Oxygen–Glucose Deprivation Promoted Fibroblast Senescence and Collagen Expression via IL11
Abstract
:1. Introduction
2. Results
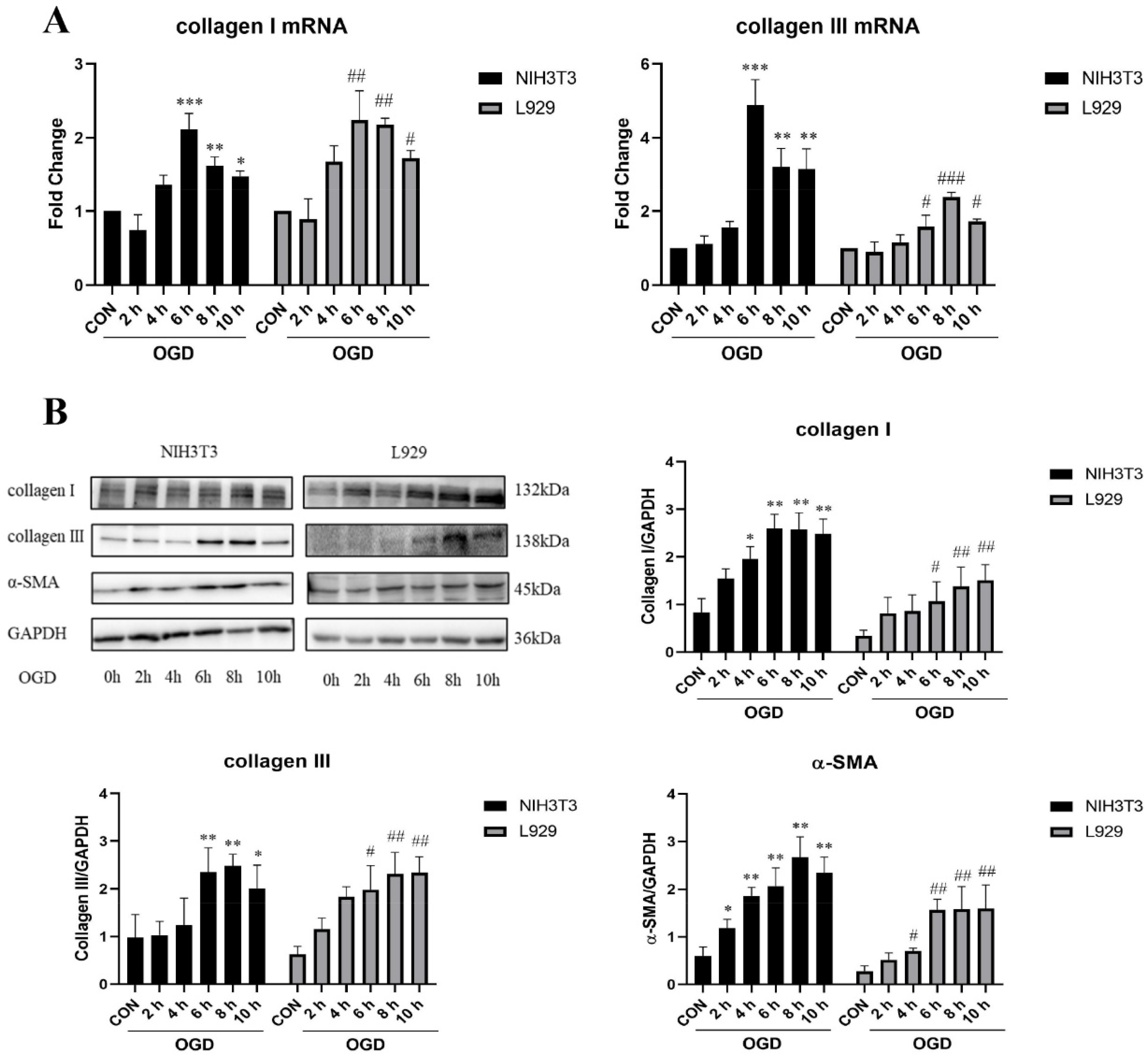
2.1. OGD-Promoted Fibroblast Senescence and Collagen-Related Protein Expression
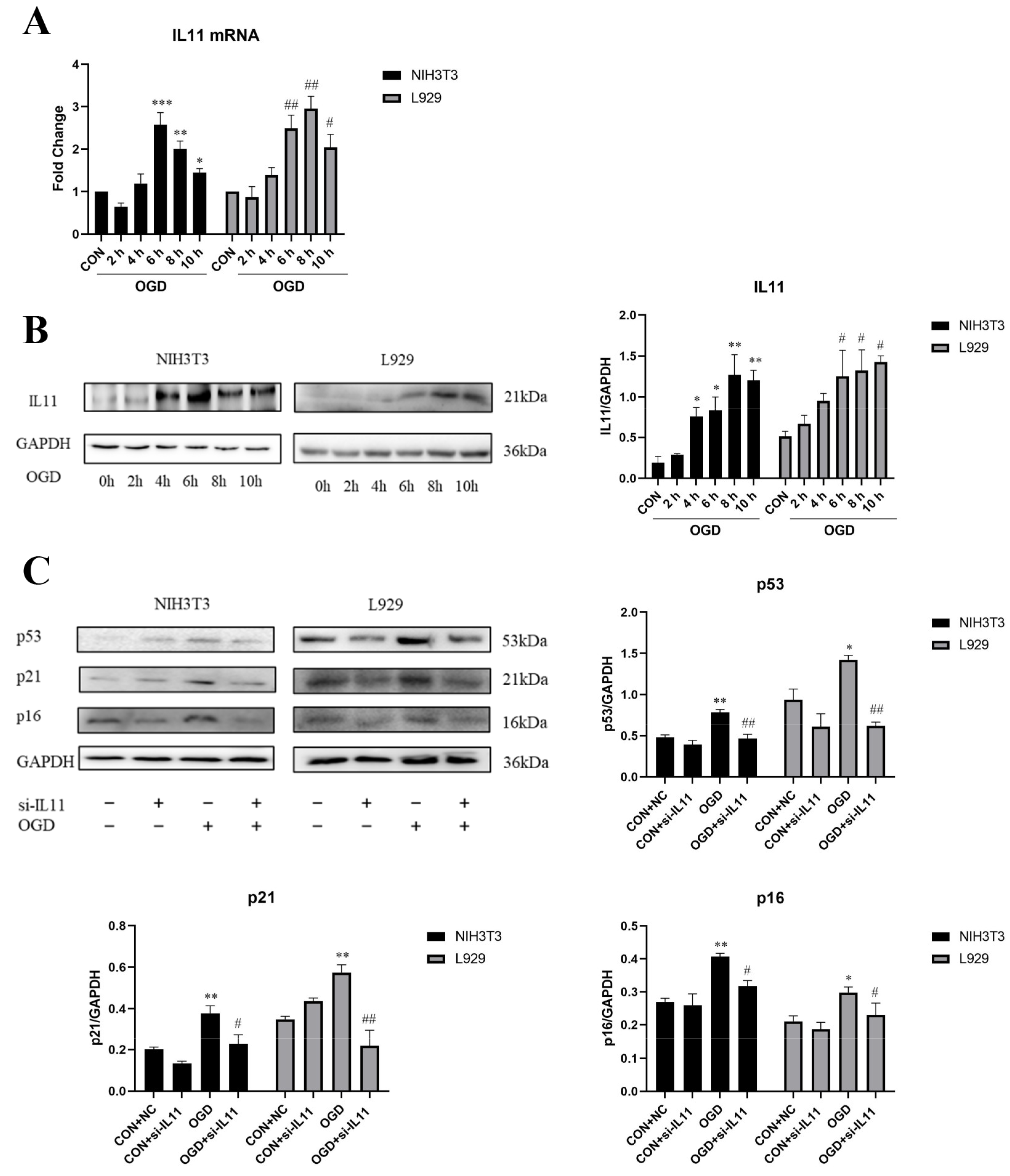
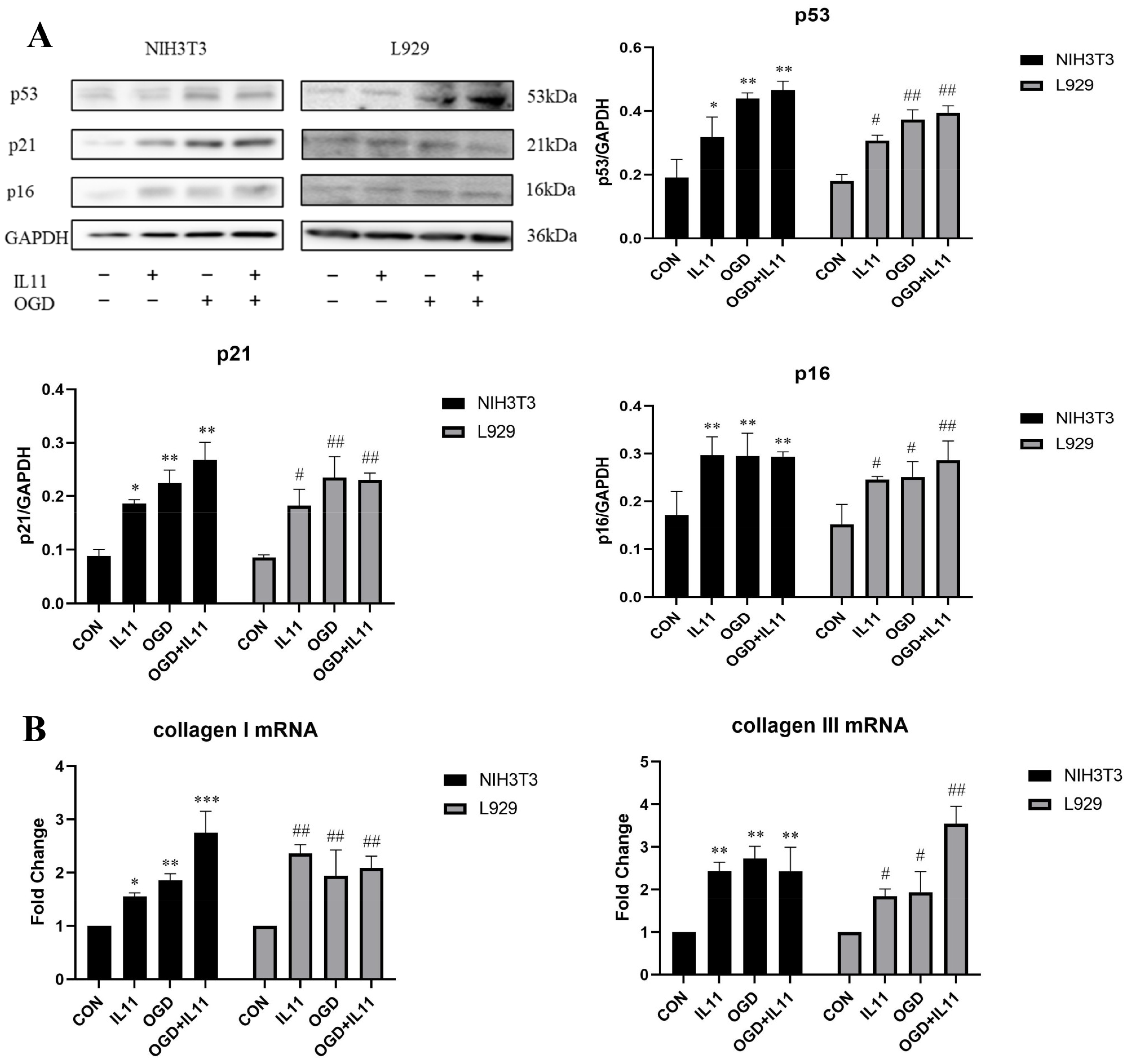
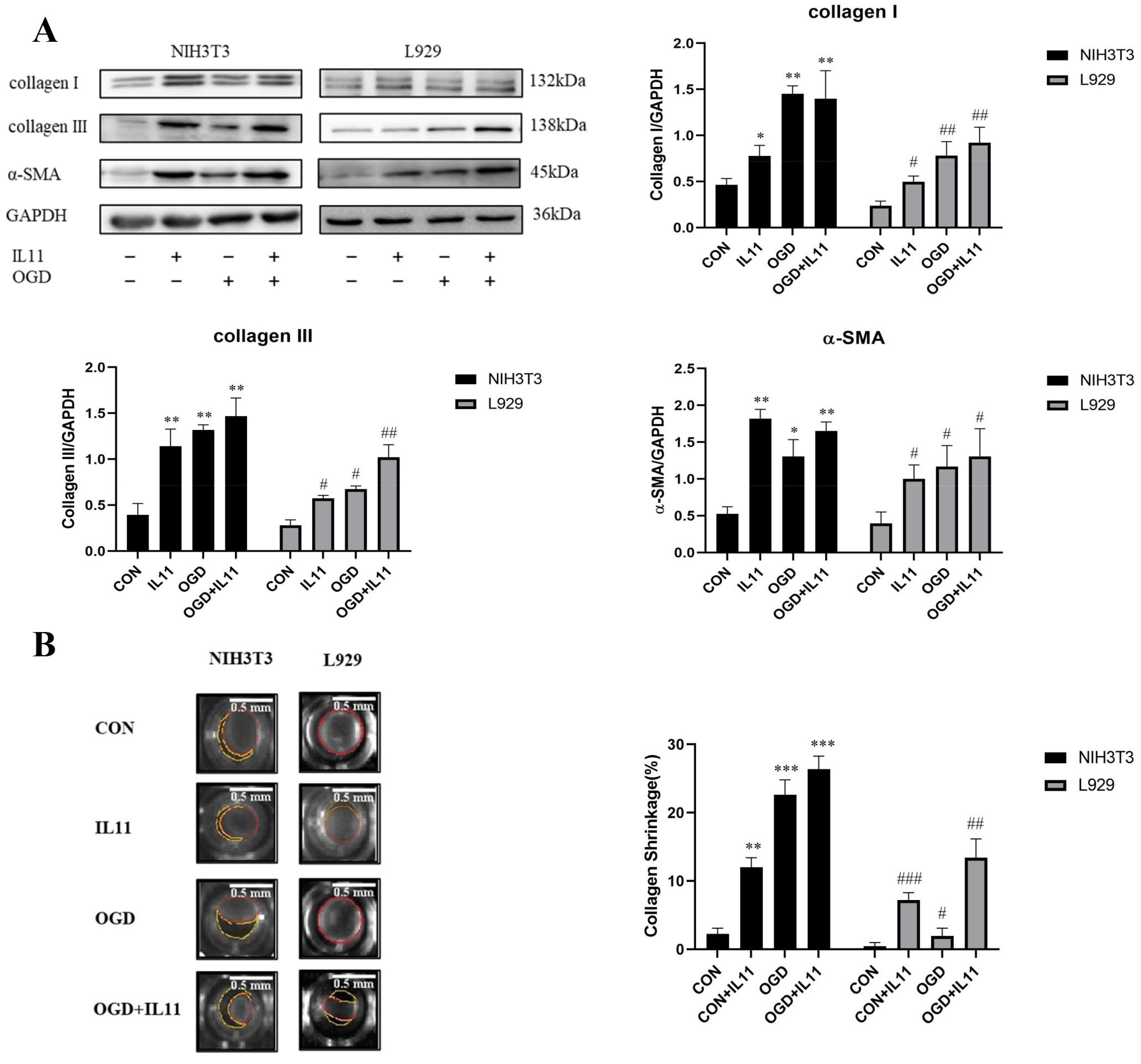
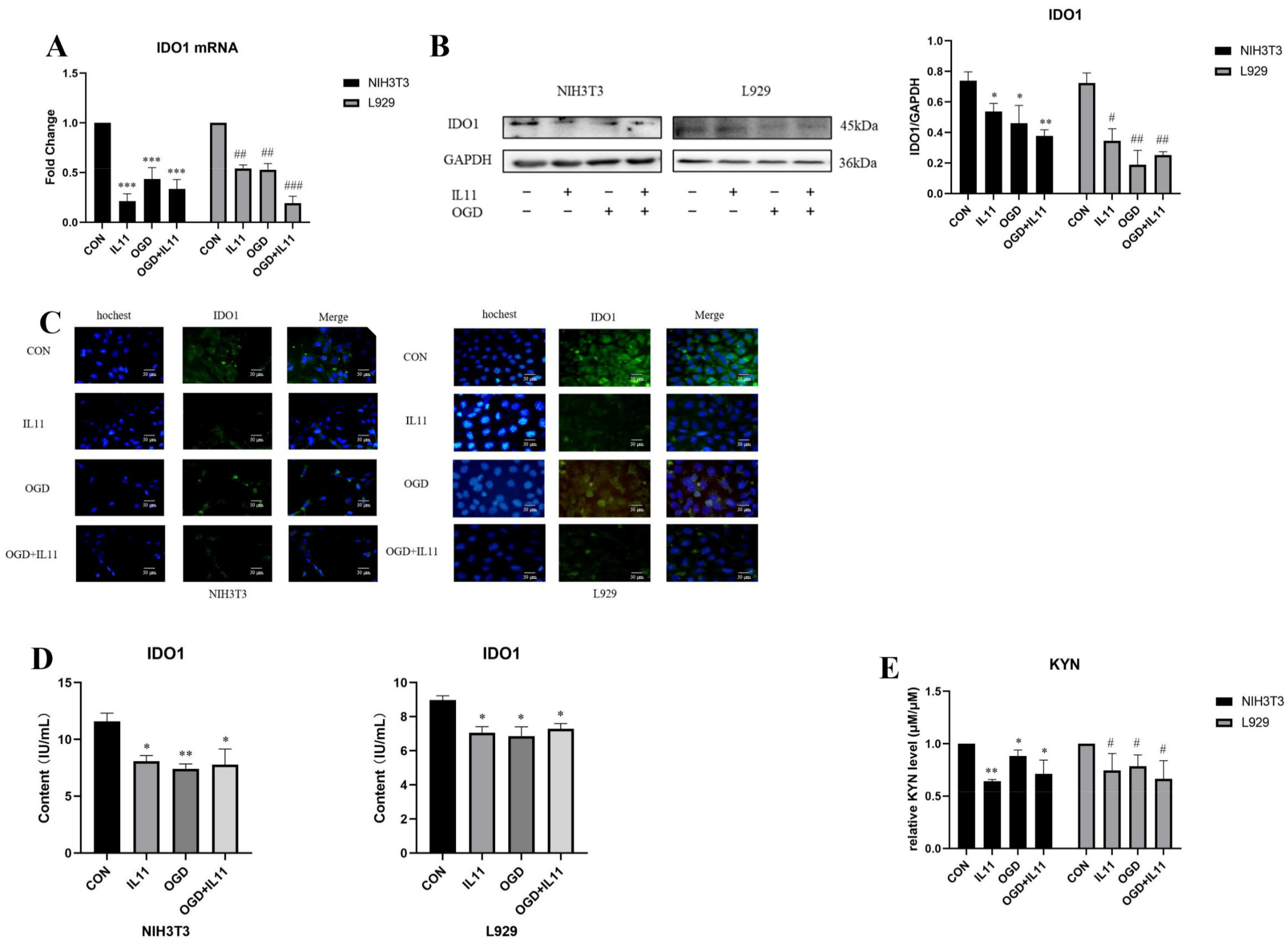
2.2. OGD Promotes Fibroblast Senescence and Collagen Expression through IL11
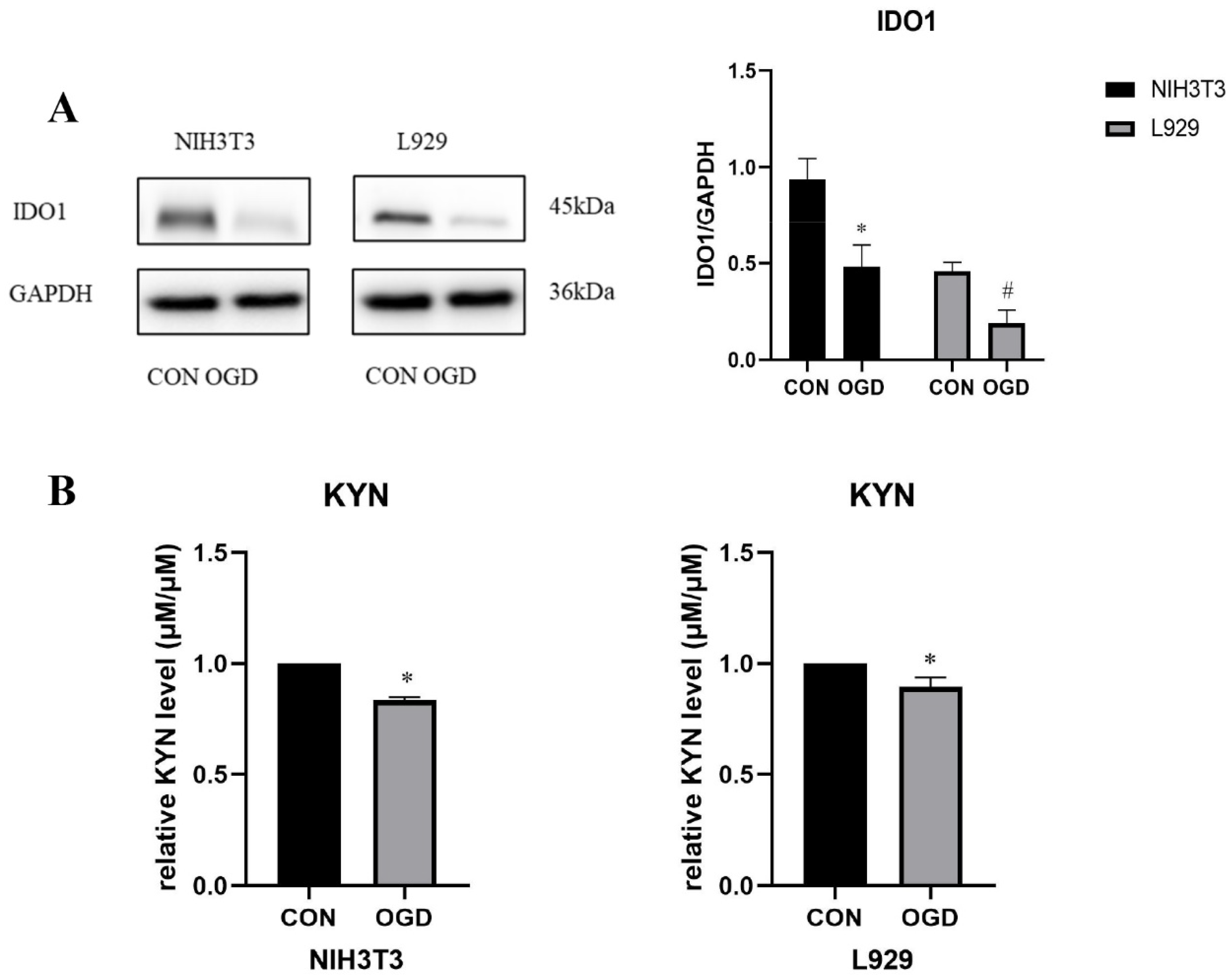
2.3. OGD Inhibits Fibroblast IDO1/KYN
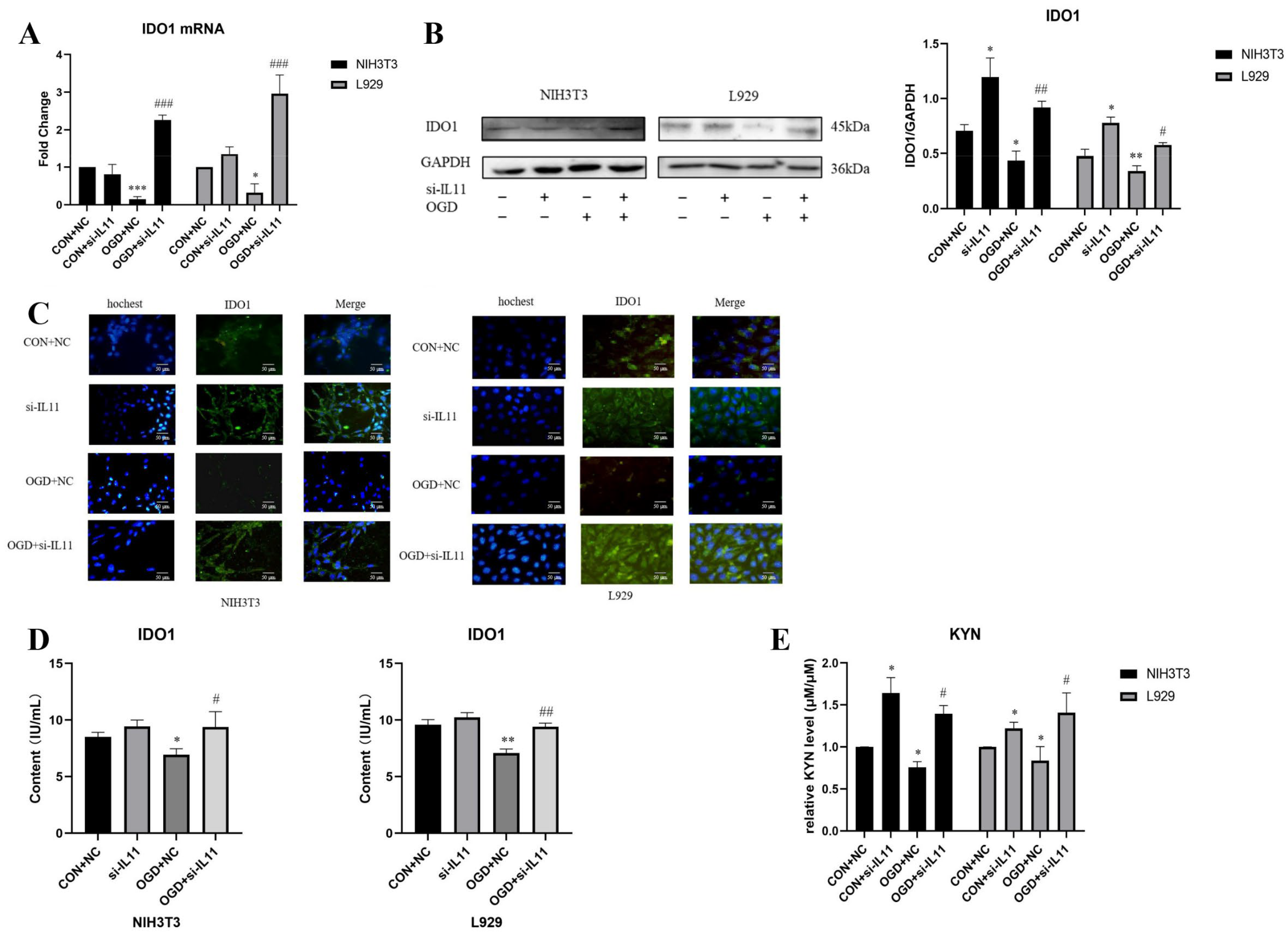
2.4. OGD Inhibits the Expression of IDO1/KYN in Fibroblasts via IL11
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Stimulations
4.2. Antibody and Reagents
4.3. Cell Transfection
4.4. RNA Isolation and Quantitative RT-PCR
4.5. Western Blot
4.6. ELISA
4.7. Collagen Contraction Assay
4.8. Immunofluorescence
4.9. MTT Assay
4.10. Ehrlich Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.S.; Lee, R.T.; Garbern, J.C. Senescence mechanisms and targets in the heart. Cardiovasc. Res. 2022, 118, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Machado-Oliveira, G.; Ramos, C.; Marques, A.R.A.; Vieira, O.V. Cell Senescence, Multiple Organelle Dysfunction and Atherosclerosis. Cells 2020, 9, 2146. [Google Scholar] [CrossRef] [PubMed]
- Notohamiprodjo, S.; Nekolla, S.G.; Robu, S.; Villagran Asiares, A.; Kupatt, C.; Ibrahim, T.; Laugwitz, K.L.; Makowski, M.R.; Schwaiger, M.; Weber, W.A.; et al. Imaging of cardiac fibroblast activation in a patient after acute myocardial infarction using (68)Ga-FAPI-04. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2021. Available online: https://link.springer.com/article/10.1007/s12350-021-02603-z (accessed on 15 April 2021).
- Haider, N.; Boscá, L.; Zandbergen, H.R.; Kovacic, J.C.; Narula, N.; González-Ramos, S.; Fernandez-Velasco, M.; Agrawal, S.; Paz-García, M.; Gupta, S.; et al. Transition of Macrophages to Fibroblast-Like Cells in Healing Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 74, 3124–3135. [Google Scholar] [CrossRef]
- Moodley, Y.P.; Misso, N.L.; Scaffidi, A.K.; Fogel-Petrovic, M.; McAnulty, R.J.; Laurent, G.J.; Thompson, P.J.; Knight, D.A. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am. J. Respir. Cell Mol. Biol. 2003, 29, 490–498. [Google Scholar] [CrossRef]
- Liu, R.M.; Liu, G. Cell senescence and fibrotic lung diseases. Exp. Gerontol. 2020, 132, 110836. [Google Scholar] [CrossRef]
- Viswanathan, S.; Ng, B.; Widjaja, A.A.; Pua, C.J.; Tham, N.; Tan, J.; Cook, S.A.; Schafer, S. Critical Conditions for Studying Interleukin-11 Signaling In Vitro and Avoiding Experimental Artefacts. Curr. Protoc. 2021, 1, e251. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; Viswanathan, S.; Widjaja, A.A.; Paleja, B.S.; Adami, E.; Ko, N.S.J.; Wang, M.; Lim, S.; Tan, J.; et al. Fibroblast-specific IL11 signaling drives chronic inflammation in murine fibrotic lung disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 11802–11815. [Google Scholar] [CrossRef]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 signaling underlies fibrosis, parenchymal dysfunction, and chronic inflammation of the airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Liang, J.; Gu, X.; Zhou, J.; Xie, C.; Lv, X.; Wang, R.; Li, Q.; Mao, Z.; et al. TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp. Mol. Med. 2020, 52, 130–151. [Google Scholar] [CrossRef]
- Pascal, T.; Debacq-Chainiaux, F.; Boilan, E.; Ninane, N.; Raes, M.; Toussaint, O. Heme oxygenase-1 and interleukin-11 are overexpressed in stress-induced premature senescence of human WI-38 fibroblasts induced by tert-butylhydroperoxide and ethanol. Biogerontology 2007, 8, 409–422. [Google Scholar] [CrossRef]
- Begley, L.A.; Kasina, S.; MacDonald, J.; Macoska, J.A. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 2008, 43, 194–199. [Google Scholar] [CrossRef] [Green Version]
- James, E.L.; Michalek, R.D.; Pitiyage, G.N.; de Castro, A.M.; Vignola, K.S.; Jones, J.; Mohney, R.P.; Karoly, E.D.; Prime, S.S.; Parkinson, E.K. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome Res. 2015, 14, 1854–1871. [Google Scholar] [CrossRef]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Zhong, F.M.; Yao, Y.; Deng, S.Q.; Xu, H.Q.; Lu, J.F.; Ruan, M.; Shen, X.C. Synergistic protection of tetramethylpyrazine phosphate and borneol on brain microvascular endothelium cells injured by hypoxia. Am. J. Transl. Res. 2019, 11, 2168–2180. [Google Scholar]
- Chen, C.H.; Hsu, S.Y.; Chiu, C.C.; Leu, S. MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats. Cells 2019, 8, 935. [Google Scholar] [CrossRef] [Green Version]
- Momtaz, S.; Baeeri, M.; Rahimifard, M.; Haghi-Aminjan, H.; Hassani, S.; Abdollahi, M. Manipulation of molecular pathways and senescence hallmarks by natural compounds in fibroblast cells. J. Cell. Biochem. 2019, 120, 6209–6222. [Google Scholar] [CrossRef]
- da Silva, B.T.A.; Peloi, K.E.; Ximenes, V.F.; Nakamura, C.V.; de Oliveira Silva Lautenschlager, S. 2-acetylphenothiazine protects L929 fibroblasts against UVB-induced oxidative damage. J. Photochem. Photobiol. B Biol. 2021, 216, 112130. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Wei Ting, J.G.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8, 740650. [Google Scholar] [CrossRef]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017, 552, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Chothani, S.; Viswanathan, S.; Goh, J.W.T.; Lim, W.W.; Cook, S.A. IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues. Int. J. Mol. Sci. 2022, 23, 8900. [Google Scholar] [CrossRef] [PubMed]
- Dookun, E.; Walaszczyk, A.; Redgrave, R.; Palmowski, P.; Tual-Chalot, S.; Suwana, A.; Chapman, J.; Jirkovsky, E.; Donastorg Sosa, L.; Gill, E.; et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 2020, 19, e13249. [Google Scholar] [CrossRef] [PubMed]
- Nishina, T.; Deguchi, Y.; Ohshima, D.; Takeda, W.; Ohtsuka, M.; Shichino, S.; Ueha, S.; Yamazaki, S.; Kawauchi, M.; Nakamura, E.; et al. Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer. Nat. Commun. 2021, 12, 2281. [Google Scholar] [CrossRef]
- Mitazaki, S.; Kato, N.; Suto, M.; Hiraiwa, K.; Abe, S. Interleukin-6 deficiency accelerates cisplatin-induced acute renal failure but not systemic injury. Toxicology 2009, 265, 115–121. [Google Scholar] [CrossRef]
- Lim, W.W.; Corden, B.; Ye, L.; Viswanathan, S.; Widjaja, A.A.; Xie, C.; Su, L.; Tee, N.G.Z.; Schafer, S.; Cook, S.A. Antibody-mediated neutralization of IL11 signalling reduces ERK activation and cardiac fibrosis in a mouse model of severe pressure overload. Clin. Exp. Pharmacol. Physiol. 2021, 48, 605–613. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Rózsa, E.; Agoston, M.; Szénási, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vécsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Gu, Y.; Hui, W.; Yang, X.; Liu, Y.; Chen, X. Oxygen–Glucose Deprivation Promoted Fibroblast Senescence and Collagen Expression via IL11. Int. J. Mol. Sci. 2022, 23, 12090. https://doi.org/10.3390/ijms232012090
Song T, Gu Y, Hui W, Yang X, Liu Y, Chen X. Oxygen–Glucose Deprivation Promoted Fibroblast Senescence and Collagen Expression via IL11. International Journal of Molecular Sciences. 2022; 23(20):12090. https://doi.org/10.3390/ijms232012090
Chicago/Turabian StyleSong, Tongtong, Yiwen Gu, Wenting Hui, Xiaoyu Yang, Yanqing Liu, and Xia Chen. 2022. "Oxygen–Glucose Deprivation Promoted Fibroblast Senescence and Collagen Expression via IL11" International Journal of Molecular Sciences 23, no. 20: 12090. https://doi.org/10.3390/ijms232012090



