribution and Storage of Vitamin D and its Metabolites in Human Tissues. Clin. Sci. 1972, 43, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.-F.; Marcotorchino, J.; Tourniaire, F. Lipophilic Micronutrients and Adipose Tissue Biology. Nutrients 2012, 4, 1622–1649. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Dallal, G.E.; Dawson-Hughes, B. Body Size and Serum 25 Hydroxy Vitamin D Response to Oral Supplements in Healthy Older Adults. J. Am. Coll. Nutr. 2008, 27, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric Dilution, Rather Than Sequestration Best Explains the Low Vitamin D Status of Obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef]
- Mai, X.-M.; Chen, Y.; Camargo, C.A.; Langhammer, A. Cross-Sectional and Prospective Cohort Study of Serum 25-Hydroxyvitamin D Level and Obesity in Adults: The HUNT Study. Am. J. Epidemiology 2012, 175, 1029–1036. [Google Scholar] [CrossRef]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutierrez, C.; Rubio, E.; Pérez-Valero, V.; Esteva, I.; De Adana, M.S.R.; Almaraz, M.C.; Colomo, N.; et al. Hypovitaminosis D and incidence of obesity: A prospective study. Eur. J. Clin. Nutr. 2013, 67, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2016, 32, 237–242. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, W.; Yang, Y.; Wu, J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulatingC/EBPβtranscription. J. Cell. Biochem. 2011, 112, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Carnevalli, L.S.; Masuda, K.; Frigerio, F.; Le Bacquer, O.; Um, S.H.; Gandin, V.; Topisirovic, I.; Sonenberg, N.; Thomas, G.; Kozma, S.C. S6K1 Plays a Critical Role in Early Adipocyte Differentiation. Dev. Cell 2010, 18, 763–774. [Google Scholar] [CrossRef]
- Jin, W.; Takagi, T.; Kanesashi, S.-N.; Kurahashi, T.; Nomura, T.; Harada, J.; Ishii, S. Schnurri-2 Controls BMP-Dependent Adipogenesis via Interaction with Smad Proteins. Dev. Cell 2006, 10, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef]
- Smas, C.M.; Sul, H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 1993, 73, 725–734. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of Adipogenesis by Wnt Signaling. Sci. 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Fujiwara, K.; Hasegawa, K.; Ohkumo, T.; Miyoshi, H.; Tseng, Y.-H.; Yoshikawa, K. Necdin Controls Proliferation of White Adipocyte Progenitor Cells. PLoS ONE 2012, 7, e30948. [Google Scholar] [CrossRef] [PubMed]
- Scimè, A.; Grenier, G.; Huh, M.S.; Gillespie, M.A.; Bevilacqua, L.; Harper, M.-E.; Rudnicki, M.A. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1α. Cell Metab. 2005, 2, 283–295. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding Adipocyte Differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–224. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Molecular Regulation of Adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Gillilan, R.E.; Ayers, S.; Noy, N. Structural Basis for Activation of Fatty Acid-binding Protein 4. J. Mol. Biol. 2007, 372, 1246–1260. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Zhang, Y.; Lazar, M.A.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; et al. PPAR and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef]
- Nielsen, R.; Pedersen, T.Å.; Hagenbeek, D.; Moulos, P.; Siersbæk, R.; Megens, E.; Denissov, S.; Børgesen, M.; Francoijs, K.-J.; Mandrup, S.; et al. Genome-wide profiling of PPARγ:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008, 22, 2953–2967. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S.; Bashour, K.T.; Tsai, J.; Shen, K.; Lee, J.-H.; Sun, E.; et al. Peroxisome Proliferator-Activated Receptor γ and C/EBPα Synergistically Activate Key Metabolic Adipocyte Genes by Assisted Loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef]
- Felicidade, I.; Sartori, D.; Coort, S.L.; Semprebon, S.C.; Niwa, A.M.; D’Epiro, G.F.R.; Biazi, B.I.; Marques, L.A.; Evelo, C.T.; Mantovani, M.S.; et al. Role of 1α,25-Dihydroxyvitamin D3 in Adipogenesis of SGBS Cells: New Insights into Human Preadipocyte Proliferation. Cell. Physiol. Biochem. 2018, 48, 397–408. [Google Scholar] [CrossRef]
- Atmani, H.; Chappard, D.; Basle, M.F. Proliferation and differentiation of osteoblasts and adipocytes in rat bone marrow stromal cell cultures: Effects of dexamethasone and calcitriol. J. Cell. Biochem. 2003, 89, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bellows, C.G.; Wang, Y.H.; Heersche, J.N.; E Aubin, J. 1,25-dihydroxyvitamin D3 stimulates adipocyte differentiation in cultures of fetal rat calvaria cells: Comparison with the effects of dexamethasone. Endocrinology 1994, 134, 2221–2229. [Google Scholar] [CrossRef]
- Cianferotti, L.; Demay, M.B. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J. Cell. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Gimble, J.M. 1,25-Dihydroxy Vitamin D3 Inhibits Adipocyte Differentiation and Gene Expression in Murine Bone Marrow Stromal Cell Clones and Primary Cultures*. Endocrinology 1998, 139, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Nagai, K.; Woo, J.-T. Insulin-Dependent Adipogenesis in Stromal ST2 Cells Derived from Murine Bone Marrow. Biosci. Biotechnol. Biochem. 2003, 67, 314–321. [Google Scholar] [CrossRef]
- Shionome, M.; Shinki, T.; Takahashi, N.; Hasegawa, K.; Suda, T. 1α,25-Dihydroxyvitamin D3 modulation in lipid metabolism in established bone marrow-derived stromal cells, MC3T3-G2/PA6. J. Cell. Biochem. 1992, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Li, Y.C. Molecular mechanism of 1,25-dihydroxyvitamin D3inhibition of adipogenesis in 3T3-L1 cells. Am. J. Physiol. Metab. 2006, 290, E916–E924. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex Role of the Vitamin D Receptor and Its Ligand in Adipogenesis in 3T3-L1 Cells. J. Biol. Chem. 2006, 281, 11205–11213. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Ambati, S.; Yang, J.-Y.; Park, H.J.; Baile, C.A. Enhanced Effects of 1,25(OH)2 D3 Plus Genistein on Adipogenesis and Apoptosis in 3T3-L1 Adipocytes. Obesity 2008, 16, 539–546. [Google Scholar] [CrossRef]
- Sakuma, S.; Fujisawa, J.; Sumida, M.; Tanigawa, M.; Inoda, R.; Sujihera, T.; Kohda, T.; Fujimoto, Y. The Involvement of Mitogen-Activated Protein Kinases in the 1^|^alpha;,25-Dihydroxy-Cholecalciferol-Induced Inhibition of Adipocyte Differentiation In Vitro. J. Nutr. Sci. Vitaminol. 2012, 58, 1–8. [Google Scholar] [CrossRef]
- Kawada, T.; Aoki, N.; Kamei, Y.; Maeshige, K.; Nishiu, S.; Sugimoto, E. Comparative investigation of vitamins and their analogues on terminal differentiation, from preadipocytes to adipocytes, of 3T3-L1 cells. Comp. Biochem. Physiol. Part A Physiol. 1990, 96, 323–326. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.; Yoon, Y. Anti-adipogenic effects of 1,25-dihydroxyvitamin D3 are mediated by the maintenance of the wingless-type MMTV integration site/β-catenin pathway. Int. J. Mol. Med. 2012, 30, 1219–1224. [Google Scholar] [CrossRef]
- Hida, Y.; Kawada, T.; Kayahashi, S.; Ishihara, T.; Fushiki, T. Counteraction of retinoic acid and 1,25-dihydroxyvitamin D3 on up-regulation of adipocyte differentiation with PPARγ ligand, an antidiabetic thiazolidinedione, in 3T3-L1 cells. Life Sci. 1998, 62, PL205–PL211. [Google Scholar] [CrossRef]
- Ishida, Y.; Taniguchi, H.; Baba, S. Possible involvement of 1α,25-dihydroxyvitamine D3 in proliferation and differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 1988, 151, 1122–1127. [Google Scholar] [CrossRef]
- Sato, M.; Hiragun, A. Demonstration of 1?,25-dihydroxyvitamin D3 receptor-like molecule in ST 13 and 3T3 L1 preadipocytes and its inhibitory effects on preadipocyte differentiation. J. Cell. Physiol. 1988, 135, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.; Ahrens, J.M.; Ntambi, J.M.; DeLuca, H.F.; Clagett-Dame, M. 2-Methylene-19-nor-1α-hydroxyvitamin D3 analogs inhibit adipocyte differentiation and PPARγ2 gene transcription. Arch. Biochem. Biophys. 2007, 460, 192–201. [Google Scholar] [CrossRef]
- Ji, S.; Doumit, M.E.; Hill, R.A. Regulation of Adipogenesis and Key Adipogenic Gene Expression by 1, 25-Dihydroxyvitamin D in 3T3-L1 Cells. PLoS ONE 2015, 10, e0126142. [Google Scholar] [CrossRef]
- Fu, M.; Sun, T.; Bookout, A.L.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. A Nuclear Receptor Atlas: 3T3-L1 Adipogenesis. Mol. Endocrinol. 2005, 19, 2437–2450. [Google Scholar] [CrossRef] [PubMed]
- Rochford, J.J.; Semple, R.K.; Laudes, M.; Boyle, K.B.; Christodoulides, C.; Mulligan, C.; Lelliott, C.J.; Schinner, S.; Hadaschik, D.; Mahadevan, M.; et al. ETO/MTG8 Is an Inhibitor of C/EBPβ Activity and a Regulator of Early Adipogenesis. Mol. Cell. Biol. 2004, 24, 9863–9872. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef]
- Shi, Y.; Hon, M.; Evans, R.M. The peroxisome proliferator-activated receptor, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Lee, J.S.; Kim, M.; Ahn, B.Y.; Jung, H.S.; Lee, H.M.; Kim, J.-W.; Park, K.S. Regulation of Wnt/β-Catenin Signaling by CCAAT/Enhancer Binding Protein β During Adipogenesis. Obesity 2012, 20, 482–487. [Google Scholar] [CrossRef]
- Ching, S.; Kashinkunti, S.; Niehaus, M.D.; Zinser, G.M. Mammary adipocytes bioactivate 25-hydroxyvitamin D3 and signal via vitamin D3 receptor, modulating mammary epithelial cell growth. J. Cell. Biochem. 2011, 112, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Lin, Y.; Yang, G. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of porcine preadipocyte in vitro. Chem. Interactions 2007, 170, 114–123. [Google Scholar] [CrossRef]
- Penkov, D.N.; Egorov, A.; Mozgovaya, M.N.; Tkachuk, V. Insulin resistance and adipogenesis: Role of transcription and secreted factors. Biochemistry 2013, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Della-Fera, M.A.; Ambati, S.; Boyan, B.; Baile, C.A. Enhanced effects of guggulsterone plus 1,25(OH)2D3 on 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 364, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Pacot, C.; Dugail, I.; Lemarchand, P.; Guichard, C.; le Lièpvre, X.; Berthelier-Lubrano, C.; Spiegelman, B.; Kim, J.B.; Ferré, P.; et al. ADD1/SREBP-1c Is Required in the Activation of Hepatic Lipogenic Gene Expression by Glucose. Mol. Cell. Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.-K.; Choi, E.; Lee, J.W. Identification of a Functional Vitamin D Response Element in the Murine Insig-2 Promoter and Its Potential Role in the Differentiation of 3T3-L1 Preadipocytes. Mol. Endocrinol. 2005, 19, 399–408. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Sun, X.; Morris, K.L.; Zemel, M.B. Role of Calcitriol and Cortisol on Human Adipocyte Proliferation and Oxidative and Inflammatory Stress: A Microarray Study. J. Nutr. Nutr. 2007, 1, 30–48. [Google Scholar] [CrossRef]
- Gharbi-Chihi, J.; Teboul, M.; Bismuth, J.; Bonne, J.; Torresani, J. Increase of adipose differentiation by hypolipidemic fibrate drugs in Ob 17 preadipocytes: Requirement for thyroid hormones. Biochim. Biophys. Acta (BBA)-Bioenerg. 1993, 1177, 8–14. [Google Scholar] [CrossRef]
- Lenoir, C.; Dace, A.; Martin, C.; Bonne, J.; Teboul, M.; Planells, R.; Torresani, J. Calcitriol down-modulates the 3,5,3’ triiodothyronine (T3) receptors and affects, in a biphasic manner, the T3-dependent adipose differentiation of Ob 17 preadipocytes. Endocrinology 1996, 137, 4268–4276. [Google Scholar] [CrossRef][Green Version]
- Matthews, D.G.; D’Angelo, J.; Drelich, J.; Welsh, J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J. Steroid Biochem. Mol. Biol. 2016, 164, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean Phenotype and Resistance to Diet-Induced Obesity in Vitamin D Receptor Knockout Mice Correlates with Induction of Uncoupling Protein-1 in White Adipose Tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Erben, R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2012, 97, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Chen, Y.; Zhu, G.; Zhao, Q.; Li, Y.C. 1,25-Dihydroxyvitamin D3 upregulates leptin expression in mouse adipose tissue. J. Endocrinol. 2012, 216, 265–271. [Google Scholar] [CrossRef]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted Expression of Human Vitamin D Receptor in Adipocytes Decreases Energy Expenditure and Induces Obesity in Mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef]
- Schutkowski, A.; Max, D.; Bönn, M.; Brandsch, C.; Grundmann, S.M.; Hirche, F.; Staege, M.S.; Stangl, G.I. Vitamin D Does Not Play a Functional Role in Adipose Tissue Development in Rodent Models. Mol. Nutr. Food Res. 2018, 62, 1700726. [Google Scholar] [CrossRef]
- Belenchia, A.M.; Jones, K.L.; Will, M.; Beversdorf, D.Q.; Vieira-Potter, V.; Rosenfeld, C.S.; Peterson, C.A. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor gamma (PPARγ) and vitamin D receptor (VDR) in lean male mice offspring. Eur. J. Nutr. 2018, 57, 723–730. [Google Scholar] [CrossRef]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Myung, K. Vitamin D3 regulation of body fat, cytokines, and calpain gene expression. J. Sci. Food Agric. 2011, 92, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Macoritto, M.; Kremer, R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARγ2). Exp. Gerontol. 2004, 39, 333–338. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Sergeev, I.N. Vitamin D Status and Vitamin D-Dependent Apoptosis in Obesity. Nutrients 2020, 12, 1392. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Sun, X. Calcitriol and energy metabolism. Nutr. Rev. 2008, 66, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. Role of uncoupling protein 2 (UCP2) expression and 1α, 25-dihydroxyvitamin D 3 in modulating adipocyte apoptosis. FASEB J. 2004, 18, 1430–1432. [Google Scholar] [CrossRef]
- Sergeev, I.N. 1,25-Dihydroxyvitamin D3 induces Ca2+-mediated apoptosis in adipocytes via activation of calpain and caspase-12. Biochem. Biophys. Res. Commun. 2009, 384, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. 1,25-Dihydroxyvitamin D3 and type 2 diabetes: Ca2+-dependent molecular mechanisms and the role of vitamin D status. Horm. Mol. Biol. Clin. Investig. 2016, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I. Calcium as a mediator of 1,25-dihydroxyvitamin D3-induced apoptosis. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 419–425. [Google Scholar] [CrossRef]
- Shi, H.; Norman, A.W.; Okamura, W.H.; Sen, A.; Zemel, M.B. 1α,25-Dihydroxyvitamin D3modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Kang, E.-J.; Lee, J.-E.; An, S.-M.; Kwon, H.S.; Kim, B.C.; Kim, S.J.; Kim, J.M.; Hwang, D.Y.; Jung, Y.-J.; Yang, S.Y.; et al. The effects of vitamin D3 on lipogenesis in the liver and adipose tissue of pregnant rats. Int. J. Mol. Med. 2015, 36, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Greenberg, A.G.; Kraemer, F.B.; Zemel, M.B. Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J. 2001, 15, 2527–2529. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrients 2016, 32, 702–708. [Google Scholar] [CrossRef]
- 1Larrick, B.M.; Kim, K.-H.; Donkin, S.S.; Teegarden, D. 1,25-Dihydroxyvitamin D regulates lipid metabolism and glucose utilization in differentiated 3T3-L1 adipocytes. Nutr. Res. 2018, 58, 72–83. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Wang, L.-M.; Lonnroth, P.; Wesslau, C.; Pierce, J.H.; Smith, U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1997, 94, 4171–4175. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Kahn, B.B. Glucose Transporters and Insulin Action — Implications for Insulin Resistance and Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Nadeau, M.; Valle, M.; Bellmann, K.; Marette, A.; Tchernof, A.; Gagnon, C. Vitamin D reduces LPS-induced cytokine release in omental adipose tissue of women but not men. Steroids 2015, 104, 65–71. [Google Scholar] [CrossRef]
- Walker, G.E.; Ricotti, R.; Roccio, M.; Moia, S.; Bellone, S.; Prodam, F.; Bona, G. Pediatric Obesity and Vitamin D Deficiency: A Proteomic Approach Identifies Multimeric Adiponectin as a Key Link between These Conditions. PLoS ONE 2014, 9, e83685. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More Than Just Another Fat Cell Hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. cDNA Cloning and Expression of a Novel Adipose Specific Collagen-like Factor, apM1 (AdiposeMost Abundant Gene Transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Adiyaman, S.C.; Ozer, M.; Saydam, B.O.; Akinci, B. The Role of Adiponectin in Maintaining Metabolic Homeostasis. Curr. Diabetes Rev. 2020, 16, 95–103. [Google Scholar] [CrossRef]
- Neyestani, T.R.; Nikooyeh, B.; Majd, H.A.; Shariatzadeh, N.; Kalayi, A.; Tayebinejad, N.; Heravifard, S.; Salekzamani, S.; Zahedirad, M. Improvement of Vitamin D Status via Daily Intake of Fortified Yogurt Drink Either with or without Extra Calcium Ameliorates Systemic Inflammatory Biomarkers, including Adipokines, in the Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 2005–2011. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Gouranton, E.; Romier, B.; Tourniaire, F.; Astier, J.; Malezet, C.; Amiot, M.-J.; Landrier, J.-F. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 2012, 56, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Cunha, C.A.; Ribeiro, E.B.; do Nascimento, C.O.; Oyama, L.M.; Mota, J.F. Supplementing Alpha-Tocopherol (Vitamin E) and Vitamin D3 in High Fat Diet Decrease IL-6 Production in Murine Epididymal Adipose Tissue and 3T3-L1 Adipocytes Following LPS Stimulation. Lipids Health Dis. 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. Calcium and 1,25-Dihydroxyvitamin D3 Regulation of Adipokine Expression*. Obesity 2007, 15, 340–348. [Google Scholar] [CrossRef]
- Dinca, M.; Serban, M.-C.; Sahebkar, A.; Mikhailidis, D.P.; Toth, P.P.; Martin, S.S.; Blaha, M.J.; Blüher, M.; Gurban, C.; Penson, P.; et al. Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 107, 360–371. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Åström, G.; Sjölin, E.; Wåhlén, K.; Carlberg, C.; Laurencikiene, J.; et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2011, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Aguado, M.; Gómez-Ambrosi, J.; Martínez, J. Lipolytic Effect ofin VivoLeptin Administration on Adipocytes of Lean andob/obMice, but Notdb/dbMice. Biochem. Biophys. Res. Commun. 1998, 250, 99–102. [Google Scholar] [CrossRef]
- Frühbeck, G.; Aguado, M.; Martınez, J.A. In VitroLipolytic Effect of Leptin on Mouse Adipocytes: Evidence for a Possible Autocrine/Paracrine Role of Leptin. Biochem. Biophys. Res. Commun. 1997, 240, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Koszowska, A.U.; Nowak, J.; Dittfeld, A.; Brończyk-Puzoń, A.; Kulpok, A.; Zubelewicz-Szkodzińska, B. Obesity, adipose tissue function and the role of vitamin D. Central Eur. J. Immunol. 2014, 2, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Wasiluk, D.; Stefańska, E.; Ostrowska, L.; Serwin, A.B.; Klepacki, A.; Chodynicka, B. Nutritive value of daily food rations of patients with psoriasis vulgaris: A preliminary report. Adv. Dermatol. Allergol. 2012, 5, 348–355. [Google Scholar] [CrossRef]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.-C.; Haussler, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef]
- Tsuji, K.; Maeda, T.; Kawane, T.; Matsunuma, A.; Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient ob/ob Mice. J. Bone Miner. Res. 2010, 25, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Lieben, L.; Watanabe, M.; Perino, A.; Auwerx, J.; Schoonjans, K.; Verstuyf, A. Vitamin D and energy homeostasis—of mice and men. Nat. Rev. Endocrinol. 2013, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Wilson, K.; Plebanski, M.; De Courten, M.P.J.; Scragg, R.; De Courten, B. Vitamin D supplementation increases adipokine concentrations in overweight or obese adults. Eur. J. Nutr. 2019, 59, 195–204. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Shab-Bidar, S.; Neyestani, T.R. Vitamin D and serum leptin: A systematic review and meta-analysis of observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 71, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 34–38. [Google Scholar] [CrossRef]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.-A.; Gabrielsson, B.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef]
- Goossens, G.H. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 2008, 94, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Vlasova, M.; Purhonen, A.K.; Jarvelin, M.R.; Rodilla, E.; Pascual, J.; Herzig, K.H. Role of adipokines in obesity-associated hypertension. Acta Physiol. 2010, 200, 107–127. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Takatsu, K. Activation and Regulation of the Pattern Recognition Receptors in Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance. Nutrients 2013, 5, 3757–3778. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.; Lim, F.-L.; Mazzatti, D.J.; Trayhurn, P. Microarray analysis identifies matrix metalloproteinases (MMPs) as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium. Pflüger’s Archiv für die Gesammte Physiologie des Menschen und der Tiere 2009, 458, 1103–1114. [Google Scholar] [CrossRef]
- Keophiphath, M.; Achard, V.; Henegar, C.; Rouault, C.; Clément, K.; Lacasa, D. Macrophage-Secreted Factors Promote a Profibrotic Phenotype in Human Preadipocytes. Mol. Endocrinol. 2009, 23, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Trayhurn, P.; Bing, C. Macrophage-secreted factors inhibit ZAG expression and secretion by human adipocytes. Mol. Cell. Endocrinol. 2010, 325, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Bing, C. Macrophage-induced expression and release of matrix metalloproteinase 1 and 3 by human preadipocytes is mediated by IL-1β via activation of MAPK signaling. J. Cell. Physiol. 2011, 226, 2869–2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M. Calcitriol and calcium regulate cytokine production and adipocyte–macrophage cross-talk. J. Nutr. Biochem. 2008, 19, 392–399. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, J.; Sun, T.; Li, G.; Szeto, F.L.; Liu, W.; Deb, D.K.; Wang, Y.; Zhao, Q.; Thadhani, R.; et al. 1,25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch. Biochem. Biophys. 2011, 507, 241–247. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Karkeni, E.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Darmon, P.; Landrier, J.-F. Vitamin D Limits Chemokine Expression in Adipocytes and Macrophage Migration In Vitro and in Male Mice. Endocrinol. 2015, 156, 1782–1793. [Google Scholar] [CrossRef]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Park, C.Y.; Kim, T.Y.; Yoo, J.S.; Seo, Y.; Pae, M.; Han, S.N. Effects of 1,25-Dihydroxyvitamin D3 on the Inflammatory Responses of Stromal Vascular Cells and Adipocytes from Lean and Obese Mice. Nutrients 2020, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Stio, M.; Martinesi, M.; Bruni, S.; Treves, C.; Mathieu, C.; Verstuyf, A.; D’Albasio, G.; Bagnoli, S.; Bonanomi, A.G. The Vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 51–60. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Trayhurn, P.; Bing, C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int. J. Obes. 2013, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Karhu, T.; Lehtonen, S.; Lehenkari, P.; Carlberg, C.; Saarnio, J.; Sebert, S.; Hyppönen, E.; Järvelin, M.; Herzig, K. Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin D 3 via the NF-κB pathway. FASEB J. 2012, 26, 4400–4407. [Google Scholar] [CrossRef]
- Ding, C.; Wilding, J.; Bing, C. 1,25-dihydroxyvitamin D3 Protects against Macrophage-Induced Activation of NFκB and MAPK Signalling and Chemokine Release in Human Adipocytes. PLoS ONE 2013, 8, e61707. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.; Rejnmark, L.; Heickendorff, L.; Møller, H.J. 25(OH)D3 and 1.25(OH)2D3 inhibits TNF-α expression in human monocyte derived macrophages. PLoS ONE 2019, 14, e0215383. [Google Scholar] [CrossRef]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. Diabetes Res. Clin. Pr. 2007, 77, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.N.; Norde, M.M.; Oki, É.; Rogero, M.M.; Marchioni, D.; Fisberg, R.; Martini, L.A. Association between 25-hydroxyvitamin D and inflammatory biomarker levels in a cross-sectional population-based study, São Paulo, Brazil. Nutr. Res. 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Wamberg, L.; Cullberg, K.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Investigations of the Anti-inflammatory Effects of Vitamin D in Adipose Tissue: Results from an In Vitro Study and a Randomized Controlled Trial. Horm. Metab. Res. 2013, 45, 456–462. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, L.; Xiao, Y.; Huang, G.; Zhang, M. Effect of Vitamin D Supplementation on Some Inflammatory Biomarkers in Type 2 Diabetes Mellitus Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2018, 73, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Wozniewicz, M.; Walkowiak, J.; Bogdański, P.; Jeszka, J.; Stelmach-Mardas, M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Zeitschrift für Ernährungswissenschaft 2016, 55, 2163–2176. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Bapat, S.P.; Suh, J.M.; Fang, S.; Liu, S.; Zhang, Y.; Cheng, A.; Zhou, C.; Liang, Y.; Leblanc, M.; Liddle, C.; et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 2015, 528, 137–141. [Google Scholar] [CrossRef]
- Lynch, L. Adipose invariant natural killer T cells. Immunology 2014, 142, 337–346. [Google Scholar] [CrossRef]
- Morin, S.O.; Poggi, M.; Alessi, M.-C.; Landrier, J.-F.; Nunès, J.A. Modulation of T Cell Activation in Obesity. Antioxid. Redox Signal. 2017, 26, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef]
- Mocanu, V.; Oboroceanu, T.; Zugun-Eloae, F. Current status in vitamin D and regulatory T cells--immunological implications. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013, 117, 965–973. [Google Scholar] [PubMed]
- Chun, R.F.; Lauridsen, A.L.; Suon, L.; Zella, L.A.; Pike, J.W.; Modlin, R.L.; Martineau, A.R.; Wilkinson, R.; Adams, J.; Hewison, M. Vitamin D-Binding Protein Directs Monocyte Responses to 25-Hydroxy- and 1,25-Dihydroxyvitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 3368–3376. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Ajibade, D.; Benn, B.S.; Feng, J.; Joshi, S.S. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J. Steroid Biochem. Mol. Biol. 2010, 121, 183–187. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef]
- Nankam, P.N.; Nguelefack, T.; Goedecke, J.; Blüher, M. Contribution of Adipose Tissue Oxidative Stress to Obesity-Associated Diabetes Risk and Ethnic Differences: Focus on Women of African Ancestry. Antioxidants 2021, 10, 622. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Vitamin C: A Review on its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Palacios, R.; Cat, A.N.D. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159. [Google Scholar] [CrossRef]
- Ionica, M.; Aburel, O.M.; Vaduva, A.; Petrus, A.; Rațiu, S.; Olariu, S.; Sturza, A.; Muntean, D.M. Vitamin D alleviates oxidative stress in adipose tissue and mesenteric vessels from obese patients with subclinical inflammation. Can. J. Physiol. Pharmacol. 2020, 98, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. 1α,25-Dihydroxyvitamin D3 Modulation of Adipocyte Reactive Oxygen Species Production. Obesity 2007, 15, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-L.; Zhu, C.; Zhao, Y.-P.; Chen, X.-H.; Ji, C.-B.; Zhang, C.-M.; Zhu, J.-G.; Xia, Z.-K.; Tong, M.-L.; Guo, X.-R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010, 320, 25–33. [Google Scholar] [CrossRef]
- Samouda, H.; De Beaufort, C.; Gilson, G.; Schritz, A.; Vaillant, M.; Ghaddhab, C.; Ruiz-Castell, M.; Huiart, L.; Dohet, F.; Weber, B.; et al. Relationship of oxidative stress to visceral adiposity in youth and role played by vitamin D. Pediatr. Diabetes 2020, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Cătoi, A.F.; Iancu, M.; Pârvu, A.E.; Cecan, A.D.; Bidian, C.; Chera, E.I.; Pop, I.D.; Macri, A.M. Relationship between 25 Hydroxyvitamin D, Overweight/Obesity Status, Pro-Inflammatory and Oxidative Stress Markers in Patients with Type 2 Diabetes: A Simplified Empirical Path Model. Nutrients 2021, 13, 2889. [Google Scholar] [CrossRef]
- Wenclewska, S.; Szymczak-Pajor, I.; Drzewoski, J.; Bunk, M.; Śliwińska, A. Vitamin D Supplementation Reduces Both Oxidative DNA Damage and Insulin Resistance in the Elderly with Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 2891. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.-F.; Ramanathan, S. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation With Obesity or Diabetes. EBioMedicine 2018, 30, 237–247. [Google Scholar] [CrossRef]
- Pricope-Veselin, A.E.; Mocanu, V.; Timofte, D. Open Surgical and Needle Biopsy to Study Abdominal Subcutaneous Adipose Tissue in Obesity. Jurnalul de Chir. 2018, 14, 101–105. [Google Scholar] [CrossRef]
- Ping-Delfos, W.C.S.; Soares, M. Diet induced thermogenesis, fat oxidation and food intake following sequential meals: Influence of calcium and vitamin D. Clin. Nutr. 2011, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Norman, A.W.; Okamura, W.H.; Sen, A.; Zemel, M. 1α,25-dihydroxyvitamin D3inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 2002, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Cheng, S.; Zhou, Y.; Ma, L. Effects of 1,25(OH) 2 D 3 on lipid droplet growth in adipocytes. BioFactors 2020, 46, 943–954. [Google Scholar] [CrossRef]
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Women may respond different from men to vitamin D supplementation regarding cardiometabolic biomarkers. Exp. Biol. Med. 2016, 241, 830–838. [Google Scholar] [CrossRef]
- Amiri, H.L.; Agah, S.; Azar, J.T.; Hosseini, S.; Shidfar, F.; Mousavi, S.N. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin. Nutr. 2017, 36, 1490–1497. [Google Scholar] [CrossRef]
- Foroughi, M.; Maghsoudi, Z.; Ghiasvand, R.; Iraj, B.; Askari, G. Effect of Vitamin D Supplementation on C-reactive Protein in Patients with Nonalcoholic Fatty Liver. Int. J. Prev. Med. 2014, 5, 969–975. [Google Scholar] [PubMed]
- Entezari, M.H.; Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H. Effect of Vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: A clinical trial study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Kampmann, U.; Stødkilde-Jørgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, A.; Hosseinpanah, F.; Shidfar, F.; Vafa, M.; Razaghi, M.; Dehghani, S.; Hoshiarrad, A.; Gohari, M. A 12-week double-blind randomized clinical trial of vitamin D3supplementation on body fat mass in healthy overweight and obese women. Nutr. J. 2012, 11, 78. [Google Scholar] [CrossRef]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Sneve, M.; Figenschau, Y.; Jorde, R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur. J. Endocrinol. 2008, 159, 675–684. [Google Scholar] [CrossRef]
- Major, G.C.; Alarie, F.; Doré, J.; Phouttama, S.; Tremblay, A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am. J. Clin. Nutr. 2007, 85, 54–59. [Google Scholar]
- Farag, H.A.M.; Hosseinzadeh-Attar, M.J.; Muhammad, B.A.; Esmaillzadeh, A.; el Bilbeisi, A.H. Effects of vitamin D supplementation along with endurance physical activity on lipid profile in metabolic syndrome patients: A randomized controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Makariou, S.E.; Elisaf, M.; Challa, A.; Tentolouris, N.; Liberopoulos, E.N. No effect of vitamin D supplementation on cardiovascular risk factors in subjects with metabolic syndrome: A pilot randomised study. Arch. Med Sci.-Atheroscler. Dis. 2017, 2, e52–e60. [Google Scholar] [CrossRef] [PubMed]
- Makariou, S.E.; Elisaf, M.; Challa, A.; Tellis, C.C.; Tselepis, A.D.; Liberopoulos, E.N. No effect of vitamin D administration plus dietary intervention on emerging cardiovascular risk factors in patients with metabolic syndrome. J. Nutr. Intermed. Metab. 2019, 16, 100093. [Google Scholar] [CrossRef]
- Salekzamani, S.; Mehralizadeh, H.; Ghezel, A.; Jafarabadi, M.A.; Bavil, A.S.; Gargari, B.P. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: A randomized controlled double-blind clinical trial. J. Endocrinol. Investig. 2016, 39, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Wongwiwatthananukit, S.; Sansanayudh, N.; Phetkrajaysang, N.; Krittiyanunt, S. Effects of vitamin D2 supplementation on insulin sensitivity and metabolic parameters in metabolic syndrome patients. J. Endocrinol. Investig. 2013, 36, 558–563. [Google Scholar] [CrossRef]
- Yin, X.; Yan, L.; Lu, Y.; Jiang, Q.; Pu, Y.; Sun, Q. Correction of hypovitaminosis D does not improve the metabolic syndrome risk profile in a Chinese population: A randomized controlled trial for 1 year. Asia Pac. J. Clin. Nutr. 2016, 25, 71–77. [Google Scholar]
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 542–547. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Chardigny, J.-M.; Boirie, Y.; Yammine, K.; Helou, M.; Walrand, S. Effect of Vitamin D Treatment on Glucose Homeostasis and Metabolism in Lebanese Older Adults: A Randomized Controlled Trial. J. Nutr. Health Aging 2018, 22, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Azadbakht, L.; Faghihimani, E.; Tabesh, M.; Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: A randomised controlled clinical trial. Diabetologia 2014, 57, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Upreti, V.; Maitri, V.; Dhull, P.; Handa, A.; Prakash, M.; Behl, A. Effect of oral vitamin D supplementation on glycemic control in patients with type 2 diabetes mellitus with coexisting hypovitaminosis D: A parellel group placebo controlled randomized controlled pilot study. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Shahar, D.; Geva, D.; Ish-Shalom, S. Differences in homeostatic model assessment (HOMA) values and insulin levels after vitamin D supplementation in healthy men: A double-blind randomized controlled trial. Diabetes Obes. Metab. 2016, 18, 633–637. [Google Scholar] [CrossRef]



| Vitamin D | Structure | Synonym | Sources |
|---|---|---|---|
| D2 |  | Ergocalciferol | Produced from ergosterol. Plants, fungi. |
| D3 |  | Cholecalciferol | Produced from 7-dehydrocholesterol. Fish, agriculture animals, dairy products, egg yolk, and skin of vertebrates. |
| D4 |  | 22-dihydroergocalciferol, 22,23-dihydroercalciol | Produced from 22,23-dihydroergosterol. Mushrooms. |
| D5 |  | Sitocalciferol | Converted from 7-dehydrositosterol found in Rauwolfia serpentina |
| Reference | Target Population | Study Design | Results on Studied Parameters |
|---|---|---|---|
| Sharifi et al. 2016 [238] | NAFLD
| Double-blind, randomized-controlled clinical trial | ↓TC, ↓LDL-C (in women) ↑TC, ↓↑LDL-C (in men) |
| Lorvand Amiri et al. 2017 [239] | NAFLD
| Randomized, placebo-controlled, double-blind clinical trial | ↓WT, ↓BMI, ↓fat mass, ↓FPG, ↓insulin, ↓HOMA-IR, ↓TG, ↑HDL-C, ↓ALT |
| Foroughi et al. 2014 [240] | NAFLD
| Randomized double-blind placebo-controlled clinical trial | ↓TG, ↓CRP, ↑Ca2+ |
| Khosravi et al. 2018 [241] | Overweight and obese women
| Double-blind placebo-controlled clinical trial | ↓WT, ↓WC, ↓BMI ↓↑TC, ↓↑TG, ↓↑LDL-C, ↓↑HDL-C, ↓↑FBS, ↓↑ insulin, ↓↑HOMA-IR, ↓↑WHR |
| Wamberg et al. 2013 [242] | Obese adults
| Randomized double-blind placebo-controlled clinical trial | ↓↑body fat, ↓↑SAT, ↓↑VAT, ↓↑IHL, ↓↑IMCL, ↓↑HOMA-IR, ↓↑blood pressure, ↓↑HDL-C, ↓↑TG, ↓↑TC, ↓↑hsCRP |
| Salehpour et al. 2012 [243] | Overweight and obese women
| Double-blind, randomized, placebo-ontrolled, parallel group trial | ↓ fat mass, ↓↑WT, ↓↑WC |
| Zittermann et al. 2009 [244] | Overweight subjects
| Double-blind placebo-controlled clinical trial | ↓PTH, ↓TG, ↓TNF-α, ↑LDL-C |
| Sneve et al. 2008 [245] | Overweight and obese subjects
| Randomized double-blind, placebo-controlled clinical trial | ↓↑WHR, ↓↑ fat, ↓↑Ca, ↓PTH |
| Major et al. 2007 [246] | Overweight or obese women
| Double-blind, clinical placebo-controlled trial | ↓LDL-C, ↓LDL:HDL ratio, ↓↑ HDL-C, ↓TG, ↓TC, ↓total HDL |
| Farag et al. 2019 [247] | Metabolic syndrome patients
| Parallel randomized placebo-controlled trial | ↓TC, ↓LDL-C (in vitamin D + physical activity group) ↓↑TG, ↓↑HDL-C (in all three groups) |
| Mikariou et al. 2017 [248] | Metabolic syndrome patients
| Prospective, randomized, open-label, blinded placebo-controlled end-point trial | ↓↑TG, ↓↑HDL-C, ↓↑LDL-C, ↓↑FTG, ↓↑ HbA1c, ↓↑HOMA-IR, ↓↑DBP, ↓SBP |
| Mikariou et al. 2019 [249] | Metabolic syndrome patients
| Prospective, randomized, open-label, blinded placebo-controlled end-point trial | ↓↑sdLDL-C, ↓↑LDL size, ↓↑LpPLA2 activity, ↓↑leptin, ↓↑ adiponectin, ↓↑leptin:adiponectin ratio |
| Salekzamani et al. 2016 [250] | Metabolic syndrome patients
| Randomized placebo-controlled, double-blind parallel trial | ↓TG, ↓↑FBG, ↓↑HOMA-IR, ↓↑LDL-C, ↓↑HDL-C, ↓↑TC, ↓↑WC, ↓↑BMI, ↓↑HC, ↓↑DBP, ↓↑SBP, ↓↑FP |
| Wongwiwatthana- nukit et al. 2013 [251] | Metabolic syndrome patients
| Prospective, randomized, double-blind, Double-dummy, placebo-controlled parallel trial | ↓↑FPG, ↓↑FPI, ↓↑HOMA-IR, ↓↑TC, ↓↑TG, ↓↑HDL-C, ↓↑LDL-C |
| Yin et al. 2016 [252] | Metabolic syndrome patients
| Randomized placebo-controlled intervention trial | ↓↑BMI, ↓↑WC, ↓↑FPG, ↓↑FPI, ↓↑HOMA-IR, ↓↑TG, ↓↑HDL-C, ↓↑LDL-C, ↓↑SBP, ↓↑DBP |
| Barzegari et al. 2019 [253] | Diabetic nephropathy patients
| Paralleled, randomized, double-blinded, placebo-controlled clinical trial | ↓TG, ↓LDL, ↓TC, ↓↑HDL ↑↓ oxidative/antioxidative markers |
| El Hajj et al. 2018 [254] | Elderly subjects (nondiabetic with vitamin D deficiency)
| Randomized placebo-controlled trial | ↓HOMA-IR, ↓FBG, ↓TC, ↓LDL-C, ↓BMI, ↓↑HDL-C |
| Tabesh et al. 2014 [255] | Nonsmoker individuals with T2DM and vitamin D insufficiency
| Randomized placebo-controlled clinical trial | ↓serum insulin, ↓HbA1c, ↓HOMA-IR, ↓LDL-C, ↓TC/HDL-C, ↑HDL-C |
| Wenclewska et al. 2019 [232] | Elderly subjects with metabolic disorders
| Randomized placebo-controlled clinical trial | ↑HDL-C, ↓HOMA-IR, ↓TG:HDL-C ratio (in vitamin D-supplemented group) ↓HbA1c (in T2DM supplemented with vitamin D group) |
| Upreti et al. 2018 [256] | T2DM patients with hypovitaminosis D
| Randomized, parallel group, placebo-controlled trial | ↓FPG, ↓PPPG, ↓HbA1c, ↓SBP, ↓DBP, ↓TC, ↓LDL-C, ↓↑TG, ↑↓HDL-C |
| Tepper et al. 2016 [257] | Healthy men without diabetes with vitamin D deficiency/insufficiency
| Double-blind randomized-controlled trial | ↓↑BMI, ↓↑glucose, ↓↑insulin, ↓↑hsCRP, ↓↑HOMA-IR, ↓↑HOMA-β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int. J. Mol. Sci. 2022, 23, 956. https://doi.org/10.3390/ijms23020956
Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Śliwińska A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? International Journal of Molecular Sciences. 2022; 23(2):956. https://doi.org/10.3390/ijms23020956
Chicago/Turabian StyleSzymczak-Pajor, Izabela, Krystian Miazek, Anna Selmi, Aneta Balcerczyk, and Agnieszka Śliwińska. 2022. "The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders?" International Journal of Molecular Sciences 23, no. 2: 956. https://doi.org/10.3390/ijms23020956
APA StyleSzymczak-Pajor, I., Miazek, K., Selmi, A., Balcerczyk, A., & Śliwińska, A. (2022). The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? International Journal of Molecular Sciences, 23(2), 956. https://doi.org/10.3390/ijms23020956






