Metabolomics-Driven Mining of Metabolite Resources: Applications and Prospects for Improving Vegetable Crops
Abstract
1. Introduction
2. Vegetable Plants as Prominent Metabolite Resources
3. Defining Metabolites, Metabolome and Metabolomics
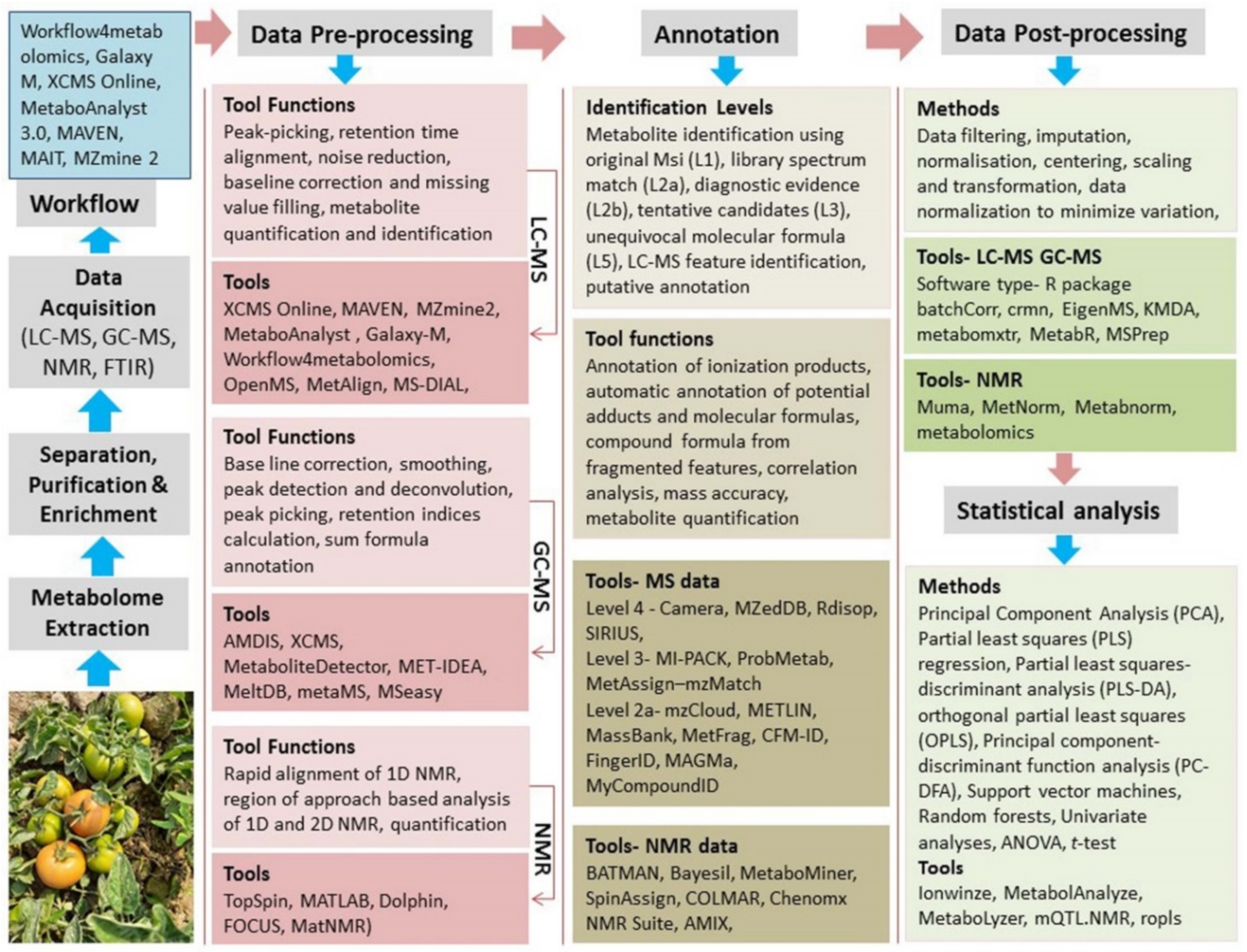
4. Application of Metabolomics in Vegetable Crops
4.1. Vegetable Crop Domestication Studies
4.2. Monitoring Development-Dependent Metabolic Changes
4.3. Nutritional Metabolomics of Vegetable Plants
4.4. Decephering Plant’s Adaptive Strategies under Abiotic Stresses
4.5. Assessing Metabolome under Plant-Microbe Interactions
4.6. Metabolomic Studies in Plant-Insect (Herbivore) Interactions
4.7. Understanding on Rhizosphere Metabolome
4.8. Metabolomic Studies on Transgenic Vegetable Plants
4.9. Metabolomic Changes in Vegetable Plants Due to Nanoparticles Impact
4.10. Metabolome Profiling and Pharmaco-Physiological Impact Assessment
| Sr. No. | Vegetable Plants/Parts | Metabolomics Platforms | Metabolome Studies/Metabolites Identified | Reference |
|---|---|---|---|---|
| Tomato | ||||
| 1. | Transgenic and non-transgenic fruit powder | 600 MHz 1H-NMR in D2O buffer solution | amino acids (9), gama-aminobutyric acid (GABA), organic acids, sugars, choline, polyamines spermine and spermidine | [168] |
| 2. | Ripened fruits of 50 cultivars including cherry and round type tomato | 1H-NMR, LC-(QTOF) MSIntra-(NMR–NMR, LCMS–LCMS) and inter-(NMR–LCMS) metabolomics-correlation analyses | phenylalanine, zeatin hexose, caffeic acid hexose, dehydrophaseic acid, caffoylquinic acid, esculeoside A, rutin, kaempferol-3-O-rutinose, dicaffeoylquinic acid, naringenin chalcone-hexose, lycoperoside A–C, tomatin, tomatoside, tricaffeoylquinic acid, naringenin, naringenin chalcone | [176] |
| 3. | Fruit volatile compounds | Headspace SPME-GC-MS | hexanol, phenylethanol, phenylacetaldehyde, hexanal, heptenel, β-Ionone, 6-Methyl-5-hepten-2-one, 2-Methylbutanal, 2-isobutylthiazole, 1-Penten-3-one, eugenol | [177] |
| 4. | Plant-bacterial wilt (Ralstonia solanacearum) interaction | 1H-NMR | amino acids, organic acids, and sugar groups, GABA, ethanolamine, glycine, choline, valine etc. | [178] |
| 5. | Midstem and xylem sap-Ralstonia solanacearum interaction | LC-MS, GC-MS | amino acids, organic acids, fatty acids, various derivatives of cinnamic acid and benzoic acids, flavonoids and steroidal glycoalkaloids, flavonoids and hydroxycinnamic acids, polyamines and tyramine. 22 compounds identified by GC-MS | [139,140] |
| 6. | Cutin | 3D HR-MAS NMR spectroscopy | lignin structures, plant derived fulvic acid, humic materials | [179] |
| 7. | Seeds treated with Trichoderma secondary metabolites 6-pentyl-2H-pyran-2-one and harzianic acid and corresponding plants leaves at 15 days | HRMAS NMR | acetylcholine, GABA | [180] |
| 8. | Leaves and roots | GC-MS, LC-MS | sugars, organic acids, oligosaccharides—α-ketoglutarate and raffinose | [181] |
| 9. | Cherry tomato (5 types)—fruits | GC-TOF-MS, UPLC-Linear Trap Quadrupole-Orbitrap-Tandem MS | amino acids, lipids, organic acids, phenylpropanoids, lycopene, β-carotene, and α-carotene | [110] |
| 10. | Mature fruits—25 cultivars | Untargeted MS/MS | 7118 accurate mass values | [182] |
| 11. | Fruits—Cryptococcus laurentii induced changes | UPLC-MS/MS analysis in Positive and Negative ion mode | 59 metabolites phenolics, flavonoids and phenylpropanoids including chlorogenic, caffeic and ferulic acids | [183] |
| 12. | Fruits | GC-MS | primary metabolites (organic acids, sugars, sugar alcohols, amino acids, phosphorylated intermediates, lipophilic compounds) | [87] |
| 13. | Fruits and leaves | 1H-NMR and LC-QTOF-MS metabolomics profiling | 70 metabolites in leaves, 56 in fruits, free amino acids, sugars, sucrose, rhamnose, erythritol, glycerate, alanine, β-alanine, glycine, tyramine, and trigonelline, hydroxycinnamate, flavonoid, or glycoalkaloid, α-tomatine, dehydrotomatine, and chlorogenate | [184] |
| 14. | Fruits Solanum pannellii | NMR, HPLC | carotenoids and tocopherols | [185] |
| 15. | Host pathogen interaction with leaf mold disease caused by Cladosporium fulvum | GC-TOF-MS, LC-PDA-QTOF-MS | polar primary metabolites (PPM)-132semi-polar secondary metabolites (SPSM)enhanced accumulation of benzoic acid, salicylic acid, tyramine, dopamine, coumaroyltyramine-isoform 1, and coumaroyldopamine | [186] |
| 16. | Predator Chrysoperla carnea larvae and herbivore interaction Tetranychus urticae and Myzus persicae | GC-MS, LC-ESI-MS | non-volatile metabolites—abscisic acid and amino acidsvolatile metabolites—monoterpenes (37%), sesquiterpenes (32%), aldehydes (5%), phenylpropanoids (11%) and fatty acid derivatives (5%) | [152] |
| 17. | Tomato-Trichoderma harzianum-Aphids | LC-ESI (+)-MS | primary and secondary semi-polar metabolitesDown-accumulation-citric acid, dihydro-caffeic acid, 2-hydroxyglutarate, phosphoenolpyruvate (PEP), coumarin, syringaldehyde, tetrahydrofolate, δ-tomatine (an alkaloid), N-isovalerylglycine and threonine/homoserine (amino acids) and protoporphyrinogen IX Over-represented metabolites—salicylate β-D-glucose ester and salicyloyl-L-aspartic acid, phenylalanine, coumaric and chorismic acids | [156] |
| 18. | Tomato—thrips-resistant (10 wild) and susceptible (10 cultivated) lines | 1H-NMR | acylsugars | [187] |
| 19. | Tomato rhizosphere | GC-MS, LC-MS | fatty acid derivatives, oxylipin isomers, azelaic and pimelic acids, glycosides, amino acids | [188] |
| 20. | Tomato leaves—Si-based biostimulant treatment | LC-MS | terpenes, polyamines, phenolic compounds, caffeic-, coumaric-, sinapoyl- and ferulic acids and conjugates, quercetin and kaempferol conjugates, hexadecadienoic acid (HDDA), hydroperoxyoctadecadienoic acid (HPDA) | [130] |
| 21. | Plant-PGPR mediated salt tolerance | GC-MS based metabolite profiling | high accumulation—xylose, rhamnose, D-mannose, L-galactose, allose, sedoheptulose, erythrose, and 2-deoxy-D-erythro-pentitol, lactate and oxalate, alanine, valine, isoleucine, glycine, serine, threonine, and cystathionine in inoculated plants | [189] |
| 22. | Fruits at different ripening stages | FT-ICR MS, NMR, HPLC | amino acids and derivatives, organic acids, sugar esters of caffeic and ferulic acids, caffeoyl- and feruloyl-hexose, monosaccharides fructose, glucose and galactose, choline and adenosine, tannins, flavonoids apigeniflavan, tetrahydroxyflavanone glucoside, polyphenol derivatives like catechin-O-glucoside, catechin-O-rutinoside, dihydrokaempferol, trihydroxy-prenyldihydrochalcone glucosyl-coumarate, quercetin glucoside-glucuronide | [190] |
| 23. | Fruits—chitosan treated plants | LC-ESI-MS/MS | benzenesulfonates, benzoates, desulfoglucosinolates, fatty acids, flavonoids, oligosaccharides, organosulfates, organosulfur compounds, phenylpropanoids, phospholipids and terpenoids | [191] |
| 24. | Tomato product—purees | HPLC-PDA and fluorescence detector, 1H-NMR, LC-QToF-MS, LC-multiple reaction monitoring, headspace GC-MS | vitamins, semi-polar and polar metabolites, oxylipins, volatile metabolites, phytonutrients | [192] |
| Capsicum | ||||
| 25. | Capsicum annuum L.—aqueous phase of Serrano peppers | 1H-NMR, 2D NMR and Chenomx database | vitamin C and 44 metabolites including sugars, organic acids, polyphenols, amino acids, alcohols | [193] |
| 26. | Capsicum annuum cv. serrano | 1H-NMR, 2D NMR and qNMR, PCA, PLS-DS | 48 metabolites including sugars (glucose, fructose, sucrose and galactose), organic acids (citric, formic, fumaric, malic), alcohols and polyphenolic acids | [194] |
| 27. | Capsicum annuum L. Serrano peppers from two geographically different regions | 1H-NMR | Veracruz region produce: rich in aspartate citrate, lactate, leucine and sucroseOaxaca region produce: rich in acetate, formate, fumarate, malonate, phosphocholine, pyruvate and succinate | [195] |
| Cucumber | ||||
| 28. | Cucumber-leaves and root exudates | 1H-NMR and GC-MS | ascorbic acid, citric acid, amino acids | [196] |
| 29. | Cucumber fruits-extract | 1H-NMR and GC-MS | nano-Cu influenced metabolite profile of sugars, amino acids, fatty acids, methylnicotinamide, trigonelline, imidazole, quinolate | [128] |
| 30. | Cucumber-fruit peel | UPLC-QTOF-MS | uniquely abundant 113 ions in resistant fruit peels | [197] |
| 31. | Cucumber grafted on different root stocks | GC-MS | volatile organic compounds (VOCs), Fructose-6-phosphate and trehalose), linoleic acid, and amino-acid (isoleucine, proline, and valine) | [198] |
| 32. | Cucumis sativus—cucurbit chlorotic yellows virus infection | UPLC, ESI-Q TRAP-MS/MS, MWDB and MRM | total identified metabolites 612. maximum accumulation—lipids, phenolic acids and flavonoidsmetabolites detected—organic acids, alkaloids, terpenoids, lignans, coumarins, and tannins. | [199] |
| 33. | Cucumber fruit-2,4-dichlorophenoxyacetic acid (2,4-D) exogenous treatment | Non-targeted metabolomics by UPLC-qTOF-MS | decreased levels of flavonoids and cinnamic acid derivatives but increased levels of amino acids | [86] |
| 34. | Luffa aegyptiaca Mill-young and mature fruits | UHPLC-MS, GC-MS | aldehydes, furans, alcohols, ketones, acids, aromatics | [95] |
| Lettuce | ||||
| 35. | Lettuce leaf | GC-MS, LC-MS, PCA | 101 metabolites from 223 peaks GC-MS, 95 peaks by LC-MS, tetracosanol and hexacosanol, sesquiterpene lactones (lactucopicrin-15-oxalate and 15-deoxylactucin-8-sulfate) | [200] |
| Eggplant | ||||
| 36. | Eggplant and Tomato—Tuta absoluta infection | UPLC-MS/MS, HS-SPME/GC-MS | identified 141 volatile organic compounds and 797 primary/secondary metabolites, dominant compounds-aldehyde, alcohols, alkanes, amine, aromatics, a heterocyclic compound, ketone, olefin, phenol, and terpenes | [154] |
| 37. | Eggplant—fruit accessions | LC-MS, GC-MS | 51 compounds (LC-MS), 207 compounds (GC-MS)Terpenes, alkaloids, fatty acids, flavonoids and terpenoids | [201] |
| 38. | Eggplant-fruits-browning mechanism | LC-MS | 946 metabolites changed dynamically, 119 common differential metabolites | [202] |
| 39. | Solanaceous crops fruit tissues (peel and pericarp) of pepper, eggplant, tomato, Capsicum annum | LC-ESI-MS | polyphenolics hydroxycinnamate and flavonoids quercetin-3-O-(2″-O-apiosyl-6″-O-rhamnosyl)glucoside-7-O-glucoside, kaempferol-3-O-(2″-O-apiosyl-6″-O-rhamnosyl)glucoside, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside; and K3R, kaempferol-3-O-rhamnoside | [203] |
| Momordica charantia | ||||
| 40. | Momordica charantia fruit (Cucurbitaceae) | LC-MS-QTOF | brevifolincarboxylic acid (new antioxidant molecule), 3-malonylmomordicin I and goyaglycoside G and other metabolites | [204] |
| Cabbage | ||||
| 41. | Chinese cabbage—root colonization by Piriformospora indica | High-throughput GC-MS | total compounds—1126, 549 identified in colonized and non-colonized cabbage roots and hyphae of P. indica | [205] |
| 42. | Cabbage (Brassica oleracea var. capitata)—taste attributes | GC-MS | primary metabolites, 4-aminobutyric acid, fructose 1-phosphate, adipic acid, 5-oxoproline, N-acetylglycine, O-phosphoethanolamine, and homovanillic acid | [206] |
| 43. | Brassica rapa subsp. chinensis | GC-TOF-MS and HPLC | 53 primary metabolites in 3 pak choi cultivars, 49 hydrophilic metabolites in others. | [207] |
| Spinach (Spinacia oleracea) | ||||
| 44. | Leaves | GC-MS | variations in carbohydrates, organic acids, amino acids content after treatment with NO−3 and Gly | [208] |
| Common beans (Phaseolus vulgaris L.) | ||||
| 45. | Contrasting genotypes of saline conditions | GC-MS | accumulation of lysine, valine and isoleucine in roots | [209] |
| 46. | Common bean Genotype (107 accessions)-heat stress | Non-targeted and targeted MS | measured high content of salicylic acid while low content of triterpene saponins, phenylpropanoids deciphered | [210] |
| 47. | Cold-tolerant (120) and sensitive (93) common bean cultivars | UPLC-MS | flavonoids, methionine and malondialdehyde as biomarkers against cold temperatures | [211] |
| Lotus (Nelumbo nucifera) | ||||
| 48. | Seeds | LC-MS | identified flavonoids (30) and alkaloids | [212] |
| 49. | Seeds | LC-MS | amino acids, organic acids, sugars | [213] |
| Moringa (Moringa oleifera) | ||||
| 50. | Leaves and stem | 1H-NMR | 30 metabolites identified, 22 common in leaf and stem tissues. 4-aminobutyrate, adenosine, guanosine, tyrosine, p-cresol | [214] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wurtzel, E.T.; Kutchan, T.M. Plant metabolism, the diverse chemistry set of the future. Science 2016, 353, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A. (Ed.) A brief review on bioactive compounds in plants. In Bioactive Compounds in Plants—Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; pp. 11–17. [Google Scholar]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–558. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, I.M. Elements of the cellular metabolic structure. Front. Mol. Biosci. 2015, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Kastenmüller, G.; Schenk, M.E.; Gasteiger, J.; Mewes, H.W. Uncovering metabolic pathways relevant to phenotypic traits of microbial genomes. Genome Biol. 2009, 10, R28. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Joosten, F.; Dijkxhoorn, Y.; Sertse, Y.; Ruben, R. How Does the Fruit and Vegetable Sector Contribute to Food and Nutrition Security? LEI Wageningen UR: The Hague, The Netherlands, 2015. [Google Scholar]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- HLPE-FAO. Impacts of COVID-19 on Food Security and Nutrition: Developing Effective Policy Responses to Address the Hunger and Malnutrition Pandemic; CFS: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Goldman, I.L. Forgotten and Future Vegetable Phytoceuticals. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 484–490. [Google Scholar]
- Solankey, S.S.; Saha, D.P.; Shyama, K.; Shirin, A.; Jha, V.B.; Sohane, R.K.; Singh, R.R. Biodiversity in Vegetable Crops for Healthier Life and Livelihood. In Proceedings-Cum-Abstract Book of International Web Conference, 27–28 August 2020; Bihar Agricultural University: Bhagalpur, India, 2020; ISBN 978-81-937815-0-0. [Google Scholar]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Ülger, T.G.; Songur, A.N.; Çırak, O.; Çakıroğlu, P.F. Role of Vegetables in Human Nutrition and Disease Prevention. In Vegetables—Importance of Quality Vegetables to Human Health; Asaduzzaman, M., Asao, T., Eds.; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant metabolites and nutritional quality of vegetables. J. Food Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef]
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Kale, V.; Sathe, A.; Sonah, H.; Jugdaohsingh, R.; et al. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food. Sci. Nutr. 2022, 62, 1003–1034. [Google Scholar] [CrossRef]
- Soto, C.; González, R.; Galmarini, C. Bioactive compounds in vegetables, Is there consistency in the published information? A systematic review. J. Hortic. Sci. Biotechnol. 2021, 96, 1–18. [Google Scholar] [CrossRef]
- Francini, A.; Pintado, M.; Manganaris, G.A.; Ferrante, A. Editorial: Bioactive compounds biosynthesis and metabolism in fruit and vegetables. Front. Plant. Sci. 2020, 11, 129. [Google Scholar] [CrossRef]
- Bowling, F.G.; Thomas, M. Analyzing the metabolome. Methods Mol. Biol. 2014, 1168, 31–45. [Google Scholar]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Perea-Domínguez, X.P.; Hernández-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato by-product: Free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of defense phenolics in tomato leaves of different age. Molecules 2020, 25, 4952. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in polyphenols contents and antioxidant capacities of organically and conventionally cultivated tomato (Solanum lycopersicum L.) fruits during ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Meyer, R.S.; Whitaker, B.D.; Little D., P.; Wu, S.-B.; Kennelly, E.J.; Long, C.-L.; Litt, A. Parallel reductions in phenolic constituents resulting from the domestication of eggplant. Phytochemistry 2015, 115, 194–206. [Google Scholar] [CrossRef]
- San José, R.; Sánchez-Mata, M.C.; Cámara, M.; Prohens, J. Eggplant fruit composition as affected by the cultivation environment and genetic constitution. J. Sci. Food Agric. 2014, 94, 2774–2784. [Google Scholar] [CrossRef]
- Ma, C.; Dastmalchi, K.; Whitaker, B.D.; Kennelly, E.J. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J. Agric. Food Chem. 2011, 59, 9645–9651. [Google Scholar] [CrossRef]
- Noda, Y.; Kneyuki, T.; Igarashi, K.; Mori, A.; Packer, L. Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology 2000, 148, 119–0123. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef]
- Azuma, K.; Ohyama, A.; Ippoushi, K.; Ichiyanagi, T.; Tekeuchi, A.; Saito, T.; Fukuoka, H. Structures and antioxidant activity of anthocyanins in many accessions of eggplant and its related species. J. Agric. Food Chem. 2008, 56, 10154–10159. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Veberic, R.; Hudina, M.; Jakopic, J.; Slatnar, A. Metabolic variation among fruits of different chili cultivars (Capsicum spp.) using HPLC/MS. Plants 2021, 11, 101. [Google Scholar] [CrossRef]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.L.; Patil, B.S. Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. J. Food Compos. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Umashankar, S.; Liang, X.; Lee, H.W.; Swarup, S.; Ong, C.N. Characterization of plant volatiles reveals distinct metabolic profiles and pathways among 12 Brassicaceae vegetables. Metabolites 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.A.; Jung, Y.H.; Lim, S.H.; Park, S.U.; Kim, J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. subsp. pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Kim, Y.J.; Kim, H.J.; Choi, J.; Kim, J.K. Phytochemical profiles of Brassicaceae vegetables and their multivariate characterization using chemometrics. Appl. Biol. Chem. 2018, 61, 131–144. [Google Scholar] [CrossRef]
- Abureidah, I.M.; Arráezromán, D.; Quirantespiné, R.; Fernándezarroyo, S.; Seguracarretero, A.; Fernándezgutiérrez, A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012, 46, 108–117. [Google Scholar] [CrossRef]
- Mcnally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, H.; Wang, W.; Xu, M.; Shi, J.; Nie, X.; Yang, G. Identification of conserved and diverse metabolic shift of the stylar, intermediate and peduncular segments of cucumber fruit during development. Int. J. Mol. Sci. 2018, 19, 135. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef]
- Iijima, Y. Recent advances in the application of metabolomics to studies of biogenic volatile organic compounds (BVOC) produced by plant. Metabolites 2014, 4, 699–721. [Google Scholar] [CrossRef]
- Mattoli, L.; Gianni, M.; Burico, M. Mass spectrometry-based metabolomic analysis as a tool for quality control of natural complex products. Mass Spectrom. Rev. 2022, e21773. [Google Scholar] [CrossRef]
- Martínez-Esteso, M.J.; Martínez-Márquez, A.; Sellés-Marchart, S.; Morante-Carriel, J.A.; Bru-Martínez, R. The role of in proteomics progressing insights into plant secondary metabolism. Front. Plant Sci. 2015, 6, 504. [Google Scholar] [CrossRef]
- Yugi, K.; Kubota, H.; Hatano, A.; Kuroda, S. Trans-omics: How to reconstruct biochemical networks across multiple ‘Omic’ layers. Trends Biotechnol. 2016, 34, 276–290. [Google Scholar] [CrossRef]
- Chan, E.K.; Rowe, H.C.; Hansen, B.G.; Kliebenstein, D.J. The complex genetic architecture of the metabolome. PLoS Genet. 2010, 6, e1001198. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.-L.; Li, F. Application of metabolomics in the study of natural Products. Nat. Prod. Bioprospect. 2018, 8, 321–334. [Google Scholar] [CrossRef]
- Salem, M.A.; De Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M. González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Comp. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Williams, S.R.; Oatley, D.L.; Abdrahman, A.; Butt, T.; Nash, R. Membrane technology for the improved separation of bioactive compounds. Procedia Eng. 2012, 44, 2112–2114. [Google Scholar] [CrossRef]
- Salam, A.M.; Lyles, J.T.; Quave, C.L. Methods in the Extraction and Chemical Analysis of Medicinal Plants. In Methods and Techniques in Ethnobiology and Ethnoecology; Albuquerque, U.P., De Lucea, R.F.P., Da Cunha, L.V.F.C., Alves, R.R.N., Eds.; Springer: New York, NY, USA, 2019; pp. 257–283. [Google Scholar]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Sharma, V.; Gupta, P.; Priscilla, K.; Kumar, S.; Hangargi, B.; Veershetty, A.; Ramrao, D.P.; Suresh, S.; Narasanna, R.; Naik, G.R.; et al. Metabolomics intervention towards better understanding of plant traits. Cells 2021, 10, 346. [Google Scholar] [CrossRef]
- Rienzo, M.; Jackson, S.J.; Chao, L.K.; Leaf, T.; Schmidt, T.J.; Navidi, A.H.; Nadler, D.C.; Ohler, M.; Leavell, M.D. High-throughput screening for high-efficiency small-molecule biosynthesis. Metab. Eng. 2021, 63, 102–125. [Google Scholar] [CrossRef]
- Di Masi, S.; De Benedetto, G.E.; Malitesta, C.; Saponari, M.; Citti, C.; Cannazza, G.; Ciccarella, G. HPLC-MS/MS method applied to an untargeted metabolomics approach for the diagnosis of “olive quick decline syndrome”. Anal. Bioanal. Chem. 2022, 414, 465–473. [Google Scholar] [CrossRef]
- Silva, M.S.; Cordeiro, C.; Roessner, U.; Figueiredo, A. Editorial: Metabolomics in crop research—Current and emerging methodologies. Front. Plant. Sci. 2019, 10, 1013. [Google Scholar] [CrossRef]
- Ma, A.; Qi, X. Mining plant metabolomes: Methods, applications, and perspectives. Plant Commun. 2021, 2, 100238. [Google Scholar] [CrossRef]
- De Souza, L.P.; Borghi, M.; Fernie, A. Plant single-cell metabolomics—Challenges and perspectives. Int. J. Mol. Sci. 2020, 21, 8987. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Hong, J.; Shi, J. Promoting human nutrition and health through plant metabolomics: Current status and challenges. Biology 2021, 10, 20. [Google Scholar] [CrossRef]
- Pinu, F.R.; Goldansaz, S.A.; Jaine, J. Translational metabolomics: Current challenges and future opportunities. Metabolites 2019, 9, 108. [Google Scholar] [CrossRef]
- Dias, D.A.; Hill, C.B.; Jayasinghe, N.S.; Atieno, J.; Sutton, T.; Roessner, U. Quantitative profiling of polar primary metabolites of two chickpea cultivars with contrasting responses to salinity. J. Chromatogr. B 2015, 1000, 1–13. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Composite score analysis for unsupervised comparison and network visualization of metabolomics data. Anal. Chim. Acta 2020, 1095, 38–47. [Google Scholar] [CrossRef]
- Turner, M.F.; Heuberger, A.L.; Kirkwood, J.S.; Collins, C.C.; Wolfrum, E.J.; Broeckling, C.D.; Prenni, J.E.; Jahn, C.E. Non-targeted metabolomics in diverse sorghum breeding lines indicates primary and secondary metabolite profiles are associated with plant biomass accumulation and photosynthesis. Front. Plant Sci. 2016, 7, 953. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, S.; Võsa, U.; Van der Graaf, A.; Franke, L.; De Magalhães, J.P. Gene co-expression analysis for functional classification and gene–disease predictions. Brief. Bioinform. 2018, 19, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Fernie, A.R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 2018, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Wei, S.; Yang, X.; Huo, G.; Ge, G.; Liu, H.; Luo, L.; Hu, J.; Huang, D.; Long, P. Distinct metabolome changes during seed germination of lettuce (Lactuca sativa L.) in response to thermal stress as revealed by untargeted metabolomics analysis. Int. J. Mol. Sci. 2020, 21, 1481. [Google Scholar] [CrossRef]
- Yizhak, K.; Benyamini, T.; Liebermeister, W.; Ruppin, E.; Shlomi, T. Integrating quantitative proteomics and metabolomics with a genome-scale metabolic network model. Bioinformatics 2010, 26, i255–i260. [Google Scholar] [CrossRef]
- Sharma, K.; Sarma, S.; Bohra, A.; Mitra, A.; Sharma, N.K.; Kumar, A. Plant Metabolomics: An Emerging Technology for Crop Improvement; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Raza, A. Metabolomics: A systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2022, 41, 741–763. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid, H.M.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gols, R.; Benrey, B. Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 2015, 60, 35–58. [Google Scholar] [CrossRef]
- Alseekh, S.; Scossa, F.; Wen, W.; Luo, J.; Yan, J.; Beleggia, R.; Klee, H.J.; Huang, S.; Papa, R.; Fernie, A.R. Domestication of crop metabolomes: Desired and unintended consequences. Trends. Plant. Sci. 2021, 26, 650–661. [Google Scholar] [CrossRef]
- Alseekh, S.; Bermudez, L.; De Haro, L.A.; Fernie, A.R.; Carrari, F. Crop metabolomics: From diagnostics to assisted breeding. Metabolomics 2018, 14, 148. [Google Scholar] [CrossRef]
- Giovannoni, J. Tomato multiomics reveals consequences of crop domestication and improvement. Cell 2018, 172, 6–8. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef]
- Tohge, T.; Scossa, F.; Wendenburg, R.; Frasse, P.; Balbo, I.; Watanabe, M.; Alseekh, S.; Jadhav, S.S.; Delfin, J.C.; Lohse, M.; et al. Exploiting natural variation in tomato to define pathway structure and metabolic regulation of fruit polyphenolics in the Lycopersicum complex. Mol. Plant 2020, 13, 127–1046. [Google Scholar] [CrossRef]
- Carrari, F.; Baxter, C.; Usadel, B.; Urbanczyk-Wochniak, E.; Zanor, M.I.; Nunes-Nesi, A.; Nikiforova, V.; Centero, D.; Ratzka, A.; Pauly, M.; et al. Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behaviour. Plant Physiol. 2006, 142, 1380–1396. [Google Scholar] [CrossRef]
- Rohrmann, J.; Tohge, T.; Alba, R.; Osorio, S.; Caldana, C.; McQuinn, R.; Arvidsson, S.; Van der Merwe, M.J.; Riaño-Pachón, D.M.; Mueller-Roeber, B.; et al. Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J. 2011, 68, 999–1013. [Google Scholar] [CrossRef]
- Ibarra-Estrada, E.; Palma-Tenango RM, S. Metabolomics as a Tool in Agriculture. In Metabolomics—Fundamentals and Applications; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Roessner-Tunali, U.; Hegemann, B.; Lytovchenko, A.; Carrari, F.; Bruedigam, C.; Granot, D.; Fernie, A.R. Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol. 2003, 133, 84–99. [Google Scholar] [CrossRef]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, H.W.M. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef]
- Abreu, A.C.; Fernández, I. NMR Metabolomics applied on the discrimination of variables influencing tomato (Solanum lycopersicum). Molecules 2020, 25, 3738. [Google Scholar] [CrossRef]
- Ntagkas, N.; De Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the tomato fruit metabolome by LED light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.K.; Jung, E.; Lee, H.-A.; Choi, D. Metabolomic characterization of Hot pepper (Capsicum annuum ‘CM334’) during fruit development. J. Agric. Food Chem. 2015, 63, 9452–9460. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Wenlong, Y.; Jian, L.; Minxuan, L.; Yaxin, S.; Xianghong, W. Characterization and discrimination of chilli peppers based on multi-element and non-targeted metabolomics analysis. LWT 2020, 131, 109742. [Google Scholar] [CrossRef]
- Aizat, W.M.; Dias, D.A.; Stangoulis, J.C.R.; Able, J.A.; Roessner, U.; Able, A.J. Metabolomics of capsicum ripening reveals modification of the ethylene related-pathway and carbon metabolism. Postharvest Biol. Technol. 2014, 89, 19–31. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Liang, Z.; Liu, W.; Jiang, B.; Yan, J.; Sun, P.; Cao, Z.; Peng, Q.; Lin, Y. Metabolome and transcriptome analyses reveal chlorophyll and anthocyanin metabolism pathway associated with cucumber fruit skin color. BMC Plant Biol. 2020, 20, 386. [Google Scholar] [CrossRef]
- Maamoun, A.A.; El-Akkad, R.H.; Farag, M.A. Mapping metabolome changes in Luffa aegyptiaca mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2021, 29, 179–189. [Google Scholar] [CrossRef]
- Brock, M.T.; Lucas, L.K.; Anderson, N.A.; Rubin, M.J.; Markelz, R.J.; Covington, M.F.; Devisetty, U.K.; Chapple, C.; Maloof, J.N.; Weinig, C. Genetic architecture, biochemical underpinnings and ecological impact of floral UV patterning. Mol. Ecol. 2016, 25, 1122–1140. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Zhao, P.J.; Li, G.H. Advances in the study of metabolomics and metabolites in some species interactions. Molecules 2021, 26, 3311. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 643–684. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gil, A. Nutrimetabolomics: An update on analytical approaches to investigate the role of plant-based foods and their bioactive compounds in non-communicable chronic diseases. Int. J. Mol. Sci. 2016, 17, 2072. [Google Scholar] [CrossRef]
- Baidoo, E.E.K. Microbial metabolomics: A general overview. Methods Protoc. 2019, 1859, 18. [Google Scholar]
- Carrera, F.P.; Noceda, C.; Maridueña-Zavala, M.G.; Cevallos-Cevallos, J.M. Metabolomics, a powerful tool for understanding plant abiotic stress. Agronomy 2021, 11, 824. [Google Scholar] [CrossRef]
- Tohge, T.; De Souza, L.P.; Fernie, A.R. Genome-enabled plant metabolomics. J. Chromatogr. B 2014, 966, 7–20. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Wang, F.; Jia, W.; Xu, Z. Combined transcriptomic and metabolomic analyses uncover rearranged gene expression and metabolite metabolism in tobacco during cold acclimation. Sci. Rep. 2020, 10, 5242. [Google Scholar] [CrossRef]
- Jones, D.P.; Park, Y.; Ziegler, T.R. Nutritional metabolomics: Progress in addressing complexity in diet and health. Annu. Rev. Nutr. 2012, 32, 183–202. [Google Scholar] [CrossRef]
- Mun, H.; Kwon, M.C.; Lee, N.-R.; Son, S.Y.; Song, D.H.; Lee, C.H. Comparing metabolites and functional properties of various tomatoes using mass spectrometry-based metabolomics approach. Front. Nutr. 2021, 8, 659646. [Google Scholar] [CrossRef]
- Reed, D.; Kumar, D.; Kumar, S.; Raina, K.; Punia, R.; Kant, R.; Saba, L.; Cruickshank-Quinn, C.; Tabakoff, B.; Reisdorph, N.; et al. Transcriptome and metabolome changes induced by bitter melon(Momordica charantia)-intake in a high-fat diet induced obesity model. J. Tradit. Complement. Med. 2021, 12, 287–301. [Google Scholar] [CrossRef]
- Emwas, A.H.M.; Al-Rifai, N.; Szczepski, K.; Alsuhaymi, S.; Rayyan, S.; Almahasheer, H.; Jaremko, M.; Brennan, L.; Lachowicz, J.I. You are what you eat: Application of metabolomics approaches to advance nutrition research. Foods 2021, 10, 1249. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savva, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and health: From nutritional crops and plant-based pharmaceuticals to profiling of human biofluids. Cell. Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.-R.; Tikunov, Y.; De Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2013, 9, 130–144. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Hu, T.; Zhang, F.; Wang, B.; Li, C.; Yang, T.; Li, H.; Lu, Y.; Giovannoni, J.J.; et al. An InDel in the promoter of Al-Activated malate transporter selected during tomato domestication determines fruit malate contents and Aluminum tolerance. Plant Cell 2017, 29, 2249–2268. [Google Scholar] [CrossRef]
- Saxena, A.; Cramer, C.S. Metabolomics: A potential tool for breeding nutraceutical vegetables. Adv. Crop Sci. Tech. 2013, 1, 106. [Google Scholar] [CrossRef]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Metabolomics-inspired insight into developmental, environmental and genetic aspects of tomato fruit chemical composition and quality. Plant Cell Physiol. 2015, 56, 1681–1696. [Google Scholar] [CrossRef]
- Pinter, M.D.; Harter, T.; McCarthy, M.J.; Augustine, M.P. Towards using NMR to screen for spoiled tomatoes stored in 1000 L, aseptically sealed, metal-lined totes. Sensors 2014, 14, 4167–4176. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Cañellas, N.; Abete, I.; Rodríguez, M.A.; Perez-Cornago, A.; Navas-Carretero, S.; Ángeles Zulet, M.; Correig, X.; Martínez, J.A. Nutri-Metabolomics: Subtle serum metabolic differences in healthy subjects by NMR-based metabolomics after a short-term nutritional intervention with two tomato sauces. OMICS J. Int. Biol. 2013, 17, 611–618. [Google Scholar] [CrossRef]
- Hohmann, M.; Christoph, N.; Wachter, H.; Holzgrabe, U. 1H NMR profiling as an approach to differentiate conventionally and organically grown Tomatoes. J. Agric. Food Chem. 2014, 33, 8530–8540. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Amat, M.; Lamuela-Raventós, R.M. A metabolomic approach differentiates between conventional and organic ketchups. J. Agric. Food Chem. 2011, 59, 11703–11710. [Google Scholar] [CrossRef]
- Novotná, H.; Kmiecik, O.; Gałązka, M.; Krtková, V.; Hurajová, A.; Schulzová, V.; Hallmann, E.; Rembiałkowska, E.; Hajšlová, J. Metabolomic fingerprinting employing DART-TOFMS for authentication of tomatoes and peppers from organic and conventional farming. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1335–1346. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Bonini, P.; Bontempo, L.; Camin, F.; Trevisan, M.; Lucini, L. The hierarchical contribution of organic vs. conventional farming, cultivar, and terroir on untargeted metabolomics phytochemical profile and functional traits of tomato fruits. Front. Plant Sci. 2022, 13, 856513. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.M.; Durán-Soria, S.; Osorio, S.; Vallarino, J.G. Combining metabolomic and transcriptomic approaches to assess and improve crop quality traits. CABI Agric. Biosci. 2021, 2, 1. [Google Scholar] [CrossRef]
- Tebani, A.; Bekri, S. Paving the way to precision nutrition through metabolomics. Front. Nutr. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Rahman, S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Zhang, W.-F.; Gong, Z.-H.; Wu, M.-B.; Chan, H.; Yuan, Y.-J.; Tang, N.; Zhang, Q.; Miao, M.-J.; Chang, W.; Li, Z.; et al. Integrative comparative analyses of metabolite and transcript profiles uncovers complex regulatory network in tomato (Solanum lycopersicum L.) fruit undergoing chilling injury. Sci. Rep. 2019, 9, 4470. [Google Scholar] [CrossRef]
- Luna-Ruiz, J.D.J.; Nabhan, G.P.; Aguilar-Meléndez, A. Shifts in plant chemical defenses of chile pepper (Capsicum annuum L.) due to domestication in mesoamerica. Front. Ecol. Evol. 2018, 6, 48. [Google Scholar] [CrossRef]
- Luna, E.; Flandin, A.; Cassan, C.; Prigent, S.; Chevanne, C.; Kadiri, F.; Gibon, Y.; Pétriacq, P. Metabolomics to exploit the primed immune system of tomato fruit. Metabolites 2020, 10, 96. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Chen, S.; Fu, D.-Q.; Jiang, C.-Z. Metabolomic and transcriptomic analyses reveal that a MADS-box transcription factor TDR4 regulates tomato fruit quality. Front. Plant Sci. 2019, 10, 792. [Google Scholar] [CrossRef]
- Sudiro, C.; Guglielmi, F.; Hochart, M.; Senizza, B.; Zhang, L.; Lucini, L.; Altissimo, A. A phenomics and metabolomics investigation on the modulation of drought stress by a biostimulant plant extract in tomato (Solanum lycopersicum). Agronomy 2022, 12, 764. [Google Scholar] [CrossRef]
- Chele, K.H.; Steenkamp, P.; Piater, L.A.; Dubery, I.A.; Huyser, J.; Tugizimana, F. A global metabolic map defines the effects of a Si-based biostimulant on tomato plants under normal and saline conditions. Metabolites 2021, 11, 820. [Google Scholar] [CrossRef]
- Min, K.; Chen, K.; Arora, R. A metabolomics study of ascorbic acid-induced in situ freezing tolerance in spinach (Spinacia oleracea L.). Plant Direct 2020, 4, e00202. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Babalola, O.O.; Loots, D.T. Metabolomic applications for understanding complex tripartite plant-microbe interactions: Strategies and perspectives. Biotechnol. Rep. 2020, 25, e00425. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2020, 11, 610065. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Labuschagne, N.; Dubery, I.A. Metabolomic evaluation of tissue-specific defense responses in tomato plants modulated by PGPR-priming against Phytophthora capsici infection. Plants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic profiling of the host response of tomato (Solanum lycopersicum) following infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Hendrich, C.G.; Von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.L.; Ricca, P.; Naidoo, J.; Cook, D.; et al. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Rossouw, L.T.; Madala, N.E.; Tugizimana, F.; Steenkamp, P.A.; Esterhuizen, L.L.; Dubery, I.A. Deciphering the resistance mechanism of tomato plants against whitefly-mediated tomato curly stunt virus infection through ultra-high-performance liquid chromatography coupled to mass spectrometry (UHPLC-MS)-based metabolomics approaches. Metabolites 2019, 9, 60. [Google Scholar] [CrossRef]
- Yogendra, K.N.; Kushalappa, A.C.; Sarmiento, F.; Rodriguez, E.; Mosquera, T. Metabolomics deciphers quantitative resistance mechanisms in diploid potato clones against late blight. Funct. Plant Biol. 2014, 42, 284–298. [Google Scholar] [CrossRef]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an emerging tool for the study of plant-pathogen interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef]
- Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.A. An overview of NMR-based metabolomics to identify secondary plant compounds involved in host plant resistance. Phytochem. Rev. 2011, 10, 205–216. [Google Scholar] [CrossRef]
- Garcia, G.P.; Dos Santos, F.N.; Zanotta, S.; Eberlin, M.N.; Carazzone, C. Metabolomics of Solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef]
- Hernández-Aparicio, F.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; López-Gresa, M.P. Signaling in the tomato immunity against Fusarium oxysporum. Molecules 2021, 26, 1818. [Google Scholar] [CrossRef]
- Bisht, H.; Bhagat, D.; Bhatnagar, M.K. Metabolic profiling of tomatoes with pest infestation using GC-MS and NMR spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 550–556. [Google Scholar]
- Afifah, E.N.; Murti, R.H.; Nuringtyas, T.R. Metabolomics approach for the analysis of resistance of four tomato genotypes (Solanum lycopersicum L.) to root-knot nematodes (Meloidogyne incognita). Open Life Sci. 2019, 14, 141–149. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Velásquez-Valle, R.; Zepeda-Vallejo, L.G.; Pérez-Hernández, N.; Velázquez-Ponce, M.; Arcos-Adame, V.M.; Becerra-Martínez, E. 1H NMR-based metabolomic profiling for identification of metabolites in Capsicum annuum cv. mirasol infected by beet mild curly top virus (BMCTV). Food Res. Int. 2018, 106, 870–877. [Google Scholar] [CrossRef]
- Moënne-Loccoz, Y.; Mavingui, P.; Combes, C.; Normand, P.; Steinberg, C. Microorganisms and Biotic Interactions. In Environmental Microbiology: Fundamentals and Applications; Bertrand, J.C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Sime-Ngando, T., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Maltese, F.; Bellés, J.M.; Conejero, V.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Metabolic response of tomato leaves upon different plant-pathogen interactions. Phytochem. Anal. 2010, 21, 89–94. [Google Scholar] [CrossRef]
- Allwood, J.W.; Ellis, D.I.; Goodacre, R. Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiol. Plant. 2008, 132, 117–135. [Google Scholar] [CrossRef]
- Maag, D.; Erb, M.; Glauser, G. Metabolomics in plant–herbivore interactions: Challenges and applications. Entomol. Exp. Appl. 2015, 157, 18–29. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.-X.; Chen, R.; He, S.Y. Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 23390–23397. [Google Scholar] [CrossRef] [PubMed]
- Errard, A.; Ulrichs, C.; Kühne, S.; Mewis, I.; Mishig, N.; Maul, R.; Drungowski, M.; Parolin, P.; Schreiner, M.; Baldermann, S. Metabolite profiling reveals a specific response in tomato to predaceous Chrysoperla carnea larvae and herbivore(s)-predator interactions with the generalist pests Tetranychus urticae and Myzus persicae. Front. Plant Sci. 2016, 7, 1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Zhang, J.; He, T.; Huang, J.; Zhang, Z.; Wang, Y.; Hafeez, M.; Zhou, S.; Ren, X.; et al. Comprehensive metabolome and volatilome analyses in eggplant and tomato reveal their differential responses to Tuta absoluta infestation. Front. Plant Sci. 2021, 12, 757230. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Li, X.W.; He, T.J.; Li, P.J.; Liu, Y.; Zhou, S.X.; Wu, Q.C.; Chen, T.T.; Lu, Y.B.; Hou, Y.M. Comparative biochemical and transcriptome analyses in tomato and eggplant reveal their differential responses to Tuta absoluta infestation. Genomics 2021, 113, 2108–2121. [Google Scholar] [CrossRef]
- Macel, M.; Visschers, I.G.S.; Peters, J.L.; Kappers, I.F.; De Vos, R.C.H.; Van Dam, N.M. Metabolomics of thrips resistance in pepper (Capsicum spp.) reveals monomer and dimer acyclic diterpene glycosides as potential chemical defenses. J. Chem. Ecol. 2019, 45, 490–501. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and metabolome reprogramming in tomato plants by Trichoderma harzianum strain T22 primes and enhances defense responses against aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Zhang, Y.; Bouwmeester, H.J.; Kappers, I.F. Combined transcriptome and metabolome analysis identifies defence responses in spider mite-infested pepper (Capsicum annuum). J. Exp. Bot. 2020, 71, 330–343. [Google Scholar] [CrossRef]
- He, J.; Bouwmeester, H.J.; Dicke, M.; Kappers, I. Transcriptional and metabolite analysis reveal a shift in direct and indirect defences in response to spider-mite infestation in cucumber (Cucumis sativus). Plant Mol. Biol. 2020, 103, 489–505. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Gong, F.; Hou, F.; Zhang, Z.; Cheng, X.; Du, W.; Zhang, L.; Wang, J.; Xu, J.; et al. The transcriptome and metabolome reveal stress responses in sulfur-fumigated Cucumber (Cucumis sativus L.). Front. Plant Sci. 2021, 12, 778956. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Wu, Q.; Yupeng, Z.; Jiang, Y.; John, A.; Wen, L.; Li, T.; Jian, Q.; Yang, B. Analyses of quality and metabolites levels of okra during postharvest senescence by 1 H-high resolution NMR. Postharvest Biol. Technol. 2017, 132, 171–178. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere tripartite interactions and PGPR-mediated metabolic reprogramming towards ISR and plant priming: A metabolomics review. Biology 2022, 11, 346. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, M.B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; De Oliveira, A.L.M. The adaptive metabolomic profile and functional activity of tomato rhizosphere are revealed upon PGPB inoculation under saline stress. Environ. Exp. Bot. 2021, 189, 104552. [Google Scholar] [CrossRef]
- Wen, T.; Zhao, M.; Liu, T.; Huang, Q.; Yuan, J.; Shen, Q. High abundance of Ralstonia solanacearum changed tomato rhizosphere microbiome and metabolome. BMC Plant Biol. 2020, 20, 166. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, J.; He, X.; Lin, Y. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 2020, 7, 154. [Google Scholar] [CrossRef]
- Wu, F.; Ding, Y.; Nie, Y.; Wang, X.-J.; An, Y.-Q.; Roessner, U.; Walker, R.; Du, B.; Bai, J.-G. Plant metabolomics integrated with transcriptomics and rhizospheric bacterial community indicates the mitigation effects of Klebsiella oxytoca P620 on p-hydroxybenzoic acid stress in cucumber. J. Hazard. Mater. 2021, 415, 125756. [Google Scholar] [CrossRef]
- Han, S.; Micallef, S.A. Environmental metabolomics of the Tomato plant surface provides insights on Salmonella enterica colonization. Appl. Environ. Microbiol. 2016, 82, 3131–3142. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yao, S.; Yang, X.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Sobolev, A.P.; Neelam, A.; Goyal, R.K.; Handa, A.K.; Segre, A.L. Nuclear Magnetic Resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol. 2006, 142, 1759–1770. [Google Scholar] [CrossRef]
- Paupière, M.J.; Tikunov, Y.; Schleiff, E.; Bovy, A.; Fragkostefanakis, S. Reprogramming of tomato leaf metabolome by the activity of heat stress transcription factor HsfB1. Front. Plant Sci. 2020, 11, 610599. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Zhang, H.; Du, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Keller, A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics reveals how cucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Adeleye, A.S.; Keller, A.A. Metabolomics reveals Cu(OH)2 nanopesticide-activated anti-oxidative pathways and decreased beneficial antioxidants in spinach leaves. Environ. Sci. Technol. 2017, 51, 10184–10194. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics reveals the “invisible” responses of spinach plants exposed to CeO2 nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef]
- Zhou, S.; Allard, P.M.; Wolfrum, C.; Ke, C.; Tang, C.; Ye, Y.M.; Wolfender, J.-L. Identification of chemotypes in bitter melon by metabolomics: A plant with potential benefit for management of diabetes in traditional Chinese medicine. Metabolomics 2019, 15, 104. [Google Scholar] [CrossRef]
- Mishra, S.; Ankit; Sharma, R.; Gogna, N.; Dorai, K. NMR-based metabolomic profiling of the differential concentration of phytomedicinal compounds in pericarp, skin and seeds of Momordica charantia (bitter melon). Nat. Prod. Res. 2022, 36, 390–395. [Google Scholar] [CrossRef]
- Moco, S.; Forshed, J.; De Vos, R.C.H.; Bino, R.J.; Vervoort, J. Intra- and inter-metabolite correlation spectroscopy of tomato metabolomics data obtained by liquid chromatography-mass spectrometry and nuclear magnetic resonance. Metabolomics 2008, 4, 202–215. [Google Scholar] [CrossRef]
- Tikunov, Y.; Lommen, A.; De Vos, C.H.R.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Arnaud, G.; Bovy, A. Novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef]
- Murti, R.H.; Afifah, E.N.; Nuringtyas, T.R. Metabolomic response of tomatoes (Solanum lycopersicum L.) against bacterial wilt (Ralstonia solanacearum) Using 1H-NMR spectroscopy. Plants 2021, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Kingery, W.L.; Hatcher, P.G. The identification of plant derived structures in humic materials using three-dimensional NMR spectroscopy. Environ. Sci. Technol. 2003, 37, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, P.; Vinale, F.; Woo, S.L.; Pascale, A.; Lorito, M.; Piccolo, A. Metabolomics by proton high-resolution magic-angle-spinning nuclear magnetic resonance of tomato plants treated with two secondary metabolites isolated from Trichoderma. J. Agric. Food Chem. 2016, 64, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Ara, T.; Sakurai, N.; Takahashi, S.; Waki, N.; Suganuma, H.; Aizawa, K.; Matsumura, Y.; Kawada, T.; Shibata, D. TOMATOMET: A metabolome database consists of 7118 accurate mass values detected in mature fruits of 25 tomato cultivars. Plant Direct 2021, 5, e00318. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, X.; Chen, W.; Ye, X.; Tu, P. Metabolomics reveals key resistant responses in tomato fruit induced by Cryptococcus laurentii. Food Chem. Mol. Sci. 2022, 4, 100066. [Google Scholar] [CrossRef]
- Bénard, C.; Bernillon, S.; Biais, B.; Osorio, S.; Maucourt, M.; Ballias, P.; Deborde, C.; Colombié, S.; Cabasson, C.; Jacob, D.; et al. Metabolomic profiling in tomato reveals diel compositional changes in fruit affected by source–sink relationships. J. Exp. Bot. 2015, 66, 3391–3404. [Google Scholar] [CrossRef]
- Perez-Fons, L.; Wells, T.; Corol, D.; Ward, J.L.; Gerrish, C.; Beale, M.H.; Seymour, G.B.; Bramley, P.M.; Fraser, P.D. A genome-wide metabolomic resource for tomato fruit from Solanum pennellii. Sci. Rep. 2014, 4, 3859. [Google Scholar] [CrossRef]
- Etalo, D.W. An Integrated Approach Involving Metabolomics and Transcriptomics for a System-Wide Understanding of the Interaction between Tomato and Cladosporium fulvum. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2014. Available online: https://www.wur.nl (accessed on 22 September 2022).
- Mirnezhad, M.; Romero-González, R.R.; Leiss, K.A.; Choi, Y.H. Metabolomic analysis of host plant cultivated tomatoes. Phytochem. Anal. 2009, 21, 110–117. [Google Scholar] [CrossRef]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, J.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signalling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative transcriptomics and metabolomics reveal an intricate priming mechanism involved in PGPR-mediated salt tolerance in tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef]
- Ingallina, C.; Maccelli, A.; Spano, M.; Di Matteo, G.; Di Sotto, A.; Giusti, A.M.; Vinci, G.; Di Giacomo, S.; Rapa, M.; Ciano, S.; et al. Chemico-Biological characterization of Torpedino Di Fondi® tomato fruits: A comparison with San Marzano cultivar at two ripeness stages. Antioxidant 2020, 9, 1027. [Google Scholar] [CrossRef]
- Fatima, E.A.; Moha, T.; Said, W.; Abdelilah, M.; Mohammed, R. Use of metabolomics data analysis to identify fruit quality markers enhanced by the application of an aminopolysaccharide. RSC Adv. 2021, 11, 35514–35524. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; De Vos, R.C.H.; Jonker, H.H.; Mumm, R.; Hall, R.D.; Bialek, L.; Leenman, R.; Strassburg, K.; Vreeken, R.; Hankemeier, T.; et al. Comprehensive metabolomics to evaluate the impact of industrial processing on the phytochemical composition of vegetable purees. Food Chem. 2015, 168, 348–355. [Google Scholar] [CrossRef]
- Florentino-Ramos, E.; Villa-Ruano, N.; Hidalgo-Martínez, D.; Ramírez-Meraz, M.; Méndez-Aguilar, R.; Velásquez-Valle, R.; Zepeda-Vallejo, L.G.; Pérez-Hernández, N.; Becerra-Martínez, E. 1H NMR-based fingerprinting of eleven Mexican Capsicum annuum cultivars. Food Res. Int. 2019, 121, 12–19. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Ramírez-Meraz, M.; Méndez-Aguilar, R.; Zepeda-Vallejo, L.G.; Álvarez-Bravo, A.; Pérez-Hernández, N.; Becerra-Martínez, E. 1H NMR-based metabolomics profiling of ten new races from Capsicum annuum cv. serrano produced in Mexico. Food Res. Int. 2019, 119, 785–792. [Google Scholar] [CrossRef]
- Becerra-Martínez, E.; Florentino-Ramos, E.; Pérez-Hernández, N.; Zepeda-Vallejo, L.G.; Villa-Ruano, N.; Velázquez-Ponce, M.; García-Mendoza, F.; Bañuelos-Hernández, A.E. 1H NMR-based metabolomic fingerprinting to determine metabolite levels in serrano peppers (Capsicum annum L.) grown in two different regions. Food Res. Int. 2017, 102, 163–170. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, J.; Huang, Y.; Wang, H.; Adeleye, A.; Ortiz, C.; Keller, A.A. 1H NMR and GC–MS based metabolomics reveal nano-Cu altered cucumber (Cucumis sativus) fruit nutritional supply. Plant Physiol. Biochem. 2017, 110, 138–146. [Google Scholar] [CrossRef]
- Mansfeld, B.; Colle, M.; Kang, Y.; Jones, A.D.; Grumet, R. Transcriptomic and metabolomic analyses of cucumber fruit peels reveal a developmental increase in terpenoid glycosides associated with age-related resistance to Phytophthora capsici. Hortic. Res. 2017, 4, 17022. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Di, Q.; Sun, T.; Li, Y.; Duan, Y.; Wang, J.; Yan, Y.; He, C.; Wang, C.; Yu, X. Integrated metabolome and transcriptome analysis provide insights into the effects of grafting on fruit flavor of cucumber with different rootstocks. Int. J. Mol. Sci 2019, 20, 3592. [Google Scholar] [CrossRef]
- Zhang, Z.; He, H.; Yan, M.; Zhao, C.; Lei, C.; Li, J.; Yan, F. Widely targeted analysis of metabolomic changes of Cucumis sativus induced by cucurbit chlorotic yellows virus. BMC Plant Biol. 2022, 22, 158. [Google Scholar] [CrossRef]
- Tamura, Y.; Mori, T.; Nakabayashi, R.; Kobayashi, M.; Saito, K.; Okazaki, S.; Wang, N.; Kusano, M. Metabolomic evaluation of the quality of leaf lettuce grown in practical plant factory to capture metabolite signature. Front. Plant Sci. 2018, 9, 665. [Google Scholar] [CrossRef]
- Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A.; Ridwani, S. Untargeted metabolomics analysis of eggplant (Solanum melongena L.) fruit and its correlation to fruit morphologies. Metabolites 2016, 8, 49. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Shang, J.; Zhu, Z.; Li, Y.; Wu, X.; Zha, D. Study on browning mechanism of fresh-cut eggplant (Solanum melongena L.) based on metabolomics, enzymatic assays and gene expression. Sci. Rep. 2021, 11, 6937. [Google Scholar] [CrossRef] [PubMed]
- Calumpang, C.L.F.; Saigo, T.; Watanabe, M.; Tohge, T. Cross-species comparison of fruit-metabolomics to elucidate metabolic regulation of fruit polyphenolics among solanaceous crops. Metabolites 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Khatib, A.; Ahmed, Q.U.; Uzir, B.F.; Abas, F.; Murugesu, S.; Saiman, M.Z.; Primaharinastiti, R.; El-Seedi, H. Antioxidants profile of Momordica charantia fruit extract analyzed using LC-MS-QTOF-based metabolomics. Food Chem. Mol. Sci. 2021, 2, 100012. [Google Scholar] [CrossRef]
- Hua, M.D.S.; Kumar, S.; Shyur, R.; Cheng, Y.B.; Tian, Z.; Oelmüller, R.; Yeh, K.W. Metabolomic compounds identified in Piriformospora indica-colonized Chinese cabbage roots delineate symbiotic functions of the interaction. Sci. Rep. 2017, 7, 9291. [Google Scholar] [CrossRef]
- Mabuchi, R.; Tanaka, M.; Nakanishi, C.; Takatani, N.; Tanimoto, S. Analysis of primary metabolites in cabbage (Brassica oleracea var capitata) varieties correlated with antioxidant activity and taste attributes by metabolic profiling. Molecules 2019, 24, 4282. [Google Scholar]
- Yeo, H.J.; Baek, S.A.; Sathasivam, R.; Kim, J.K.; Park, S.U. Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa subsp chinensis). Appl. Biol. Chem. 2021, 64, 3. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Wang, L.; Duan, Q.; Huang, D. Comparative analysis of metabolites profile in spinach (Spinacia oleracea L.) affected by different concentrations of gly and nitrate. Sci. Hortic. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Niron, H.; Barlas, N.; Salih, B.; Türet, M. Comparative transcriptome, metabolome, and ionome analysis of two contrasting common bean genotypes in saline conditions. Front. Plant Sci. 2020, 11, 599501. [Google Scholar] [CrossRef]
- Mecha, E.; Erny, G.L.; Guerreiro, A.C.L.; Feliciano, R.P.; Barbosa, I.; Da Silva, A.B.; Leitão, S.T.; Veloso, M.M.; Rubiales, D.; Rodriguez-Mateos, A.; et al. Metabolomics profile responses to changing environments in a common bean (Phaseolus vulgaris L.) germplasm collection. Food Chem. 2022, 370, 131003. [Google Scholar] [CrossRef]
- Mengdi, L.; Xiaoxu, Y.; Chang, L.; Yanmei, L.; Zhishan, Y.; Guojun, F.; Dajun, L. Integrated transcriptomics and metabolomics analysis to characterize cold stress responses in common bean (Phaseolus vulgaris L.). BMC Plant Biol. 2022; preprint. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, T.; Guo, M. Current advances in the metabolomics study on Lotus seeds. Front. Plant Sci. 2016, 7, 891. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, J.; Li, M.; Fragner, L.; Weckwerth, W.; Yang, P. Metabolomic and proteomic profiles reveal the dynamics of primary metabolism during seed development of lotus (Nelumbo nucifera). Front. Plant Sci. 2016, 7, 750. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, I.; Chowdhury, K.; Boroujerdi, A. Tissue-specific metabolic profile study of Moringa oleifera L. using nuclear magnetic resonance spectroscopy. Plant Tissue Cult. Biotechnol. 2014, 24, 77–86. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.P.; Bisen, M.S.; Shukla, R.; Prabha, R.; Maurya, S.; Reddy, Y.S.; Singh, P.M.; Rai, N.; Chaubey, T.; Chaturvedi, K.K.; et al. Metabolomics-Driven Mining of Metabolite Resources: Applications and Prospects for Improving Vegetable Crops. Int. J. Mol. Sci. 2022, 23, 12062. https://doi.org/10.3390/ijms232012062
Singh DP, Bisen MS, Shukla R, Prabha R, Maurya S, Reddy YS, Singh PM, Rai N, Chaubey T, Chaturvedi KK, et al. Metabolomics-Driven Mining of Metabolite Resources: Applications and Prospects for Improving Vegetable Crops. International Journal of Molecular Sciences. 2022; 23(20):12062. https://doi.org/10.3390/ijms232012062
Chicago/Turabian StyleSingh, Dhananjaya Pratap, Mansi Singh Bisen, Renu Shukla, Ratna Prabha, Sudarshan Maurya, Yesaru S. Reddy, Prabhakar Mohan Singh, Nagendra Rai, Tribhuvan Chaubey, Krishna Kumar Chaturvedi, and et al. 2022. "Metabolomics-Driven Mining of Metabolite Resources: Applications and Prospects for Improving Vegetable Crops" International Journal of Molecular Sciences 23, no. 20: 12062. https://doi.org/10.3390/ijms232012062
APA StyleSingh, D. P., Bisen, M. S., Shukla, R., Prabha, R., Maurya, S., Reddy, Y. S., Singh, P. M., Rai, N., Chaubey, T., Chaturvedi, K. K., Srivastava, S., Farooqi, M. S., Gupta, V. K., Sarma, B. K., Rai, A., & Behera, T. K. (2022). Metabolomics-Driven Mining of Metabolite Resources: Applications and Prospects for Improving Vegetable Crops. International Journal of Molecular Sciences, 23(20), 12062. https://doi.org/10.3390/ijms232012062







