MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression?
Abstract
:1. Overview of Diabetic Retinopathy
1.1. Retinal Neural Dysfunction and Degeneration in DR
1.2. Retinal Vascular Degeneration and Complications in DR
2. Pathogenesis of Diabetes-Induced Degeneration in Retinal Neurons and Microvasculature: Oxidative Stress, Inflammation, and Glutamate Excitotoxicity
2.1. Oxidative Stress in the Diabetic Retina
2.2. Inflammation in the Diabetic Retina
2.3. Glutamate Toxicity in the Diabetic Retina
3. MicroRNAs and DR
3.1. Overview of microRNAs and DR
3.2. Evidence of miRs Involved in Inflammation, Oxidative Stress, and Apoptosis in DR
4. MicroRNA-150 and DR
4.1. Decreased microRNA-150 (miR-150) Is Correlated with the Development of DR
4.2. The Targets of miR-150 in Diabetes
4.3. miR-150 and Inflammation in the Diabetic Retina
4.4. miR-150 and Neural Apoptosis in the Diabetic Retina
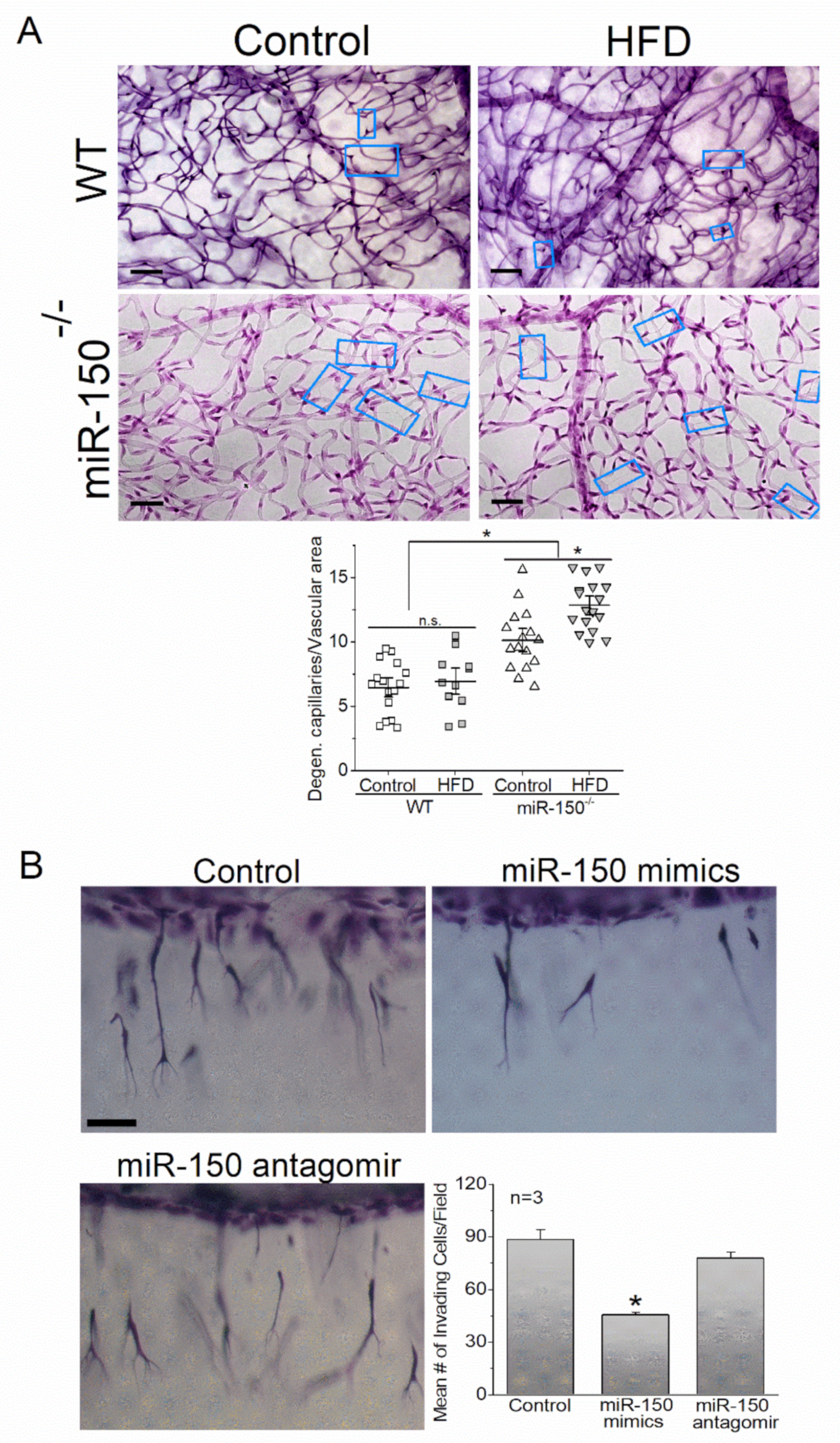
4.5. miR-150 and Angiogenesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Barker, L.E.; Williamson, D.F. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 2010, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelgau, M.M.; Geiss, L.S.; Saaddine, J.B.; Boyle, J.P.; Benjamin, S.M.; Gregg, E.W.; Tierney, E.F.; Rios-Burrows, N.; Mokdad, A.H.; Ford, E.S.; et al. The evolving diabetes burden in the United States. Ann. Intern. Med. 2004, 140, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsegian, A.; Kotlyar, B.; Lee, J.; Salifu, M.O.; McFarlane, S.I. Diabetic Retinopathy: Focus on Minority Populations. Int. J. Clin. Endocrinol. Metab. 2017, 3, 034–045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, A.J. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef]
- Moran, E.P.; Wang, Z.; Chen, J.; Sapieha, P.; Smith, L.E.; Ma, J.X. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H738–H749. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Ma, J.-X. Angiogenesis in diabetes and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Cao, D.; Yang, D.; Zhuang, X.; Hu, Y.; He, M.; Yu, H.; Wang, J.; Yang, C.; Zhang, L. Retinal vasculature–function correlation in non-proliferative diabetic retinopathy. Doc. Ophthalmol. 2020, 140, 129–138. [Google Scholar] [CrossRef]
- Simo, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef]
- Gallemore, R.P.; Nguyen, D. When Anti-VEGF Treatment Fails. Review of Ophthalmology 2008, March. Available online: http://www.reviewofophthalmology.com/content/d/retinal_insider/i/1230/c/23141/ (accessed on 19 May 2019).
- Lux, A.; Llacer, H.; Heussen, F.M.; Joussen, A.M. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br. J. Ophthalmol. 2007, 91, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.; Wong, I.Y.; Wong, T.Y. Ocular anti-VEGF therapy for diabetic retinopathy: Overview of clinical efficacy and evolving applications. Diabetes Care 2014, 37, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, R.V.; Farrell, U.; Banford, D.; Jones, C.; Gregory, J.W.; Butler, G.; Owens, D.R. Visual function in young IDDM patients over 8 years of age. A 4-year longitudinal study. Diabetes Care 1997, 20, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, P.T.; Karschny, L.; Stolpe, V.; Wolf, E.; Berger, M. Color discrimination and accuracy of blood glucose self-monitoring in type I diabetic patients. Diabetes Care 1991, 14, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Sakurai, Y.; Sato, H.; Chihara, E.; Takeuchi, M. Do type 2 diabetes patients without diabetic retinopathy or subjects with impaired fasting glucose have impaired colour vision? The Okubo Color Study Report. Diabet. Med. 2011, 28, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.W.; Bearse, M.A., Jr.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Lecleire-Collet, A.; Audo, I.; Aout, M.; Girmens, J.F.; Sofroni, R.; Erginay, A.; Le Gargasson, J.F.; Mohand-Said, S.; Meas, T.; Guillausseau, P.J.; et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2861–2867. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kamiyama, M.; Nitta, K.; Yamada, T.; Hayasaka, S. Selective reduction of the S cone electroretinogram in diabetes. Br. J. Ophthalmol. 1996, 80, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Tzekov, R.; Arden, G.B. The Electroretinogram in Diabetic Retinopathy. Surv. Ophthalmol. 1999, 44, 53–60. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lopes De Faria, J.M.; Russ, H.; Costa, V.P. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br. J. Ophthalmol. 2002, 86, 725–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dijk, H.W.; Verbraak, F.D.; Stehouwer, M.; Kok, P.H.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vis. Res 2011, 51, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, E.; Hernandez, C.; Miralles, A.; Huguet, P.; Farres, J.; Simo, R. Lower Somatostatin Expression Is an Early Event in Diabetic Retinopathy and Is Associated with Retinal Neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ramírez, M.; Hernández, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; García-Arumí, J.; Simo, R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef] [Green Version]
- El-Asrar, A.M.A.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of Apoptosis Markers in the Retinas of Human Subjects with Diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760. [Google Scholar] [CrossRef]
- Bronson-Castain, K.W.; Bearse, M.A., Jr.; Neuville, J.; Jonasdottir, S.; King-Hooper, B.; Barez, S.; Schneck, M.E.; Adams, A.J. Adolescents with Type 2 diabetes: Early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina 2009, 29, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Tavares Ferreira, J.; Proença, R.; Alves, M.; Dias-Santos, A.; Santos, B.O.; Cunha, J.P.; Papoila, A.L.; Abegão Pinto, L. Retina and Choroid of Diabetic Patients Without Observed Retinal Vascular Changes: A Longitudinal Study. Am. J. Ophthalmol. 2017, 176, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.B.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; Devries, J.H.; Schlingemann, R.O.; Abràmoff, M.D. Early Neurodegeneration in the Retina of Type 2 Diabetic Patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715. [Google Scholar] [CrossRef] [Green Version]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence from Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydkjaer-Olsen, U.; Hansen, R.S.; Peto, T.; Grauslund, J. Structural neurodegeneration correlates with early diabetic retinopathy. Int. Ophthalmol. 2018, 38, 1621–1626. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef] [Green Version]
- Ning, X.; Baoyu, Q.; Yuzhen, L.; Shuli, S.; Reed, E.; Li, Q. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int. J. Mol. Med. 2004, 13, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Y.; Xie, P.; Cheng, H.; Song, Q.; Su, T.; Yuan, S.; Liu, Q. Retinal Neurodegeneration in db/db Mice at the Early Period of Diabetes. J. Ophthalmol. 2015, 2015, 757412. [Google Scholar] [CrossRef] [Green Version]
- Ahmadieh, H.; Behbahani, S.; Safi, S. Continuous wavelet transform analysis of ERG in patients with diabetic retinopathy. Doc. Ophthalmol. 2021, 142, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, R.Y.; Park, W.; Park, Y.G.; Kim, I.B.; Park, Y.H. Electroretinography and retinal microvascular changes in type 2 diabetes. Acta Ophthalmol. 2020, 98, e807–e813. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.W.; Park, S.J.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 2003, 46, 1260–1268. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Jin, Y.; Ji, F.; Sinclair, S.H.; Luo, Y.; Xu, G.; Lu, L.; Dai, W.; Yanoff, M.; et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 732–742. [Google Scholar] [CrossRef] [PubMed]
- McAnany, J.J.; Park, J.C. Cone Photoreceptor Dysfunction in Early-Stage Diabetic Retinopathy: Association Between the Activation Phase of Cone Phototransduction and the Flicker Electroretinogram. Investig. Ophthalmol. Vis. Sci. 2019, 60, 64. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.J.; Chang, J.Y.; Shi, L.; Chang, R.C.; Ko, M.L.; Ko, G.Y. The Effects of Metformin on Obesity-Induced Dysfunctional Retinas. Investig. Ophthalmol. Vis. Sci. 2017, 58, 106–118. [Google Scholar] [CrossRef]
- Karaca, C.; Karaca, Z. Beyond Hyperglycemia, Evidence for Retinal Neurodegeneration in Metabolic Syndrome. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1360–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Énzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, Á.; Németh, J.; Lukáts, Á. Pathologic Alterations of the Outer Retina in Streptozotocin-Induced Diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arden, G.B. The absence of diabetic retinopathy in patients with retinitis pigmentosa: Implications for pathophysiology and possible treatment. Br. J. Ophthalmol. 2001, 85, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahdenranta, J.P.R.; Schlingemann, R.O.; Hagedorn, M.; Stallcup, W.B.; Bucana, C.D.; Sidman, R.L.; Arap, W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc. Natl. Acad. Sci. USA 2001, 98, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tang, J.; Du, Y.; Saadane, A.; Tonade, D.; Samuels, I.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Influence Retinal Vascular Degeneration in Mouse Models of Retinal Degeneration and Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4272–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef]
- Li, W.; Myron, Y.; Liu, X.; Ye, X. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin. Med. J. 1997, 110, 659–663. [Google Scholar] [PubMed]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef] [Green Version]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Tang, J.; Mizutani, M.; Kowluru, R.A.; Nagaraj, R.H.; Romeo, G.; Podesta, F.; Lorenzi, M. Response of Capillary Cell Death to Aminoguanidine Predicts the Development of Retinopathy: Comparison of Diabetes and Galactosemia. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3972–3978. [Google Scholar]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Cai, W.Y.; Suzuma, I.; Pak, J.; Ju, S.T.; Rook, S.L.; Esser, P.; Mitsiades, C.; et al. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J. 2003, 17, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Podestà, F.; Romeo, G.; Liu, W.-H.; Krajewski, S.; Reed, J.C.; Gerhardinger, C.; Lorenzi, M. Bax Is Increased in the Retina of Diabetic Subjects and Is Associated with Pericyte Apoptosis in Vivo and in Vitro. Am. J. Pathol. 2000, 156, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Kobayashi, M.; Kawamura, H.; Li, Q.; Puro, D.G. Enhancement of P2 × 7-Induced Pore Formation and Apoptosis: An Early Effect of Diabetes on the Retinal Microvasculature. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.K.H.; Fung, M.K.L.; Lo, A.C.Y.; Lam, T.T.L.; So, K.F.; Chung, S.S.M.; Chung, S.K. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes 2005, 54, 3119–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, Y.; Krothapalli, P.; Desta, T.; Dipiazza, A.; Roy, S.; Graves, D.T. Diabetes-Enhanced Tumor Necrosis Factor-α Production Promotes Apoptosis and the Loss of Retinal Microvascular Cells in Type 1 and Type 2 Models of Diabetic Retinopathy. Am. J. Pathol. 2008, 172, 1411–1418. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFα Is Required for Late BRB Breakdown in Diabetic Retinopathy, and Its Inhibition Prevents Leukostasis and Protects Vessels and Neurons from Apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, J.T.; Herman, I.M. Microvascular Modifications in Diabetic Retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox. Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Letts, J.A.; Sazanov, L.A. Clarifying the supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017, 24, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Kizub, I.V.; Klymenko, K.I.; Soloviev, A.I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 2014, 174, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Neginskaya, M.A.; Pavlov, E.V.; Sheu, S.S. Electrophysiological properties of the mitochondrial permeability transition pores: Channel diversity and disease implication. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148357. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Usui, Y.; Shibauchi, Y.; Uchino, H.; Goto, H. Increased levels of 8-hydroxydeoxyguanosine in the vitreous of patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2010, 89, e59–e61. [Google Scholar] [CrossRef]
- Kanwar, M.; Chan, P.-S.; Kern, T.S.; Kowluru, R.A. Oxidative Damage in the Retinal Mitochondria of Diabetic Mice: Possible Protection by Superoxide Dismutase. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3805. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Odenbach, S. Effect of Long-Term Administration of-Lipoic Acid on Retinal Capillary Cell Death and the Development of Retinopathy in Diabetic Rats. Diabetes 2004, 53, 3233–3238. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Diabetic retinopathy and dysregulated innate immunity. Vis. Res. 2017, 139, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Su, S.B. Neuroinflammatory responses in diabetic retinopathy. J. NeuroInflamm. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulfaul, K.; Ozaki, E.; Fernando, N.; Brennan, K.; Chirco, K.R.; Connolly, E.; Greene, C.; Maminishkis, A.; Salomon, R.G.; Linetsky, M.; et al. Toll-like Receptor 2 Facilitates Oxidative Damage-Induced Retinal Degeneration. Cell Rep. 2020, 30, 2209–2224.e2205. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Wang, P.-W.; Yang, I.H.; Huang, H.-M.; Chang, C.-S.; Wu, C.-L.; Chuang, J.-H. High-Fat Diet Induces Toll-Like Receptor 4-Dependent Macrophage/Microglial Cell Activation and Retinal Impairment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3041. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, J.G.; Cardona, S.M.; Pouw, C.E.; Muniz, A.; Mendiola, A.S.; Tsin, A.T.C.; Allen, D.M.; Cardona, A.E. The Role of Microglia in Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 705783. [Google Scholar] [CrossRef] [Green Version]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-Induced Reactive Oxygen Species Toxicity to Endothelial Cells Is Dependent on Paracrine Mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Esselman, W.J.; Jump, D.B.; Busik, J.V. Anti-inflammatory Effect of Docosahexaenoic Acid on Cytokine-Induced Adhesion Molecule Expression in Human Retinal Vascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4342. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.A.; Mohr, S. Inhibition of Caspase-1/Interleukin-1 Signaling Prevents Degeneration of Retinal Capillaries in Diabetes and Galactosemia. Diabetes 2007, 56, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Joussen, A.M.; Doehmen, S.; Le, M.L.; Koizumi, K.; Radetzky, S.; Krohne, T.U.; Poulaki, V.; Semkova, I.; Kociok, N. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol. Vis. 2009, 15, 1418–1428. [Google Scholar] [PubMed]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.-A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J. Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Syeda, S.; Patel, A.K.; Lee, T.; Hackam, A.S. Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88. Exp. Neurol. 2015, 267, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.S.; Huang, Q.; Gurel, Z.; Sorenson, C.M.; Sheibani, N. High Glucose Alters Retinal Astrocytes Phenotype through Increased Production of Inflammatory Cytokines and Oxidative Stress. PLoS ONE 2014, 9, e103148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Luo, J. Role of MCP-1 and CCR2 in alcohol neurotoxicity. Pharmacol. Res. 2019, 139, 360–366. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Costa, M.B.S.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of Acute-Phase Response Proteins in Retinal Muüller Cells in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, F.; Xiong, H.; Hu, D.N.; Limb, G.A.; Xie, T.; Peng, L.; Zhang, P.; Wei, Y.; Zhang, W.; et al. IL-1beta induces IL-6 production in retinal Muller cells predominantly through the activation of p38 MAPK/NF-kappaB signaling pathway. Exp. Cell Res. 2015, 331, 223–231. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, J.J.; Gao, G.; Shao, C.; Mott, R.; Ma, J.X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006, 20, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhang, X.-M.; Liu, J.-J.; Dong, L.; Feng, Z.-L. Effect of high glucose concentration on VEGF and PEDF expression in cultured retinal Müller cells. Mol. Biol. Rep. 2009, 36, 2147–2151. [Google Scholar] [CrossRef]
- Lange, J.; Yafai, Y.; Reichenbach, A.; Wiedemann, P.; Eichler, W. Regulation of Pigment Epithelium–Derived Factor Production and Release by Retinal Glial (Muüller) Cells under Hypoxia. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5161. [Google Scholar] [CrossRef] [Green Version]
- Le, Y.Z. VEGF production and signaling in Muller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis. Res. 2017, 139, 108–114. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, M.; Le, Y.Z. Functions of Muller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J. Diabetes 2015, 6, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W. Glutamate receptors and circuits in the vertebrate retina. Prog. Retin. Eye Res. 1999, 18, 765–810. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Ambati, J. Elevated γ-Aminobutyric Acid, Glutamate, and Vascular Endothelial Growth Factor Levels in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Arch. Ophthalmol. 1997, 115, 1161. [Google Scholar] [CrossRef]
- Santiago, A.R.; Hughes, J.M.; Kamphuis, W.; Schlingemann, R.O.; Ambrósio, A.F. Diabetes changes ionotropic glutamate receptor subunit expression level in the human retina. Brain Res. 2008, 1198, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Zhao, S.; Zhang, J.; Sun, T.; Fan, Y.; Zheng, Z. High Glucose-Induced TRPC6 Channel Activation Decreases Glutamate Uptake in Rat Retinal Muller Cells. Front. Pharm. 2019, 10, 1668. [Google Scholar] [CrossRef]
- Li, Q.; Puro, D.G. Diabetes-Induced Dysfunction of the Glutamate Transporter in Retinal Muüller Cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Rao, V.R.; Finkbeiner, S. NMDA and AMPA receptors: Old channels, new tricks. Trends Neurosci. 2007, 30, 284–291. [Google Scholar] [CrossRef]
- Ureshino, R.P.; Erustes, A.G.; Bassani, T.B.; Wachilewski, P.; Guarache, G.C.; Nascimento, A.C.; Costa, A.J.; Smaili, S.S.; da Silva Pereira, G.J. The Interplay between Ca2+ Signaling Pathways and Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 6004. [Google Scholar]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S. microRNA expression in the eyes and their significance in relation to functions. Prog. Retin. Eye Res. 2009, 28, 87–116. [Google Scholar] [CrossRef]
- Humphreys, D.T.; Westman, B.J.; Martin, D.I.; Preiss, T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. USA 2005, 102, 16961–16966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathonnet, G.; Fabian, M.R.; Svitkin, Y.V.; Parsyan, A.; Huck, L.; Murata, T.; Biffo, S.; Merrick, W.C.; Darzynkiewicz, E.; Pillai, R.S.; et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 2007, 317, 1764–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semler, B.L.; Waterman, M.L. IRES-mediated pathways to polysomes: Nuclear versus cytoplasmic routes. Trends Microbiol. 2008, 16, 1–5. [Google Scholar] [CrossRef]
- Aitken, C.E.; Lorsch, J.R. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 2012, 19, 568–576. [Google Scholar] [CrossRef]
- Nottrott, S.; Simard, M.J.; Richter, J.D. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 2006, 13, 1108–1114. [Google Scholar] [CrossRef]
- Vislovukh, A.; Kratassiouk, G.; Porto, E.; Gralievska, N.; Beldiman, C.; Pinna, G.; El’skaya, A.; Harel-Bellan, A.; Negrutskii, B.; Groisman, I. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor eEF1 is a target of miR-663 and miR-744. Br. J. Cancer. 2013, 108, 2304–2311. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.D.; Coller, J. Pausing on Polyribosomes: Make Way for Elongation in Translational Control. Cell 2015, 163, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W.; Tseng, A.; Chang, R.C.; Wang, H.; Lin, Y.L.; Kanameni, S.; Brehm, T.; Morin, A.; Jones, B.; Splawn, T.; et al. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci. Rep. 2016, 6, 20176. [Google Scholar]
- Joglekar, M.V.; Januszewski, A.S.; Jenkins, A.J.; Hardikar, A.A. Circulating microRNA Biomarkers of Diabetic Retinopathy. Diabetes 2016, 65, 22–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Su, G. Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci. Rep. 2017, 37, BSR20171157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, C.; Muraleedharan, C.K.; O’Donnell, J.J., 3rd; Singh, P.K.; Lum, H.; Kumar, A.; Xu, S. MicroRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4944–4951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Muraleedharan, C.K.; Xu, S. Intraocular Delivery of miR-146 Inhibits Diabetes-Induced Retinal Functional Defects in Diabetic Rat Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1646–1655. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Chang, J.; Zhang, G.; Fang, Y. MicroRNAs in type 1 diabetes: New research progress and potential directions. Biochem. Cell Biol. 2018, 96, 498–506. [Google Scholar] [CrossRef]
- Barutta, F.; Bruno, G.; Matullo, G.; Chaturvedi, N.; Grimaldi, S.; Schalkwijk, C.; Stehouwer, C.D.; Fuller, J.H.; Gruden, G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta. Diabetol. 2017, 54, 133–139. [Google Scholar] [CrossRef]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaker, O.G.; Abdelaleem, O.O.; Mahmoud, R.H.; Abdelghaffar, N.K.; Ahmed, T.I.; Said, O.M.; Zaki, O.M. Diagnostic and prognostic role of serum miR-20b, miR-17–3p, HOTAIR, and MALAT1 in diabetic retinopathy. IUBMB Life 2019, 71, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Mastrocola, R.; Bruno, G.; Gruden, G. MicroRNA and Microvascular Complications of Diabetes. Int. J. Endocrinol. 2018, 2018, 6890501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yang, Y.; Feng, Z. Suppression of microRNA-495 alleviates high-glucose-induced retinal ganglion cell apoptosis by regulating Notch/PTEN/Akt signaling. Biomed. Pharm. 2018, 106, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, X.; Zhang, Q.Q.; Zhang, Y.Y.; Chu, Z.Y.; Zhang, J.L.; Ren, Q. MicroRNA-93–5p participates in type 2 diabetic retinopathy through targeting Sirt1. Int. Ophthalmol. 2021, 41, 3837–3848. [Google Scholar] [CrossRef]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.S.; Yang, Y.; Moran, E.; Ma, J.X. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARalpha. Diabetes 2017, 66, 1671–1682. [Google Scholar] [CrossRef] [Green Version]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Hui, Y.; Yin, Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-kappaB signaling. Life Sci. 2018, 207, 212–218. [Google Scholar] [CrossRef]
- Kanwar, M.; Kowluru, R.A. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes 2009, 58, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Kowluru, R.A. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes 2011, 60, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Mortuza, R.; Feng, B.; Chakrabarti, S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 2014, 57, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Mensa, E.; Giuliani, A.; Matacchione, G.; Gurau, F.; Bonfigli, A.R.; Romagnoli, F.; De Luca, M.; Sabbatinelli, J.; Olivieri, F. Circulating miR-146a in healthy aging and type 2 diabetes: Age- and gender-specific trajectories. Mech. Ageing Dev. 2019, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.A.; Steinle, J.J. miR-146a Attenuates Inflammatory Pathways Mediated by TLR4/NF-kappaB and TNFalpha to Protect Primary Human Retinal Microvascular Endothelial Cells Grown in High Glucose. Mediat. Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roganovic, J. Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med. Hypotheses 2021, 146, 110448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Navitskaya, S.; Chakravarthy, H.; Huang, C.; Kady, N.; Lydic, T.A.; Chen, Y.E.; Yin, K.J.; Powell, F.L.; Martin, P.M.; et al. Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy. EBioMedicine 2016, 11, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Du, S.; Lv, Y.; Wang, W.; Zhang, F. Elevated microRNA-20b-3p and reduced thioredoxin-interacting protein ameliorate diabetic retinopathy progression by suppressing the NLRP3 inflammasomes. IUBMB Life 2020, 72, 1433–1448. [Google Scholar] [CrossRef]
- Yury, O.; Lopez, N.; Garufi, G.; Seyhan, A. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol. BioSyst. 2017, 13, 106–121. [Google Scholar]
- Assmann, T.S.; Recamonde-Mendoza, M.; De Souza, B.M.; Crispim, D. MicroRNA expression profiles and type 1 diabetes mellitus: Systematic review and bioinformatic analysis. Endocr. Connect. 2017, 6, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Gu, Y.; Xu, N.; Zhang, M.; Yang, T. Decreased expression of miR-150, miR146a and miR424 in type 1 diabetic patients: Association with ongoing islet autoimmunity. Biochem. Biophys. Res. Commun. 2018, 498, 382–387. [Google Scholar] [CrossRef]
- Shi, L.; Kim, A.J.; Chang, R.C.; Chang, J.Y.; Ying, W.; Ko, M.L.; Zhou, B.; Ko, G.Y. Deletion of miR-150 Exacerbates Retinal Vascular Overgrowth in High-Fat-Diet Induced Diabetic Mice. PLoS ONE 2016, 11, e0157543. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Chapman, S.; Pham, D.L.; Ko, M.L.; Zhou, B.; Ko, G.Y. Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res. Care 2020, 8, e001446. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhou, B.; Su, H.; Liu, Y.; Du, C. miR-150 regulates high glucose-induced cardiomyocyte hypertrophy by targeting the transcriptional co-activator p300. Exp. Cell Res. 2013, 319, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, X.; Xie, B.; Chen, Y.; Swaim, M.; Hackett, S.F.; Campochiaro, P.A. MicroRNAs regulate ocular neovascularization. Mol. Ther. 2008, 16, 1208–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yao, K.; Guo, J.; Shi, H.; Ma, L.; Wang, Q.; Liu, H.; Gao, W.; Sun, A.; Zou, Y.; et al. miR-181a and miR-150 regulate dendritic cell immune inflammatory responses and cardiomyocyte apoptosis via targeting JAK1-STAT1/c-Fos pathway. J. Cell Mol. Med. 2017, 21, 2884–2895. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Guo, W.L.; Chen, X.M. Overexpressing microRNA-150 attenuates hypoxia-induced human cardiomyocyte cell apoptosis by targeting glucose-regulated protein-94. Mol. Med. Rep. 2018, 17, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Linsenmeier, R.A.; Zhang, H.F. Retinal oxygen: From animals to humans. Prog. Retin. Eye Res. 2017, 58, 115–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, W.S.; McElhatten, R.M.; Messina, J.E.; Harris, N.R. Hypoxia and the expression of HIF-1alpha and HIF-2alpha in the retina of streptozotocin-injected mice and rats. Exp. Eye Res. 2010, 90, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Sun, Y.; Li, J.; Gong, Y.; Tian, K.T.; Evans, L.P.; Morss, P.C.; Fredrick, T.W.; Saba, N.J.; Chen, J. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc. Natl. Acad. Sci. USA 2015, 112, 12163–12168. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yan, L.N.; Sui, Z. MicroRNA-150 affects endoplasmic reticulum stress via MALAT1-miR-150 axis-mediated NF-kappaB pathway in LPS-challenged HUVECs and septic mice. Life Sci. 2021, 265, 118744. [Google Scholar] [CrossRef]
- Ruvkun, G. The perfect storm of tiny RNAs. Nat. Med. 2008, 14, 1041–1045. [Google Scholar] [CrossRef]
- Agrawal, S.; Chaqour, B. MicroRNA signature and function in retinal neovascularization. World J. Biol. Chem. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xing, W.; Gong, F.; Wang, W.; Yan, Y.; Zhang, Y.; Xie, C.; Fu, S. MiR-150–5p retards the progression of myocardial fibrosis by targeting EGR1. Cell Cycle 2019, 18, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Huang, S.; Shen, L.; Tang, Y.; Li, H.; Shi, Y. Long noncoding RNA NONHSAG053901 promotes diabetic nephropathy via stimulating Egr-1/TGF-beta-mediated renal inflammation. J. Cell Physiol. 2019, 234, 18492–18503. [Google Scholar] [CrossRef]
- Zha, F.; Qu, X.; Tang, B.; Li, J.; Wang, Y.; Zheng, P.; Ji, T.; Zhu, C.; Bai, S. Long non-coding RNA MEG3 promotes fibrosis and inflammatory response in diabetic nephropathy via miR-181a/Egr-1/TLR4 axis. Aging 2019, 11, 3716–3730. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, X.; Kong, C.; Zhu, Y.; Li, H.; Pan, D.; Zhang, X.; Liu, Y.; Yin, F.; Qin, H. c-Myb promotes growth and metastasis of colorectal cancer through c-fos-induced epithelial-mesenchymal transition. Cancer Sci. 2019, 110, 3183–3196. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jia, K.Y.; Zhang, H.L.; Fu, J. MiR-195 enhances cardiomyocyte apoptosis induced by hypoxia/reoxygenation injury via downregulating c-myb. Eur. Rev. Med. Pharm. Sci. 2016, 20, 3410–3416. [Google Scholar]
- Barrett, L.E.; Van Bockstaele, E.J.; Sul, J.Y.; Takano, H.; Haydon, P.G.; Eberwine, J.H. Elk-1 associates with the mitochondrial permeability transition pore complex in neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 5155–5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, L.E.; Sul, J.Y.; Takano, H.; Van Bockstaele, E.J.; Haydon, P.G.; Eberwine, J.H. Region-directed phototransfection reveals the functional significance of a dendritically synthesized transcription factor. Nat. Methods 2006, 3, 455–460. [Google Scholar] [CrossRef]
- Lu, Z.; Miao, Z.; Zhu, J.; Zhu, G. ETS-domain containing protein (Elk1) suppression protects cortical neurons against oxygen-glucose deprivation injury. Exp. Cell Res. 2018, 371, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, S.H.; Choi, H.S.; Rhee, Y.; Lim, S.K. Fibroblast growth factor 2-induced cytoplasmic asparaginyl-tRNA synthetase promotes survival of osteoblasts by regulating anti-apoptotic PI3K/Akt signaling. Bone 2009, 45, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef]
- Yoon, T.M.; Kim, S.A.; Lee, D.H.; Lee, J.K.; Park, Y.L.; Lee, K.H.; Chung, I.J.; Joo, Y.E.; Lim, S.C. EGR1 regulates radiation-induced apoptosis in head and neck squamous cell carcinoma. Oncol. Rep. 2015, 33, 1717–1722. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Sakamoto, H.; Shimoda, Y.; Fujimoto, T.; Nishikawa, S.; Ogawa, M. Over-expression of c-Myb increases the frequency of hemogenic precursors in the endothelial cell population. Genes Cells 2006, 11, 859–870. [Google Scholar] [CrossRef]
- Nagarajan, R.; Svaren, J.; Le, N.; Araki, T.; Watson, M.; Milbrandt, J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 2001, 30, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Joseph, L.J.; Le Beau, M.M.; Jamieson, G.A., Jr.; Acharya, S.; Shows, T.B.; Rowley, J.D.; Sukhatme, V.P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with “zinc-binding finger” structure. Proc. Natl. Acad. Sci. USA 1988, 85, 7164–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell Immunol. 2013, 11 (Suppl. 1), 1–12. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Wong, T.Y.; Simo, R. Vascular endothelial growth factor and diabetic complications. Prog. Retin Eye Res. 2008, 27, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.N.; Alfaro, D.V., 3rd; Kerrison, J.B.; Jablon, E.P. Diabetic retinopathy and angiogenesis. Curr. Diabetes Rev. 2009, 5, 8–13. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research, N. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA. Ophthalmol. 2013, 131, 283–293. [Google Scholar]
- Patel, N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 222–229. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Tonade, D.; Liu, H.; Kern, T.S. Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Tonade, D.; Liu, H.; Palczewski, K.; Kern, T.S. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 2017, 60, 2111–2120. [Google Scholar] [CrossRef] [Green Version]
- Mima, A.; Qi, W.; Hiraoka-Yamomoto, J.; Park, K.; Matsumoto, M.; Kitada, M.; Li, Q.; Mizutani, K.; Yu, E.; Shimada, T.; et al. Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8424–8432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ola, M.S.; Nawaz, M.I.; Siddiquei, M.M.; Al-Amro, S.; Abu El-Asrar, A.M. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complicat. 2012, 26, 56–64. [Google Scholar] [CrossRef]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis. Model Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Alibrahim, E.; Islam, F.M.; Klein, R.; Klein, B.E.; Cotch, M.F.; Shea, S.; Wong, T.Y. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: The multi-ethnic study of atherosclerosis. Diabetes Care 2009, 32, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.S.; Tai, E.S.; Mitchell, P.; Wang, J.J.; Tay, W.T.; Lamoureux, E.; Wong, T.Y. C-reactive protein, body mass index, and diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Roca, M.; Molins, B.; Sala-Puigdollers, A.; Matas, J.; Vinagre, I.; Rios, J.; Adan, A. Peripheral blood metabolic and inflammatory factors as biomarkers to ocular findings in diabetic macular edema. PLoS ONE 2017, 12, e0173865. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Simo, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO68–BIO75. [Google Scholar] [CrossRef]
- Arden, G.B.; Wolf, J.E.; Tsang, Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vis. Res. 1998, 38, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, P., Jr.; Landers, M.B., 3rd; Wolbarsht, M. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. Am. J. Ophthalmol. 1984, 97, 788–789. [Google Scholar] [CrossRef]
- Landers, M.B., 3rd; Stefansson, E.; Wolbarsht, M. Panretinal photocoagulation and retinal oxygenation. Retina 1982, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- de Gooyer, T.E.; Stevenson, K.A.; Humphries, P.; Simpson, D.A.; Gardiner, T.A.; Stitt, A.W. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5561–5568. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [Green Version]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2017, 13, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-kappaB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018, 144, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Li, M.X. MicroRNA-150 protects against cigarette smoke-induced lung inflammation and airway epithelial cell apoptosis through repressing p53: MicroRNA-150 in CS-induced lung inflammation. Hum. Exp. Toxicol. 2018, 37, 920–928. [Google Scholar] [CrossRef]
- Bousquet, M.; Zhuang, G.; Meng, C.; Ying, W.; Cheruku, P.S.; Shie, A.T.; Wang, S.; Ge, G.; Wong, P.; Wang, G.; et al. miR-150 blocks MLL-AF9-associated leukemia through oncogene repression. Mol. Cancer Res. 2013, 11, 912–922. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc. Natl. Acad. Sci. USA 2007, 104, 7080–7085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. Metazoan MiroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Ko, M.L.; Ko, G.Y. Decreased MicroRNA-150 Exacerbates Neuronal Apoptosis in the Diabetic Retina. Biomedicines 2021, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Kasza, A.W.P.; Horwacik, I.; Tymoszuk, P.; Mizgalska, D.; Palmer, K.; Rokita, H.; Sharrocks, A.D.; Jura, J. Transcription factors Elk-1 and SRF are engaged in IL1-dependent regulation of ZC3H12A expression. BMC Mol. Biol. 2010, 11, 14. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, W.N.; Hou, W.C.; Hsiao, L.D.; Yang, C.M. Endothelin-1 induces VCAM-1 expression-mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L211–L225. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Bao, S.; Liu, T.; Wei, L.; Wang, D.; Ye, W.; Wang, N.; Song, S.; Li, J.; Chudhary, M.; et al. Sulforaphane delays diabetes-induced retinal photoreceptor cell degeneration. Cell Tissue Res. 2020, 382, 477–486. [Google Scholar] [CrossRef]
- Sharma, A.; Callahan, L.M.; Sul, J.Y.; Kim, T.K.; Barrett, L.; Kim, M.; Powers, J.M.; Federoff, H.; Eberwine, J. A neurotoxic phosphoform of Elk-1 associates with inclusions from multiple neurodegenerative diseases. PLoS ONE 2010, 5, e9002. [Google Scholar] [CrossRef]
- Halestrap, A.P.M.G.; Clarke, S.J. The permeability transition pore complex: Another view. Biochimie 2002, 84, 14. [Google Scholar] [CrossRef]
- Lavaur, J.; Bernard, F.; Trifilieff, P.; Pascoli, V.; Kappes, V.; Pages, C.; Vanhoutte, P.; Caboche, J. A TAT-DEF-Elk-1 peptide regulates the cytonuclear trafficking of Elk-1 and controls cytoskeleton dynamics. J. Neurosci. 2007, 27, 14448–14458. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Mosteller, R.D.; Broek, D. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Mol. Cell. Biol. 2004, 24, 7359–7369. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Wesolowski, E.; Smith, L.E. Effect of light on oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994, 35, 112–119. [Google Scholar]
- Yu, F.; Ko, M.L.; Ko, G.Y. MicroRNA-150 and its target ETS-domain transcription factor 1 contribute to inflammation in diabetic photoreceptors. J. Cell Mol. Med. 2021, 25, 10724–10735. [Google Scholar] [CrossRef] [PubMed]
- Maric, G.; Rose, A.A.; Annis, M.G.; Siegel, P.M. Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer. Onco. Targets 2013, 6, 839–852. [Google Scholar]
- Becker, P.M.; Waltenberger, J.; Yachechko, R.; Mirzapoiazova, T.; Sham, J.S.; Lee, C.G.; Elias, J.A.; Verin, A.D. Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circ. Res. 2005, 96, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasaraju, T.; Shukla, D.; More, S.; Huang, C.; Zhang, L.; Xiao, X.; Liu, L. Role of microRNA-150 and glycoprotein nonmetastatic melanoma protein B in angiogenesis during hyperoxia-induced neonatal lung injury. Am. J. Respir. Cell Mol. Biol. 2015, 52, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, M.; El-Mounayri, O.; Kattman, S.; Zandstra, P.; Sakamoto, H.; Ogawa, M.; Keller, G.; Husain, M. Regulated expression and role of c-Myb in the cardiovascular-directed differentiation of mouse embryonic stem cells. Circ. Res. 2012, 110, 253–264. [Google Scholar] [CrossRef]

| Upregulated miR | Downregulated miR | |
|---|---|---|
| In T1D patients | miR-29a, miR-30b, miR-148a, miR-181a, and miR-200a [119,121] | miR-21a, miR-93, miR-126, miR-146a, and miR-150 [119,120,121] |
| In STZ-induced T1D rodents | miR-195 [130,131], miR-495 [125]. | |
| In STZ-induced diabetic rats, at least 86 miRs are significantly altered in the retina [115,117,118] | ||
| In T2D patients and T2D mice | miR-15a, miR-20b [137], miR-21 [127], miR-24, miR-93, miR-126, miR-146a, miR-150, miR-191, miR-197, miR-320, and miR-486 [122,123] | |
| Direct Target of miR-150 | Reference |
|---|---|
| Cxcr4 (C-X-C chemokine receptor type 4) | Liu (2015) [149] |
| Dll4 (Delta like ligand 4) | Liu (2015) [149] |
| Egr1 (Early growth response 1) | Ying (2016) [113] Shen (2019) [153] |
| Egr2 (Early growth response 2): verified downstream angiogenic targets are VEGF and VEGFR2 | Nagarajan (2001) [165] Joseph (1988) [166] |
| Elk1 (ETS-domain transcription factor) | Shi (2016) [141] Zhu (2017) [145] Ying (2016) [113] Yu (2020, 2021) [196,207] |
| Etf1 (Eukaryotic translation termination factor 1) | Ying (2016) [113] |
| Fzd4 (Frizzled-4) | Liu 2015 [149] |
| GPNMB (Glycoprotein nonmetastatic melanoma protein B): verified downstream angiogenic target is Neuropilin-1 (NRP-1) | Maric (2013) [208] Becker (2005) [209] Narasaraju (2015) [210] |
| Myb (MYB proto-oncogene): verified downstream angiogenic target is VEGFR2 | Ishida (2012) [211] Dai (2006) [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, G.Y.-P.; Yu, F.; Bayless, K.J.; Ko, M.L. MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression? Int. J. Mol. Sci. 2022, 23, 12099. https://doi.org/10.3390/ijms232012099
Ko GY-P, Yu F, Bayless KJ, Ko ML. MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression? International Journal of Molecular Sciences. 2022; 23(20):12099. https://doi.org/10.3390/ijms232012099
Chicago/Turabian StyleKo, Gladys Y.-P., Fei Yu, Kayla J. Bayless, and Michael L. Ko. 2022. "MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression?" International Journal of Molecular Sciences 23, no. 20: 12099. https://doi.org/10.3390/ijms232012099
APA StyleKo, G. Y.-P., Yu, F., Bayless, K. J., & Ko, M. L. (2022). MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression? International Journal of Molecular Sciences, 23(20), 12099. https://doi.org/10.3390/ijms232012099






