Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review
Abstract
:1. Introduction
2. Results
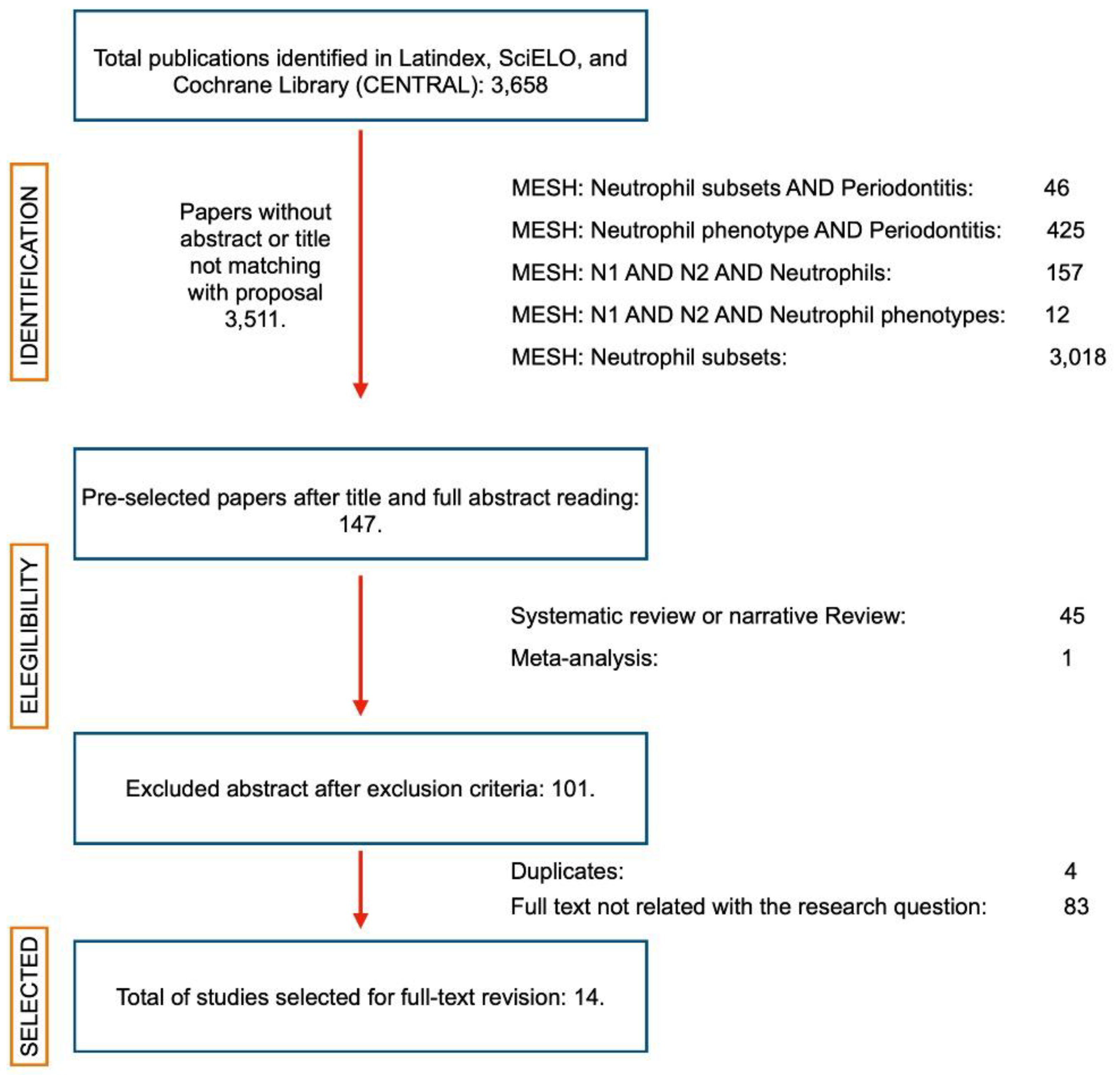
2.1. Search Results
2.2. Inclusion of Sources of Evidence
2.3. Review Findings
2.3.1. Phenotypic Characterization of Infiltrating Neutrophils in Periodontitis
2.3.2. Para- and Pro-Inflammatory Neutrophils in Periodontitis
2.3.3. Degranulation and NETosis
3. Methods
3.1. Review Question
3.2. Participants, Concept, and Context
3.3. Source of Evidence
3.4. Search Strategy
3.5. Source of Evidence Screening and Selection
3.6. Data Extraction
3.7. Outcome Measures
4. Discussion
5. Future Research Directions
6. Conclusions
7. Implication of the Findings for Research
8. Implication of the Findings for Practice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ost, M.; Singh, A.; Peschel, A.; Mehling, R.; Rieber, N.; Hartl, D. Myeloid-Derived suppresor cells in bacterial infections. Front. Cell. Infect. Microbiol. 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Pillay, J.; den Braber, I.; BVrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-inducible factors as essential regulators of inflammation. Curr. Top. Microbiol. Immunol. 2010, 345, 20. [Google Scholar]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Goldschmeding, R.; van Dalen, C.M.; Faber, N.; Calafat, J.; Huizinga, T.W.; van der Schoot, C.E.; Clement, L.T.; von dem Borne, A.E. Further characterization of the NB 1 antigen as a variably expressed 56–62 kD GPI- linked glycoprotein of plasma membranes and specific granules of neu- trophils. Br. J. Haematol. 1992, 81, 336–345. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, V. Tumor Associated Neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Perez de Puig, I.; Miro-Mur, F.; Ferrer-Ferrer, M.; Gelpi, E.; Pedragosa, J.; Justicia, C.; Urra, X.; Chamorro, A.; Planas, A.M. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015, 129, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Liu, S.; Hu, M.; Huang, F.; Zhu, Q.; Qiu, W.Q.; Hu, X.; Colello, J.; Zheng, S.G.; Lu, Z. Functional dynamics of neutrophils after ischemic stroke. Transl. Stroke Res. 2020, 11, 108–121. [Google Scholar] [CrossRef]
- Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Nombela, F.; Vivancos, J.; Hamilton, J.A.; Corbí, A.L.; Lizacoain, I.; Moro, M.A. N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the PPARgamma agonist rosiglitazone. Stroke 2013, 44, 3498–3508. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Theilgaard-Mönch, K.; Knudsen, S.; Follin, P.; Borregaard, N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 2004, 172, 7684–7693. [Google Scholar] [CrossRef] [Green Version]
- Naegelen, I.; Beaume, N.; Plançon, S.; Schenten, V.; Tschirhart, E.J.; Bréchard, S. Regulation of neutrophil degranulation and cytokine secretion: A novel model approach based on linar fitting. J. Immunol. Res. 2015, 2015, 817038. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2006, 147, 255–264. [Google Scholar] [CrossRef]
- Yagi, M.; Kantarçi, A.; Iwata, T.; Omori, K.; Ayilavarapu, S.; Ito, K.; Hasturk, H.; van Dyke, T.E. PDK1 regulates chemotaxis in human neutrophils. J. Dent. Res. 2009, 88, 1119–1124. [Google Scholar] [CrossRef] [Green Version]
- Aboodi, G.M.; Goldberg, M.B.; Glogauer, M. Refractory periodontitis population characterized by a hyperactive oral neutrophil phenotype. J. Periodontol. 2011, 82, 726–733. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Aboodi, G.M.; Glogauer, M. Oral neutrophils display a site-specific phenotype characterized by expression of T-cell receptors. J. Periodontol. 2013, 84, 1493–1503. [Google Scholar] [CrossRef]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar]
- Borenstein, A.; Fine, N.; Hassanpour, S.; Sun, C.; Oveisi, M.; Tenenbaum, H.C.; Glogauer, M. Morphological characterization of para- and proinflammatory neutrophil phenotypes using transmission electron microscopy. J. Periodontal Res. 2018, 53, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Papantonopoulos, G.; Delatola, C.; Takahashi, K.; Laine, M.L.; Loos, B.G. Hidden noise in immunologic parameters might explain rapid progression in aerly-onset peeriodontitis. PLoS ONE 2019, 14, e0224615. [Google Scholar] [CrossRef]
- Medara, N.; Lenzo, J.C.; Walsh, K.A.; Reynolds, E.C.; O’Brien-Simpson, N.M.; Darby, I.B. Peripheral neutrophil phenotypes during management of periodontitis. J. Periodontal Res. 2020, 56, 58–68. [Google Scholar]
- Johnstone, A.M.; Koh, A.; Goldberg, M.B.; Glogauer, M. A hyperactive neutrophil phenotype in patients with refractory periodontitis. J. Periodontol. 2007, 78, 1788–1794. [Google Scholar] [CrossRef]
- Wright, H.J.; Matthews, J.B.; Chapple, I.; Ling-Mountford, N.; Cooper, P. Periodontitis associates with type 1 IFN signature in peripheral blood neutrophils. J. Immunol. 2008, 181, 5775–5784. [Google Scholar] [CrossRef] [Green Version]
- Thorbert-Mros, S.; Larsson, L.; Berglundh, T. Cellular composition of long-standing gingivitis and periodontitis lesions. J. Periodontal Res. 2014, 50, 535–543. [Google Scholar] [CrossRef]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct oral neutrophil subsets define health and periodontal disease states. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef]
- White, P.; Sakellari, D.; Roberts, H.; Risafi, I.; Ling, M.; Cooper, P.; Milward, M.; Chapple, I. Peripheral blood neutrophil extracellular trap production and degradation in chronic periodontitis. J. Clin. Periodontol. 2016, 43, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Rudin, A.D.; Amirbeagi, F.; Davidsson, L.; Khamzeh, A.; Thorbert-Mros, S.; Thulin, P.; Welin, A.; Björkman, L.; Christenson, K.; Bylund, J. The neutrophil subset defined by CD177 expression is preferentially recruited to gingival crevicular fluid in periodontitis. J. Leukoc. Biol. 2020, 109, 349–362. [Google Scholar]
- Moonen, C.G.J.; de Vries, T.J.; Rijkschroeff, P.; Poubelle, P.E.; Nicu, E.A.; Loos, B.G. The possible role of neutrophils in the induction of osteoclastogenesis. J. Immunol. Res. 2019, 2019, 8672604. [Google Scholar]
- Moutsopoulos, N.M.; Konkel, J.E.; Sarmadi, M.; Eskan, M.A.; Wild, T.; Dutzan, N.; Abusleme, L.; Zenobia, C.; Hosur, K.B.; Abe, T.; et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014, 6, 229ra40. [Google Scholar] [CrossRef] [Green Version]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Armitage, G. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–110. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Seguier, S.; Godeau, G.; Leborgne, M.; Pivert, G.; Brousse, N. Quantitative morphological analysis of Langerhans cells in healthy and diseased human gingiva. Arch. Oral Biol. 2000, 45, 1073–1081. [Google Scholar]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Knopf, J.; Maueröder, C.; Kienhöfer, D.; Leppkes, M.; Herrmann, M. Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin. Exp. Rheumatol. 2016, 34, 6–8. [Google Scholar]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2017, 67, 1052–1063. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Matsuo, K.; Lin, A.; Procter, J.L.; Clement, L.T.; Stroncek, D. Variations in the expression of granulocyte antigen NB1. Transfusion 2000, 40, 654–662. [Google Scholar] [CrossRef]
- Taniguchi, K.; Kobayashi, M.; Harada, H.; Hiraoka, A.; Tanihiro, M.; Takata, N.; Kimura, A. Human neutrophil antigen-2a expression on neutrophils from healthy adults in western Japan. Transfusion 2002, 42, 651–657. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrohpil surface marker changes in health and inflammation using high-thourhput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef]
- Holers, V.M. Complement and its receptors: New insights into human disease. Annu. Rev. Immunol. 2014, 32, 433–459. [Google Scholar] [CrossRef]
- van Kessel, K.P.M.; Bestebroer, J.; van Strijp, J.A.G. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front. Immunol. 2014, 5, 467. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2012, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemmensen, S.N.; Bohr, C.T.; Rorvig, S.; Glenthoj, A.; Mora-Jensen, H.; Cramer, E.P.; Jacobsen, L.C.; Larsen, M.T.; Cowland, J.B.; Tanassi, T.; et al. Olfactomedin 4 defines a subset of human neutrophils. J. Leukoc. Biol. 2012, 91, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkeltaub, R.; Baird, S.; Sears, P.; Santiago, R.; Boisvert, W. The murine homolog if the interleukin-8 receptor CXCR2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum. 1998, 41, 900–909. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.K.D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; Holmdahl, R.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Morthe, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Vernal, R.; Dutzan, N.; Chaparro, A.; Puente, J.; Valenzuela, M.; Gamonal, J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005, 32, 383–389. [Google Scholar] [CrossRef]
- Vernal, R.; Dutzan, N.; Hernández, M.; Chandía, S.; Puente, J.; León, R.; García, L.; Del Valle, I.; Silva, A.; Gamonal, J. High expression levels of receptor activator of nuclear factor-kappa B ligand associated with human chronic periodontitis are mainly secreted by CD4+ T lymphocytes. J. Periodontol. 2006, 77, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Dutzan, N.; Vernal, R.; Hernández, M.; Dezerega, A.; Rivera, O.; Silva, N.; Aguillon, J.C.; Puente, J.; Pozo, P.; Gamonal, J. Levels of interferon-gamma and transcription factor T-bet in progressive periodontal lesions in patients with chronic periodontitis. J. Periodontol. 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Hernández, M.; Dutzan, N.; García-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [PubMed]
- Hajishengallis, G. Immuno-microbial pathogenesis of periodontitis: Keystones, pathobionts, and the host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; García-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, J.; Lechner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-B regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar]
- Zou, J.M.; Qin, J.; Li, Y.C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.S.; Chi, G.; Guo, F.; et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef] [Green Version]
- Shigematsu, K.; Asai, A.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. Enterococcus faecalis translocation in mice with severe burn injury: A pathogenic role of CCL2 and alternatively activated macrophages (M2aMo abd M2cMc). J. Leukoc. Biol. 2009, 86, 999–1005. [Google Scholar] [CrossRef]
- Neely, C.J.; Kartchner, L.B.; Mendoza, A.E.; Linz, B.M.; Frelinger, J.A.; Wolfgang, M.C.; Maile, R.; Cairns, B.A. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+IL-12- neutrophil polarization. PLoS ONE 2014, 9, e85623–e85633. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M1/M2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, S.; Calzetti, F.; Russo, M.P.; de Gironcoli, M.; Cassatella, M.A. Regulation of GRO alpha production in human granulocytes. J. Inflamm. 1995, 45, 143–151. [Google Scholar]
- van Gisbergen, K.P.J.M.; Geijtenbeek, T.B.H.; van Kooyk, Y. Close encounters of neutrofils and DCs. Trends Immunol. 2005, 26, 626–631. [Google Scholar] [CrossRef]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFB modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef] [Green Version]
- Hergott, C.B.; Roche, A.M.; Tamashiro, E.; Clarke, T.B.; Bailey, A.G.; Laughlin, A.; Bushman, F.D.; Weiser, J.N. Peptidoglycan from the gut microbiota governs the lifespan of circulatinf phagocytes at homeostasis. Blood 2016, 127, 2460–2471. [Google Scholar] [CrossRef] [Green Version]
- Gorlino, C.V.; Ranocchia, R.P.; Harman, M.F.; García, I.A.; Crespo, M.I.; Morón, G.; Maletto, B.A.; Pistoresi-Palencia, M.C. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J. Immunol. 2014, 193, 1966–1974. [Google Scholar]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastasic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Rayner, B.S.; Love, D.T.; Hawkins, C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free. Radic. Biol. Med. 2014, 71, 240–255. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Strieter, R.M.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Polverini, P.J. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C- chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 1995, 210, 51–57. [Google Scholar]
- Zhang, X.; Shi, H.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 146. [Google Scholar]
- Ohms, M.; Möller, S.; Laskay, T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Scaverlandi, M.V.; Peinetti, N.; Leimgruber, C.; Cuello Rubio, M.M.; Nicola, J.P.; Menezes, G.B.; Maldonado, C.A.; Quintar, A.A. Inefficient N2-Like neutrophils are promoted by androgens during infection. Front. Immunol. 2018, 9, 1980. [Google Scholar]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar]


| . | Author/Year | Objective | Participants | Concept | Context | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Number | Groups | Sex (Female %) | Age | Periodontal Diagnosis Criteria | Interventions or Phenomena of Interest | Samples | Results | Origin | |||
| 1 | Aboodi et al., 2011 [18] | To identify the presence of oral neutrophil hyperactivity among refractory periodontitis patients, and to determine if the hyperactivity is related to a history of periodontal disease severity. | 13 | No | 46.15 | 32 to 73 years | Not specified | Periodontal screening | Venous blood samples | Characterization of high-responder neutrophils in patients with periodontitis, and low-responder neutrophils in healthy subjects. | Refractory Disease Unit, Dental Research Institute, University of Toronto, Canada. |
| Oral rinse sample | |||||||||||
| 2 | Borenstein et al., 2018 [21] | To determine the morphological diversity between different groups of patients, as well as the response induced in naïve neutrophils after incubation with bacteria. | 5 | Health | 40 | 46.1 ± 17.9 | Not specified | None | Blood and oral expectorated samples | Oral neutrophils had lower number of granules per cytoplasm area compared to blood neutrophils. Bacteria-stimulated oral neutrophils were more granular, had more phagosomes than the blood neutrophils. Neutrophils that migrated into the connective tissue have a lighter cytoplasmic density, fewer granules and higher euchromatin fraction. Gingival tissue neutrophils had increased euchromatin/heterochromatin ratio. | Toronto General Hospital 2 Nephrology Center and University of Toronto’s Graduate Periodontology Clinic, Toronto, Canada. |
| 6 | Diseased | 50 | 58.0 ± 15.1 | ||||||||
| 3 | Dutzan et al., 2016 [20] | To characterize the human immunological cell network patrolling the oral barrier in health with a particular focus on the gingival area. | 50 | Health | 70 | 26.5 | PPD, CAL, BOP and dental radiographs | None | Biopsies | An increase was observed in the proportion of CD15+CD16+ neutrophils in gingival biopsies. Additionally, CD15+CD16+ cells increased in gingival tissues affected with periodontitis. | NIH Clinical Center |
| 6 | Diseased | 66.67 | 38.5 | ||||||||
| 4 | Fine et al., 2016 [27] | To demonstrate the presence of para-inflammatory neutrophils in the healthy oral cavity and pro-inflammatory neutrophils in patients with periodontitis. | 11 | Health | 54 | 26 to 84 | PPD, CAL, BOP, and recession | None | Blood and oral samples | Oral neutrophils from patients with periodontitis are in a pro-inflammatory activation state when compared with healthy oral neutrophils. Neutrophils from patients affected with periodontitis were characterized by elevated degranulation, phagocytosis, ROS production, and NETosis. | Toronto General Hospital’s Nephrology Center and the University of Toronto’s Graduate Periodontology Clinic |
| 17 | Diseased | 52 | 26 to 82 | ||||||||
| 5 | Johnstone et al., 2007 [24] | To compare the generation of oxygen radicals in peripheral neutrophils from patients with aggressive, chronic, and periodontally healthy after stimulation with phorbol myristate acetate (PMA). Additionally, to examine the phagocytotic ability of the neutrophils. | 22 | Diseased | 59% | 45.83 | CAL loss despite adequate maintenance therapy | None | Blood samples | Activated neutrophils demonstrated hyperactive NADPH oxidase activity in patients with refractory aggressive periodontitis compared to patients successfully treated and healthy. | Graduate Periodontal Clinic, Faculty of Dentistry, University of Toronto |
| 13 | Healthy | 46.15% | 33.25 | ||||||||
| 6 | Lakschevitz et al., 2013 [19] | To present a rapid method for oral neutrophil isolation and to characterize and compare the neutrophil gene expression profile in the blood and oral compartment of healthy individuals. | 5 | Healthy | 40% | 23 to 38 | PPD, OP, PI. | None | Blood samples | Oral neutrophils expressed TCRs. Neutrophils presented a site-specific gene expression profile in the oral cavity of healthy individuals when compared with blood neutrophils. | Faculty of Dentistry, University of Toronto |
| 7 | Matthews et al., 2006 [16] | To confirm the reported FcgR hyper-reactivity of peripheral neutrophils in chronic periodontitis using more relevant physiological conditions, and to determine whether the ROS could be detected. | 18 | Diseased | 72.2% | 47.2% +/− 6.1 | PPD, BOP, CAL, Radiography | None | Blood sample | Peripheral neutrophils from patients with chronic periodontitis exhibited both hyper-reactivity and hyperactivity. | Birmingham Dental Hospital |
| 8 | Medara et al., 2020 [23] | To assess the longitudinal variation in the expression of the adhesion and activation markers of neutrophils, to determine the neutrophil maturation stage based on CD surface markers, and to evaluate the suppressive neutrophil phenotypes. | 40 | Health | 75% | 49.3% +/− 10.6 | PPD, BOP | Non-surgical periodontal treatment. | Blood sample | PD and BOP correlated with neutrophil subsets. In particular, neutrophils over-expressed CD11b, CD16b, and CD66b, and under-expressed CD62L. | The Royal Dental Hospital of Melbourne and Melbourne Dental Clinic, The University of Melbourne. |
| 54 | Diseased | 63% | 53.28% +/− 11.4 | ||||||||
| 9 | Papantonopoulos et al., 2019 [22] | To investigate in datasets of immunologic parameters from early onset and late-onset periodontitis patients (EOP and LOP), the existence of hidden random fluctuations (anomalies or noise), which may be the source for increased frequencies and longer periods of exacerbation, resulting in rapid progression in EOP. | 18 | Early onset localized periodontitis | 50% | 19.9 +/− 6.5 | Not specified | None | Raw data set | In early-onset periodontitis, there was an increase in IL-1, IL-4, and IFN-γ compared with late-onset periodontitis. CD8, CD20, CD4/CD8 ratio and IL-2 were higher in late-onset periodontitis. Additionally, chemotaxis, phagocytosis, and adhesion of neutrophils were higher in late-onset periodontitis, than early-onset. | Okayama University Dental Hospital |
| 50 | Early onset generalized periodontitis | 43.2% | 28.3 +/− 5.8 | ||||||||
| 43 | Late-onset periodontitis | 65.4% | 47.0 +/− 11.0 | ||||||||
| 10 | Rudin et al., 2020 [29] | To determine whether the CD177+ and CD177− neutrophil subsets differ in their propensity to migrate to both aseptic- and microbe-triggered inflamed human tissues. | Not specified | Buffy coats | CD177+ neutrophil subtype was recruited to inflammatory exudate in periodontitis. Increased levels of CD177+ neutrophils in blood of periodontitis patients were detected, as compared to healthy controls. | Specialist Clinic of Periodontics in Gothenburg, Public Dental Health Services, Region Västra Götaland, Sweden | |||||
| 11 | Thorbert-Mros et al., 2014 [26] | To analyze differences in cell characteristics between lesions representing long- standing gingivitis and severe periodontitis | 28 | Gingivitis | 50% | 59.5% +/− 7.9 | PPD, CAL, BOP, and recession | None | Periodontal tissues biopsies | In periodontitis lesions, there existed an increase in CD138+ and elastase+ cells compared with gingivitis. Elastase+ cells were detected in proximity to the pocket epithelium. | Clinics of Periodontics in Gothenburg and Mölndal, the Clinic for Undergraduate Training in Gothenburg, Public Dental Health Services, Region Västra Götaland and Institute of Odontology |
| 36 | Periodontitis | 47% | 52.3% +/− 9.5 | ||||||||
| 12 | White et al., 2016 [28] | To investigate ex vivo peripheral neutrophil extracellular trap (NET) production and their subsequent degradation by plasma in chronic periodontitis patients, and periodontally and systemically healthy-matched controls. | 40 | Healthy UK | 46 +/− 8 | Not indicated | PPD, CAL, BOP, GI, PI. | Prevention | Blood samples | NET degradation was lower in patients with periodontitis compared with controls. | Specialist Periodontal Centres in Birmingham, UK and Thessaloniki, Greece. |
| Healthy Greece | 50 +/− 11 | ||||||||||
| 40 | Periodontitis UK | 46 +/− 8 | Periodontal treatment | ||||||||
| Periodontitis Greece | 52 +/− 8 | ||||||||||
| 13 | Wright et al., 2008 [16] | To use microarray technology to analyze the gene expression signature of hyperresponsive PBN from periodontitis patients to identify factors potentially important for disease pathogenesis. | 19 | Healthy | 46.4 +/− 5.4 | 73.6% | Previously, reported | None | Blood samples | In patients with periodontitis, there was an increase in the IFN-stimulated genes associated with neutrophils. Additionally, IFN-γ prime neutrophils for ROS generation. | Birmingham Dental Hospital (Birmingham, U.K.) |
| 19 | Periodontitis | 47.2 +/− 6.1 | 73.6% | ||||||||
| 14 | Yagi et al., 2009 [17] | To test the hypothesis that PDK1 regulates chemotaxis in neutrophils and is responsible for the abnormal neutrophil chemotaxis in LAP. | Not specified | None | Blood samples | PDK1 is an absolute requirement for neutrophil chemotaxis. PDK1 is not involved in superoxide production. Thus, inhibition of PDK1 blocked neutrophil migration but not Superoxide production. | Boston University Institutional Review Board, Boston, USA. | ||||
| Primary Outcome | Secondary Outcome | Tertiary Outcome | ||
|---|---|---|---|---|
| Author/Year | To determine the population of neutrophils in the periodontal tissues of patients with or without periodontal disease | To isolate neutrophils from peripheral blood samples and evaluate phenotypes. | To determine the neutrophils phenotype or cytokine analysis in patients with inflammatory diseases | |
| 1 | Aboodi et al., 2011 [18] |  |  | |
| 2 | Borenstein et al., 2018 [21] |  | ||
| 3 | Dutzan et al., 2016 [20] |  |  | |
| 4 | Fine et al., 2016 [27] |  |  | |
| 5 | Johnstone et al., 2007 [24] |  |  |  |
| 6 | Lakschevitz et al., 2013 [19] |  |  | |
| 7 | Matthews et al., 2006 [16] |  |  | |
| 8 | Medara et al., 2020 [23] |  |  |  |
| 9 | Papantonopoulos et al., 2019 [22] |  | ||
| 10 | Rudin et al., 2020 [29] |  |  | |
| 11 | Thorbert-Mros et al., 2014. [26] |  | ||
| 12 | White et al., 2016 [28] |  |  | |
| 13 | Wright et al., 2008 [16] |  |  | |
| 14 | Yagi et al., 2009 [17] |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansores-España, L.D.; Melgar-Rodríguez, S.; Vernal, R.; Carrillo-Ávila, B.A.; Martínez-Aguilar, V.M.; Díaz-Zúñiga, J. Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review. Int. J. Mol. Sci. 2022, 23, 12068. https://doi.org/10.3390/ijms232012068
Sansores-España LD, Melgar-Rodríguez S, Vernal R, Carrillo-Ávila BA, Martínez-Aguilar VM, Díaz-Zúñiga J. Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review. International Journal of Molecular Sciences. 2022; 23(20):12068. https://doi.org/10.3390/ijms232012068
Chicago/Turabian StyleSansores-España, Luis Daniel, Samanta Melgar-Rodríguez, Rolando Vernal, Bertha Arelly Carrillo-Ávila, Víctor Manuel Martínez-Aguilar, and Jaime Díaz-Zúñiga. 2022. "Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review" International Journal of Molecular Sciences 23, no. 20: 12068. https://doi.org/10.3390/ijms232012068
APA StyleSansores-España, L. D., Melgar-Rodríguez, S., Vernal, R., Carrillo-Ávila, B. A., Martínez-Aguilar, V. M., & Díaz-Zúñiga, J. (2022). Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review. International Journal of Molecular Sciences, 23(20), 12068. https://doi.org/10.3390/ijms232012068






