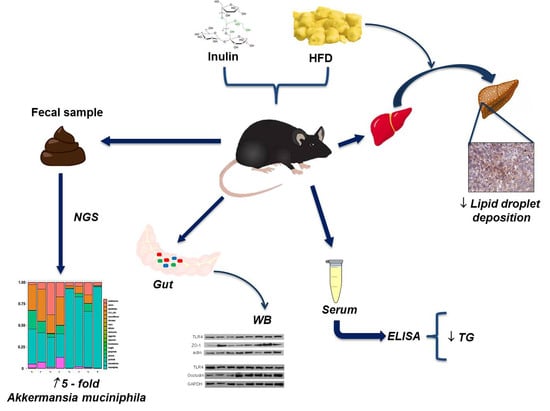
Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level
Abstract
:1. Introduction
2. Results
2.1. Body Weight Gain and Food Intake
2.2. Biochemical Parameters
2.3. Inulin Supplementation Improves Liver Steatosis under High Fat Diet Regime
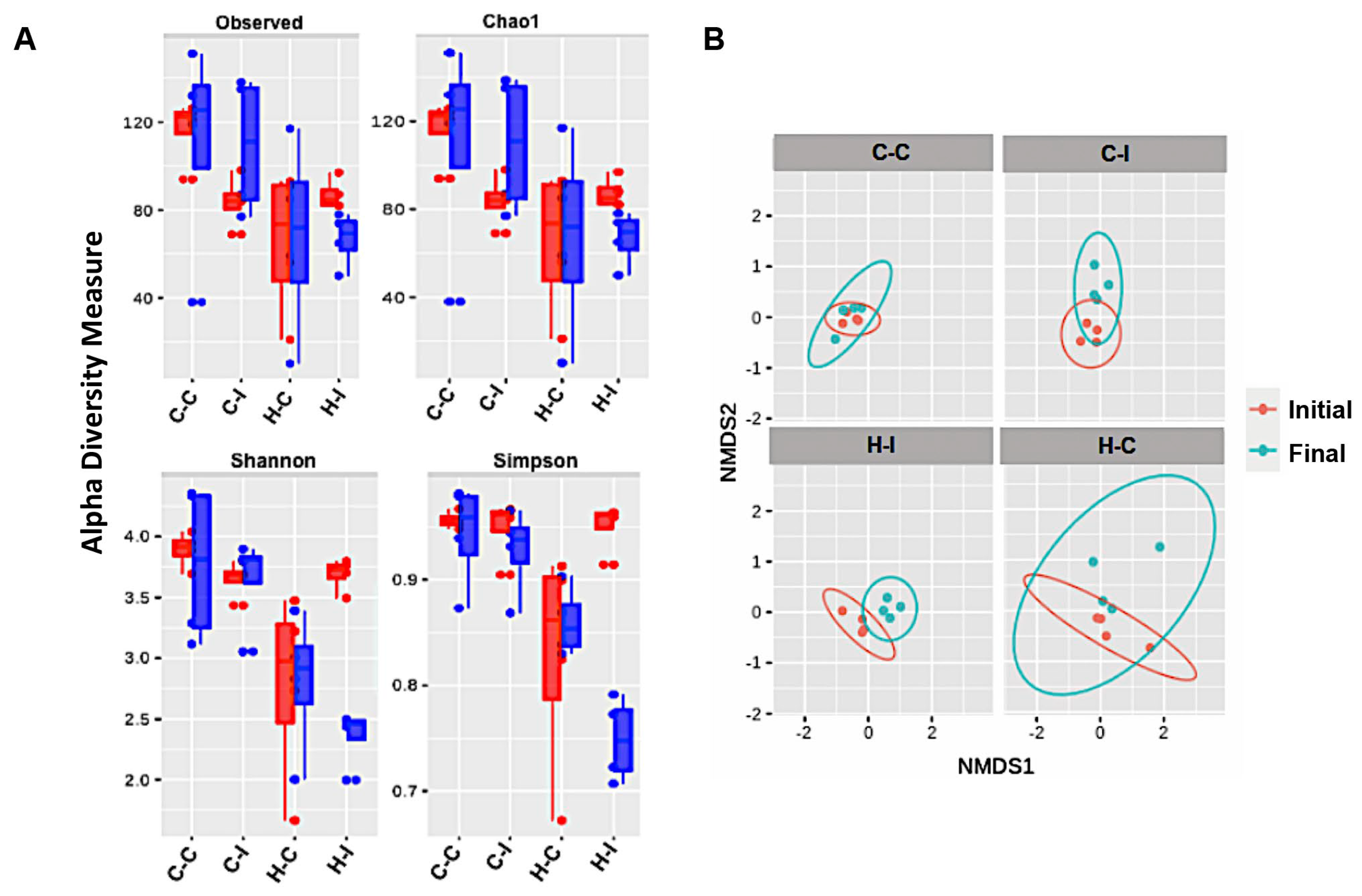
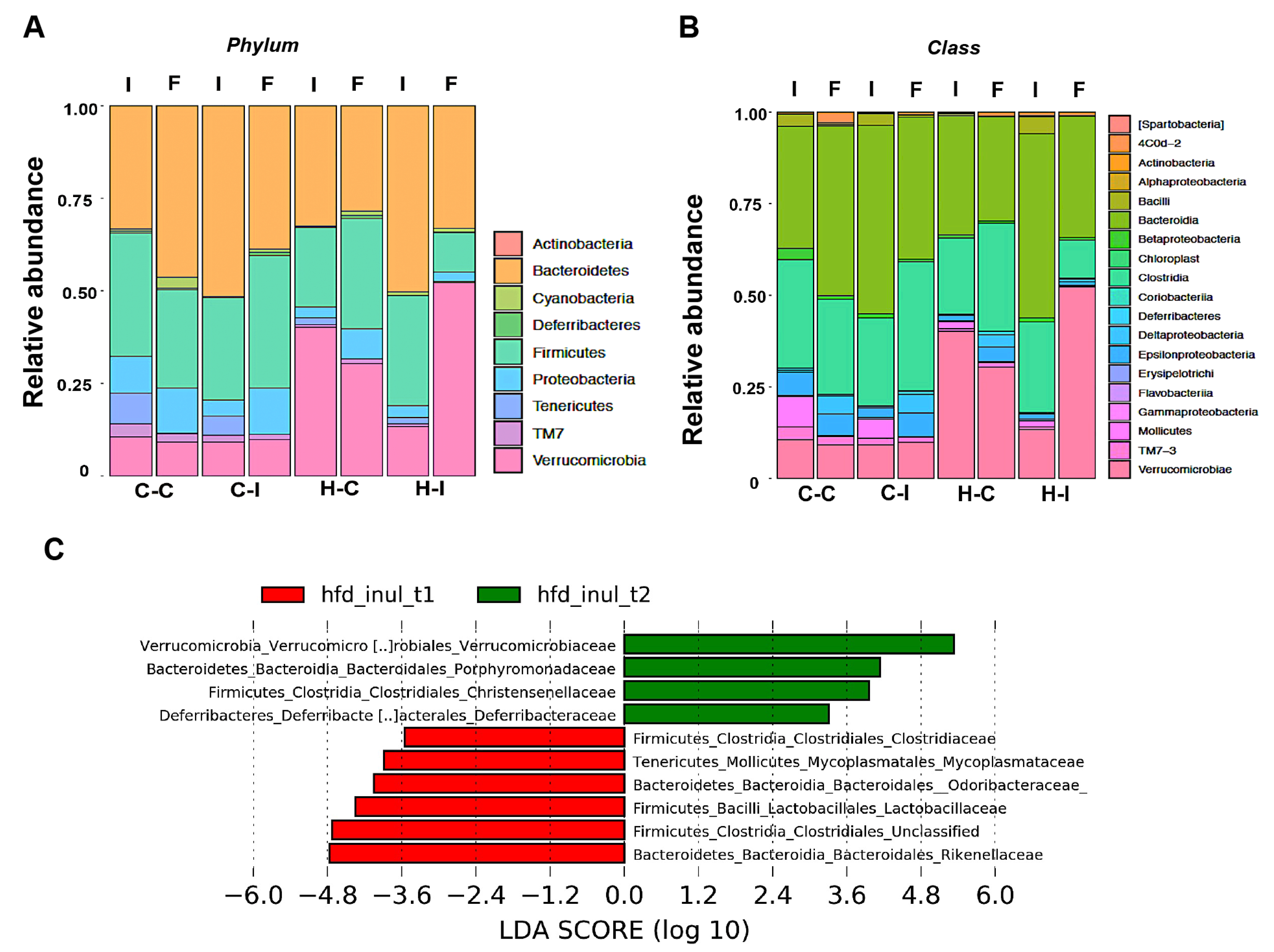
2.4. Inulin Supplementation Re-Shapes the Gut Microbiota in High-Fat Diet Treated Mice
2.5. Effect of Inulin Supplementation on Gut and Liver Tight Junction Proteins
2.6. Metagenome Inference
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Biochemical Parameters
4.3. Histology
4.4. Quantitation of Liver Fat and Triglycerides
4.5. Faecal Genomic DNA Extraction
4.6. 16S rRNA Gene Sequencing and Data Analysis
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadde, K.M.; Martin, C.K.; Berthoud, H.-R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Koopman, N.; Molinaro, A.; Nieuwdorp, M.; Holleboom, A.G. Review Article: Can Bugs Be Drugs? The Potential of Probiotics and Prebiotics as Treatment for Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2019, 50, 628–639. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 3379. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and Oligofructose as Dietary Fiber: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.A.; Horsmans, Y.; Lambert, P.; Danse, E.; Delzenne, N.M. Effects of Oligofructose on Glucose and Lipid Metabolism in Patients with Nonalcoholic Steatohepatitis: Results of a Pilot Study. Eur. J. Clin. Nutr. 2005, 59, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Xiao, G.; Li, Y.; Xie, H.; Chen, Y. Fructooligosaccharides Enhance the Therapeutic Effect of Xiao-Zhi-Fang on Non-Alcoholic Fatty Liver Disease via Regulating Intestinal Flora. Liver Res. 2021, 5, 217–223. [Google Scholar] [CrossRef]
- Takai, A.; Kikuchi, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Matsumoto, K.; Tsunashima, H.; Onda, T.; Kuniyoshi, N.; Nariyama, T.; et al. Fructo-Oligosaccharides Ameliorate Steatohepatitis, Visceral Adiposity, and Associated Chronic Inflammation via Increased Production of Short-Chain Fatty Acids in a Mouse Model of Non-Alcoholic Steatohepatitis. BMC Gastroenterol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Rugyendo, A.; Morrison, D.J.; Preston, T.; Tedford, C.; Bell, J.D.; Thomas, L.; Akbar, A.N.; Riddell, N.E.; et al. The Effects of Dietary Supplementation with Inulin and Inulin-Propionate Ester on Hepatic Steatosis in Adults with Non-Alcoholic Fatty Liver Disease. Diabetes Obes. Metab. 2019, 21, 372–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugatani, J.; Osabe, M.; Wada, T.; Yamakawa, K.; Yamazaki, Y.; Takahashi, T.; Ikari, A.; Miwa, M. Comparison of Enzymatically Synthesized Inulin, Resistant Maltodextrin and Clofibrate Effects on Biomarkers of Metabolic Disease in Rats Fed a High-Fat and High-Sucrose (Cafeteria) Diet. Eur. J. Nutr. 2008, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Huang, H.-L.; Li, Y.-Q.; Xu, H.-M.; Zhou, Y.-J. Therapeutic Advances in Non-Alcoholic Fatty Liver Disease: A Microbiota-Centered View. World J. Gastroenterol. 2020, 26, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic Fibres Dose-Dependently Increase Satiety Hormones and Alter Bacteroidetes and Firmicutes in Lean and Obese JCR:LA-Cp Rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Chassard, C.; Delmas, E.; Robert, C.; Bernalier-Donadille, A. The Cellulose-Degrading Microbial Community of the Human Gut Varies According to the Presence or Absence of Methanogens. FEMS Microbiol. Ecol. 2010, 74, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chan, Z.; Magkos, F. Lean, but Not Healthy: The ‘Metabolically Obese, Normal-Weight’ Phenotype. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 408–417. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with Different Degrees of Polymerization Modulates Composition of Intestinal Microbiota in Mice. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Biruete, A.; Cross, T.-W.L.; Allen, J.M.; Kistler, B.M.; de Loor, H.; Evenepoel, P.; Fahey, G.C.; Bauer, L.; Swanson, K.S.; Wilund, K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522. [Google Scholar] [CrossRef]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating Gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mψ-Nuclear Factor-ΚB-Nod-Like Receptor Protein 3 Pathway via Gut-Liver Axis in Mice. Front. Pharmacol. 2020, 11, 1787. [Google Scholar] [CrossRef]
- Xia, B.; Wu, W.; Zhang, L.; Wen, X.; Xie, J.; Zhang, H. Gut Microbiota Mediates the Effects of Inulin on Enhancing Sulfomucin Production and Mucosal Barrier Function in a Pig Model. Food Funct. 2021, 12, 10967–10982. [Google Scholar] [CrossRef]
- Corazziari, E.S. Intestinal Mucus Barrier in Normal and Inflamed Colon. J. Pediat. Gastroenterol. Nutr. 2009, 48, S54. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia Muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe−/− Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [Green Version]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective Stimulation of Bifidobacteria in the Human Colon by Oligofructose and Inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Vahedi, K.; Achour, L.; Attar, A.; Salfati, J.; Pochart, P.; Marteau, P.; Flourié, B.; Bornet, F.; Rambaud, J.-C. Short-Chain Fructo-Oligosaccharide Administration Dose-Dependently Increases Fecal Bifidobacteria in Healthy Humans. J. Nutr. 1999, 129, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.L.; Ley, R.E. The Human Gut Bacteria Christensenellaceae Are Widespread, Heritable, and Associated with Health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, e6978936. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid–Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-Induced Increase in Intestinal Epithelial Tight Junction Permeability Requires NF-ΚB Activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, D.; Phan, V.; Wang, A.; McKay, D.M. Interferon-γ-Induced Increases in Intestinal Epithelial Macromolecular Permeability Requires the Src Kinase Fyn. Lab. Invest. 2011, 91, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, e2645465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; An, B.S.; Yang, H.; Kang, H.S.; Jung, E.M.; Jeung, E.B. Tissue-Specific Expression of Occludin, Zona Occludens-1, and Junction Adhesion Molecule A in the Duodenum, Ileum, Colon, Kidney, Liver, Lung, Brain, and Skeletal Muscle of C57BL Mice. J. Physiol. Pharmacol. 2013, 64, 11–18. [Google Scholar] [PubMed]
- Xin, D.; Zong-Shun, L.; Bang-Mao, W.; Lu, Z. Expression of Intestinal Tight Junction Proteins in Patients with Non-Alcoholic Fatty Liver Disease. Hepatogastroenterology 2014, 61, 136–140. [Google Scholar]
- Verbrugghe, A.; Hesta, M.; Gommeren, K.; Daminet, S.; Wuyts, B.; Buyse, J.; Janssens, G.P. Oligofructose and Inulin Modulate Glucose and Amino Acid Metabolism through Propionate Production in Normal-Weight and Obese Cats. Br. J. Nutr. 2009, 102, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia Muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia Muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef] [Green Version]
- Hagi, T.; Belzer, C. The Interaction of Akkermansia Muciniphila with Host-Derived Substances, Bacteria and Diets. Appl. Microbiol. Biotechnol. 2021, 105, 4833–4841. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Holle, A.V.; François, P.; de Vos, W.M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets That Differ in Their FODMAP Content Alter the Colonic Luminal Microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota with Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Vonk, R.J.; Slooff, M.J.H.; Kuipers, F.; Smit, M.J. Differences in Propionate-Induced Inhibition of Cholesterol and Triacylglycerol Synthesis between Human and Rat Hepatocytes in Primary Culture. Br. J. Nutr. 1995, 74, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019, 63, 1800930. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, C.; Torres, N.; Gutierrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Diaz, A.M.; Antunes-Ricardo, M.; Marquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A.; et al. The Effect of Isorhamnetin Glycosides Extracted from Opuntia Ficus-Indica in a Mouse Model of Diet Induced Obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Mopuri, R.; Kalyesubula, M.; Rosov, A.; Edery, N.; Moallem, U.; Dvir, H. Improved Folch Method for Liver-Fat Quantification. Front. Vet. Sci. 2021, 7, 1185. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Perez-Monter, C.; Martinez-Armenta, M.; Miquelajauregui, A.; Furlan-Magaril, M.; Varela-Echavarria, A.; Recillas-Targa, F.; May, V.; Charli, J.L.; Perez-Martinez, L. The Kruppel-like Factor 4 Controls Biosynthesis of Thyrotropin-Releasing Hormone during Hypothalamus Development. Mol. Cell Endocrinol. 2011, 333, 127–133. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Monter, C.; Álvarez-Arce, A.; Nuño-Lambarri, N.; Escalona-Nández, I.; Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level. Int. J. Mol. Sci. 2022, 23, 991. https://doi.org/10.3390/ijms23020991
Pérez-Monter C, Álvarez-Arce A, Nuño-Lambarri N, Escalona-Nández I, Juárez-Hernández E, Chávez-Tapia NC, Uribe M, Barbero-Becerra VJ. Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level. International Journal of Molecular Sciences. 2022; 23(2):991. https://doi.org/10.3390/ijms23020991
Chicago/Turabian StylePérez-Monter, Carlos, Alejandro Álvarez-Arce, Natalia Nuño-Lambarri, Ivonne Escalona-Nández, Eva Juárez-Hernández, Norberto C. Chávez-Tapia, Misael Uribe, and Varenka J. Barbero-Becerra. 2022. "Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level" International Journal of Molecular Sciences 23, no. 2: 991. https://doi.org/10.3390/ijms23020991
APA StylePérez-Monter, C., Álvarez-Arce, A., Nuño-Lambarri, N., Escalona-Nández, I., Juárez-Hernández, E., Chávez-Tapia, N. C., Uribe, M., & Barbero-Becerra, V. J. (2022). Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level. International Journal of Molecular Sciences, 23(2), 991. https://doi.org/10.3390/ijms23020991