Ex Vivo Fluorescence Confocal Microscopy (FCM) Ensures Representative Tissue in Prostate Cancer Biobanking: A Feasibility Study
Abstract
:1. Introduction
2. Results
2.1. Available MRI Data
2.2. Tumor Extension in the Prostatectomy Preparations
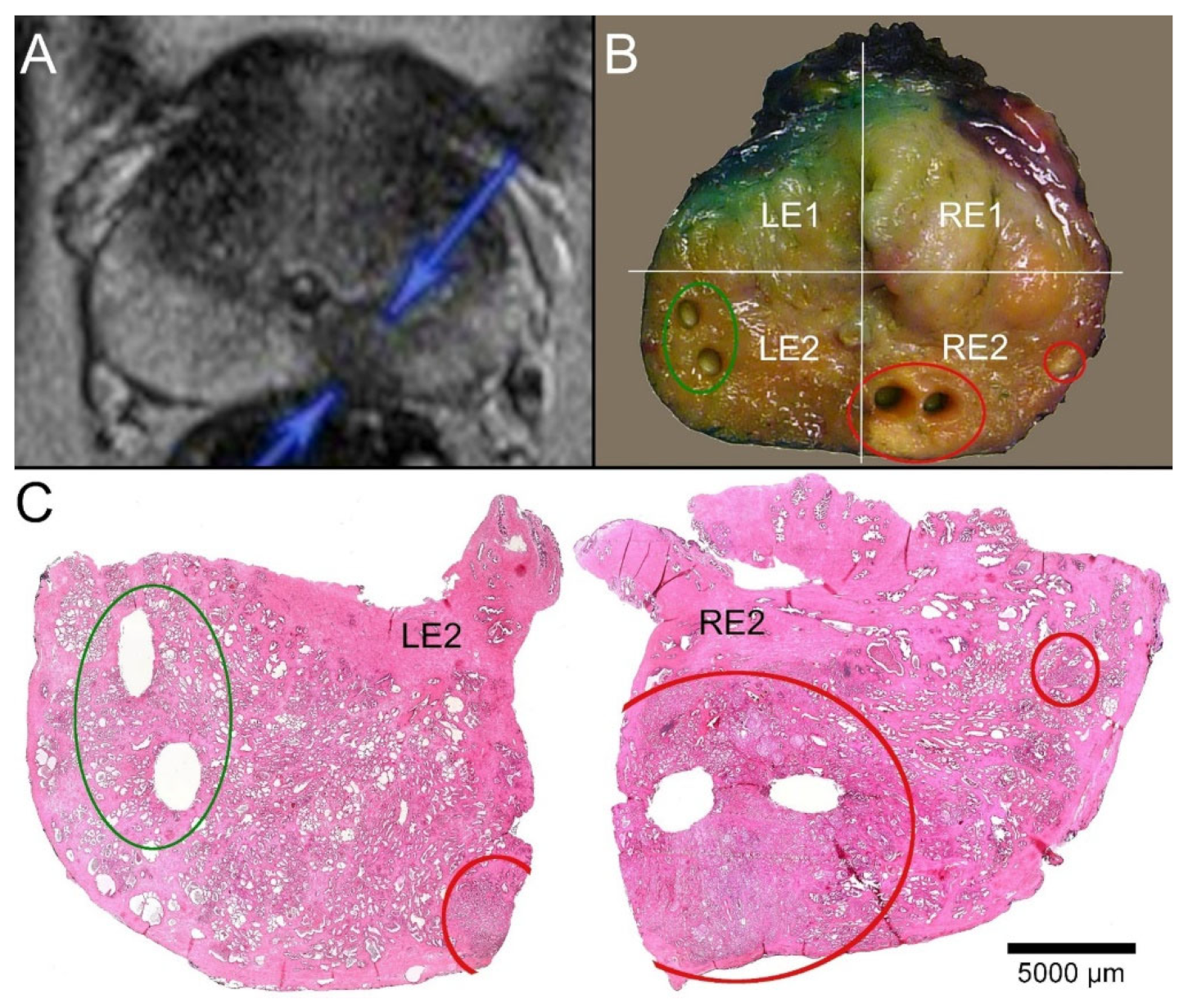
2.3. Identification of Tumor Foci
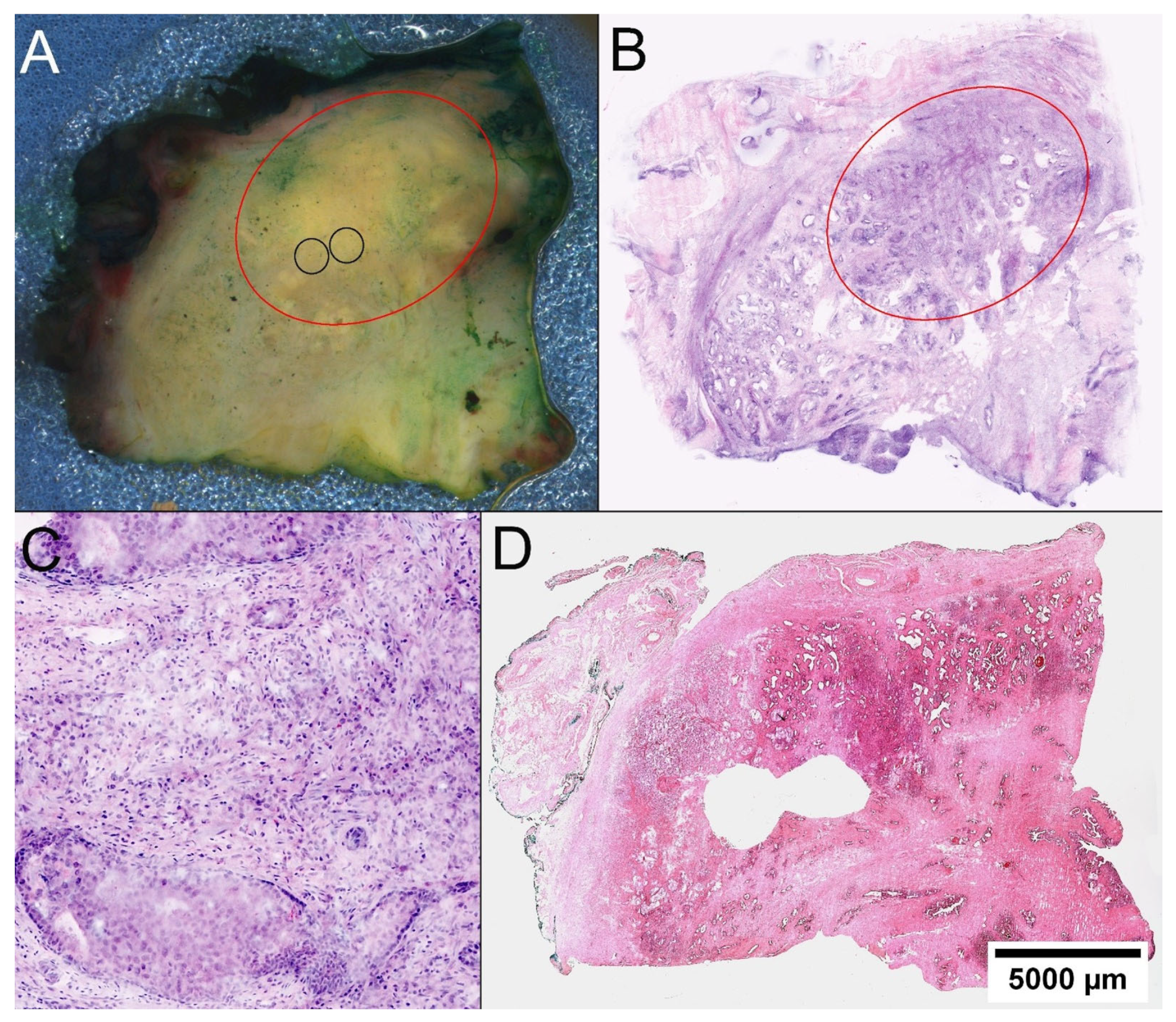
2.4. FCM Analysis of the Biobank Samples
2.5. Processing Times for FCM-Analyses
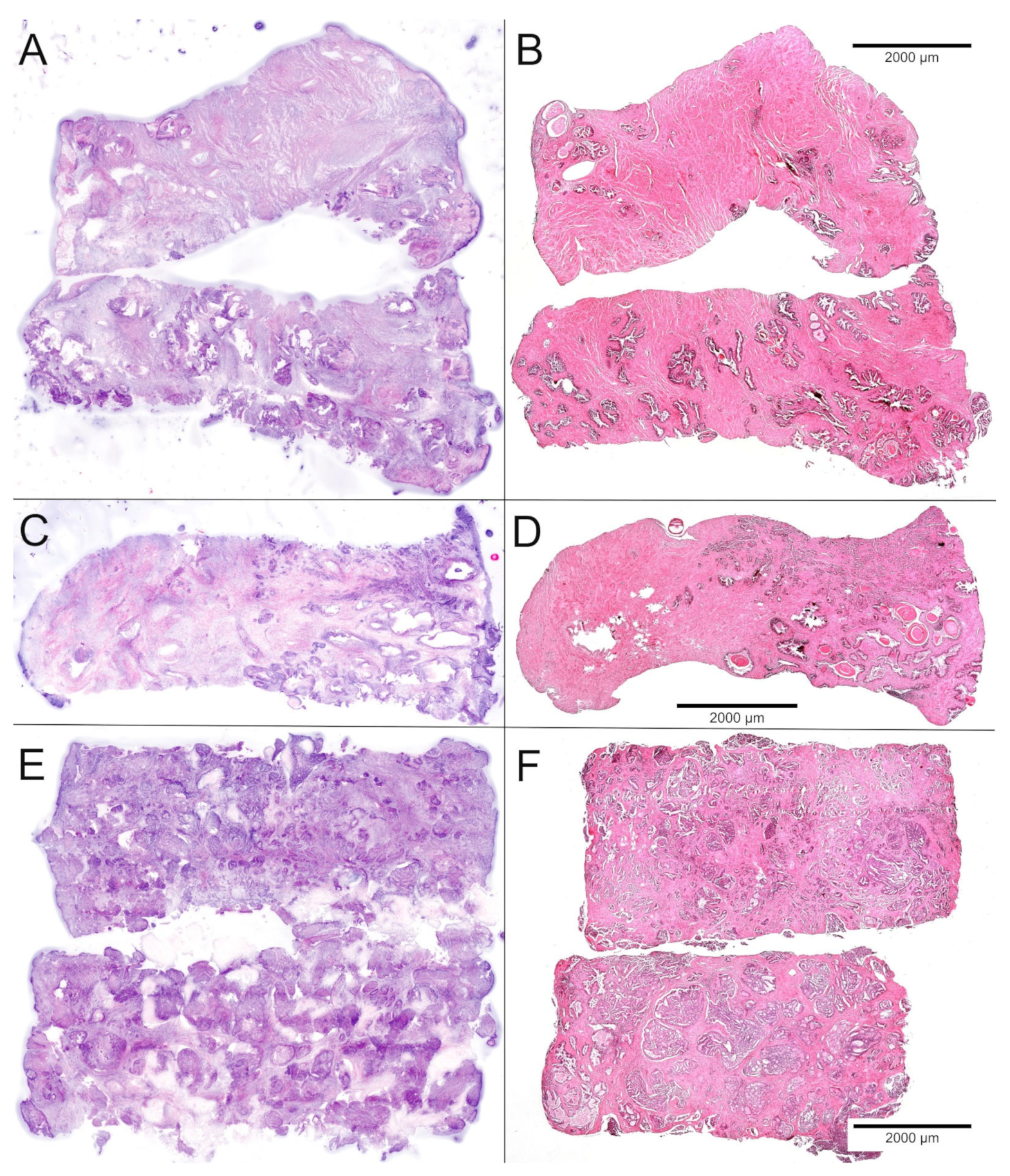
2.6. Histological Quality Control of the Biobank Material and Comparison with the Prostatectomy Specimens
2.7. DNA Content of Biobank Samples
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Study Design
4.3. Processing of the Prostatectomy Specimens
4.4. Obtaining Biobank Samples
4.5. Ex Vivo Confocal Microscopy
4.6. Identification of Tumor Areas
4.7. Examinations of Prostatectomy Specimens
4.8. Assessment of Tumor Content in the Biobank Samples
4.9. Statistical Analysis
4.10. Assessment of DNA Content in Biobank Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Andreoiu, M.; Cheng, L. Multifocal prostate cancer: Biologic, prognostic, and therapeutic implications. Hum. Pathol. 2010, 41, 781–793. [Google Scholar] [CrossRef]
- Haffner, M.C.; Mosbruger, T.; Esopi, D.M.; Fedor, H.; Heaphy, C.M.; Walker, D.A.; Adejola, N.; Gurel, M.; Hicks, J.; Meeker, A.K.; et al. Tracking the clonal origin of lethal prostate cancer. J. Clin. Investig. 2013, 123, 4918–4922. [Google Scholar] [CrossRef] [Green Version]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Hognas, G.; Annala, M.; et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Harmon, S.A.; Terrigino, N.T.; Karzai, F.; Pinto, P.A.; Madan, R.A.; VanderWeele, D.J.; Lake, R.; Atway, R.; Bright, J.R.; et al. A case report of multiple primary prostate tumors with differential drug sensitivity. Nat. Commun. 2020, 11, 837. [Google Scholar] [CrossRef] [Green Version]
- Coppola, L.; Cianflone, A.; Grimaldi, A.M.; Incoronato, M.; Bevilacqua, P.; Messina, F.; Baselice, S.; Soricelli, A.; Mirabelli, P.; Salvatore, M. Biobanking in health care: Evolution and future directions. J. Transl. Med. 2019, 17, 172. [Google Scholar] [CrossRef] [Green Version]
- Miserocchi, G.; Mercatali, L.; Liverani, C.; De Vita, A.; Spadazzi, C.; Pieri, F.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. Management and potentialities of primary cancer cultures in preclinical and translational studies. J. Transl. Med. 2017, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Van de Merbel, A.F.; van der Horst, G.; van der Mark, M.H.; van Uhm, J.I.M.; van Gennep, E.J.; Kloen, P.; Beimers, L.; Pelger, R.C.M.; van der Pluijm, G. An ex vivo Tissue Culture Model for the Assessment of Individualized Drug Responses in Prostate and Bladder Cancer. Front. Oncol. 2018, 8, 400. [Google Scholar] [CrossRef]
- Le, J.D.; Tan, N.; Shkolyar, E.; Lu, D.Y.; Kwan, L.; Marks, L.S.; Huang, J.; Margolis, D.J.; Raman, S.S.; Reiter, R.E. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur. Urol. 2015, 67, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Gröbler, B.; Gluch, M.; Heinz, H. Confocal Laser Scanning Microscopy. Principles. Carl Zeiss GmbH. 1998. Available online: http://zeiss-campus.magnet.fsu.edu/referencelibrary/pdfs/ZeissConfocalPrinciples.pdf (accessed on 28 September 2019).
- Ragazzi, M.; Longo, C.; Piana, S. Ex Vivo (Fluorescence) Confocal Microscopy in Surgical Pathology: State of the Art. Adv. Anat. Pathol. 2016, 23, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Titze, U.; Sievert, K.D.; Titze, B.; Schulz, B.; Schlieker, H.; Madarasz, Z.; Weise, C.; Hansen, T. Ex Vivo Fluorescence Confocal Microscopy in Specimens of the Liver: A Proof-of-Concept Study. Cancers 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Nackenhorst, M.C.; Kasiri, M.; Gollackner, B.; Regele, H. Ex vivo fluorescence confocal microscopy: Chances and changes in the analysis of breast tissue. Diagn Pathol. 2022, 17, 55. [Google Scholar] [CrossRef]
- Rocco, B.; Sighinolfi, M.C.; Sandri, M.; Spandri, V.; Cimadamore, A.; Volavsek, M.; Mazzucchelli, R.; Lopez-Beltran, A.; Eissa, A.; Bertoni, L.; et al. Digital Biopsy with Fluorescence Confocal Microscope for Effective Real-time Diagnosis of Prostate Cancer: A Prospective, Comparative Study. Eur. Urol. Oncol. 2020, 4, 784–791. [Google Scholar] [CrossRef]
- Selvaggio, O.; Falagario, U.G.; Bruno, S.M.; Recchia, M.; Sighinolfi, M.C.; Sanguedolce, F.; Milillo, P.; Macarini, L.; Rastinehad, A.R.; Sanchez-Salas, R.; et al. Intraoperative Digital Analysis of Ablation Margins (DAAM) by Fluorescent Confocal Microscopy to Improve Partial Prostate Gland Cryoablation Outcomes. Cancers 2021, 13, 4382. [Google Scholar] [CrossRef]
- Falagario, U.G.; Selvaggio, O.; Sanguedolce, F.; Milillo, P.; Sighinolfi, M.C.; Bruno, S.M.; Recchia, M.; Bettocchi, C.; Busetto, G.M.; Macarini, L.; et al. One-Day Prostate Cancer Diagnosis: Biparametric Magnetic Resonance Imaging and Digital Pathology by Fluorescence Confocal Microscopy. Diagnostics 2022, 12, 277. [Google Scholar] [CrossRef]
- Wheeler, T.M.; Lebovitz, R.M. Fresh tissue harvest for research from prostatectomy specimens. Prostate 1994, 25, 274–279. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Henderson, A.; Denham, P.; Langley, S.E. A novel method of obtaining prostate tissue for gene expression profiling. Int. J. Surg. Pathol. 2009, 17, 238–243. [Google Scholar] [CrossRef]
- Dev, H.; Rickman, D.; Sooriakumaran, P.; Srivastava, A.; Grover, S.; Leung, R.; Kim, R.; Kitabayashi, N.; Esqueva, R.; Park, K.; et al. Biobanking after robotic-assisted radical prostatectomy: A quality assessment of providing prostate tissue for RNA studies. J. Transl. Med. 2011, 9, 121. [Google Scholar] [CrossRef]
- Esgueva, R.; Park, K.; Kim, R.; Kitabayashi, N.; Barbieri, C.E.; Dorsey, P.J., Jr.; Abraham, C.; Banerjee, S.; Leung, R.A.; Tewari, A.K.; et al. Next-generation prostate cancer biobanking: Toward a processing protocol amenable for the International Cancer Genome Consortium. Diagn Mol. Pathol. 2012, 21, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolkach, Y.; Eminaga, O.; Wotzel, F.; Huss, S.; Bettendorf, O.; Eltze, E.; Abbas, M.; Imkamp, F.; Semjonow, A. Blind Biobanking of the Prostatectomy Specimen: Critical Evaluation of the Existing Techniques and Development of the New 4-Level Tissue Extraction Model With High Sampling Efficacy. Prostate 2017, 77, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Heavey, S.; Costa, H.; Pye, H.; Burt, E.C.; Jenkinson, S.; Lewis, G.R.; Bosshard-Carter, L.; Watson, F.; Jameson, C.; Ratynska, M.; et al. PEOPLE: PatiEnt prOstate samPLes for rEsearch, a tissue collection pathway utilizing magnetic resonance imaging data to target tumor and benign tissue in fresh radical prostatectomy specimens. Prostate 2019, 79, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.Y.; Whitaker, H.C.; Haynes, B.; Sangan, T.; McDuffus, L.A.; Kay, J.D.; Neal, D.E. Method for sampling tissue for research which preserves pathological data in radical prostatectomy. Prostate 2013, 73, 194–202. [Google Scholar] [CrossRef]
- Bertoni, L.; Puliatti, S.; Reggiani Bonetti, L.; Maiorana, A.; Eissa, A.; Azzoni, P.; Bevilacqua, L.; Spandri, V.; Kaleci, S.; Zoeir, A.; et al. Ex vivo fluorescence confocal microscopy: Prostatic and periprostatic tissues atlas and evaluation of the learning curve. Virchows Arch. 2020, 476, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Rocco, B.; Cimadamore, A.; Sarchi, L.; Bonetti, L.R.; Bertoni, L.; Azzoni, P.; Assumma, S.; Turri, F.; Bozzini, G.; Eissa, A.; et al. Current and future perspectives of digital microscopy with fluorescence confocal microscope for prostate tissue interpretation: A narrative review. Transl. Androl. Urol. 2021, 10, 1569–1580. [Google Scholar] [CrossRef]
- Marenco, J.; Calatrava, A.; Casanova, J.; Claps, F.; Mascaros, J.; Wong, A.; Barrios, M.; Martin, I.; Rubio, J. Evaluation of Fluorescent Confocal Microscopy for Intraoperative Analysis of Prostate Biopsy Cores. Eur. Urol. Focus 2021, 7, 1254–1259. [Google Scholar] [CrossRef]
- Titze, U.; Hansen, T.; Brochhausen, C.; Titze, B.; Schulz, B.; Gunnemann, A.; Rocco, B.; Sievert, K.D. Diagnostic Performance of Ex Vivo Fluorescence Confocal Microscopy in the Assessment of Diagnostic Biopsies of the Prostate. Cancers 2021, 13, 5685. [Google Scholar] [CrossRef]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef] [Green Version]
- Udovich, J.A.; Besselsen, D.G.; Gmitro, A.F. Assessment of acridine orange and SYTO 16 for in vivo imaging of the peritoneal tissues in mice. J. Microsc. 2009, 234, 124–129. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Takai, T.; Yoshimura, H.; Inoue, K.; Takai, S.; Baldini, N. Clinical trial of radiotherapy after intravenous injection of acridine orange for patients with cancer. Anticancer. Res. 2018, 38, 481–489. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Acridine orange. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man; IARC Press: Lyon, France, 1978; Volume 16, pp. 145–152. [Google Scholar]
- Lauretti, F.; Lucas de Melo, F.; Benati, F.J.; de Mello Volotao, E.; Santos, N.; Linhares, R.E.; Nozawa, C. Use of acridine orange staining for the detection of rotavirus RNA in polyacrylamide gels. J. Virol. Methods 2003, 114, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelenin, A.V. Acridine orange as a probe for molecular and cell biology. In Fluorescent and Luminescent Probes for Biological Activity: A Practical Guide to Technology for Quantitative Real-Time Analysis; Mason, W.T., Ed.; Academic Press: London, UK, 1993; pp. 83–99. [Google Scholar]
- Titze, U.; Hansen, T.; Titze, B.; Schulz, B.; Gunnemann, A.; Rocco, B.; Sievert, K.D. Feasibility study for ex vivo fluorescence confocal microscopy (FCM) on diagnostic prostate biopsies. Quant. Imaging Med. Surg. 2021, 11, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Quiroga-Garza, G.M.; Bien, L.; Heled, R.; Laifenfeld, D.; Linhart, C.; Sandbank, J.; Albrecht Shach, A.; Shalev, V.; Vecsler, M.; et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: A blinded clinical validation and deployment study. Lancet Digit. Health 2020, 2, e407–e416. [Google Scholar] [CrossRef]
- Luo, R.; Zeng, Q.; Chen, H. Artificial Intelligence Algorithm-Based MRI for Differentiation Diagnosis of Prostate Cancer. Comput. Math. Methods Med. 2022, 2022, 8123643. [Google Scholar] [CrossRef]
- Jhavar, S.G.; Fisher, C.; Jackson, A.; Reinsberg, S.A.; Dennis, N.; Falconer, A.; Dearnaley, D.; Edwards, S.E.; Edwards, S.M.; Leach, M.O.; et al. Processing of radical prostatectomy specimens for correlation of data from histopathological, molecular biological, and radiological studies: A new whole organ technique. J. Clin. Pathol. 2005, 58, 504–508. [Google Scholar] [CrossRef]
- Srigley, J.R. Key issues in handling and reporting radical prostatectomy specimens. Arch. Pathol. Lab. Med. 2006, 130, 303–317. [Google Scholar] [CrossRef]
- Gill, P.S.; Roberts, I.S.; Browning, L.; Perera, R.; Warren, A.Y.; Hamdy, F.C.; Verrill, C. The handling and sampling of radical prostatectomy specimens for reporting and research: The Oxford approach. J. Clin. Pathol. 2012, 65, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Kasten, F.H. Cytochemical studies with acridine orange and the influence of dye contaminants in the staining of nucleic acids. Int. Rev. Cytol. 1967, 21, 141–202. [Google Scholar] [CrossRef]
- Gareau, D.S. Feasibility of digitally stained multimodal confocal mosaics to simulate histopathology. J. Biomed. Opt. 2009, 14, 034050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettendorf, O.; Oberpenning, F.; Kopke, T.; Heinecke, A.; Hertle, L.; Boecker, W.; Semjonow, A. Implementation of a map in radical prostatectomy specimen allows visual estimation of tumor volume. Eur. J. Surg. Oncol. 2007, 33, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Eminaga, O.; Hinkelammert, R.; Semjonow, A.; Neumann, J.; Abbas, M.; Koepke, T.; Bettendorf, O.; Eltze, E.; Dugas, M. Clinical map document based on XML (cMDX): Document architecture with mapping feature for reporting and analysing prostate cancer in radical prostatectomy specimens. BMC Med. Inform. Decis. Mak. 2010, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleiss, J.; Cohen, J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ. Psychol. Meas. 1973, 33, 613–619. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
| FCM-Ratings | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | |||
| FFPE- ratings | 68 | 0 | 66 | 1 | 1 |
| 58 | 1 | 1 | 11 | 5 | |
| 2 | 1 | 3 | 37 | ||
| n = 126 | 68 | 15 | 43 | ||
| Tumor detection (0 vs. 1; 2) | 96.8% | ĸ = 0.94 | (Nearly perfect) | ||
| Tumor content (1 vs. 2) | 85.7% | ĸ = 0.64 | (Substantial) | ||
| Overall rating (0 vs. 1 vs. 2) | 90.5% | ĸ = 0.84 | (Nearly perfect) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titze, U.; Sommerkamp, J.; Stege, C.; Schneider, F.; Brochhausen, C.; Schulz, B.; Titze, B.; Abd Ali, F.; Pokupic, S.; Sievert, K.-D.; et al. Ex Vivo Fluorescence Confocal Microscopy (FCM) Ensures Representative Tissue in Prostate Cancer Biobanking: A Feasibility Study. Int. J. Mol. Sci. 2022, 23, 12103. https://doi.org/10.3390/ijms232012103
Titze U, Sommerkamp J, Stege C, Schneider F, Brochhausen C, Schulz B, Titze B, Abd Ali F, Pokupic S, Sievert K-D, et al. Ex Vivo Fluorescence Confocal Microscopy (FCM) Ensures Representative Tissue in Prostate Cancer Biobanking: A Feasibility Study. International Journal of Molecular Sciences. 2022; 23(20):12103. https://doi.org/10.3390/ijms232012103
Chicago/Turabian StyleTitze, Ulf, Johannes Sommerkamp, Clara Stege, Fried Schneider, Christoph Brochhausen, Birte Schulz, Barbara Titze, Furat Abd Ali, Sasa Pokupic, Karl-Dietrich Sievert, and et al. 2022. "Ex Vivo Fluorescence Confocal Microscopy (FCM) Ensures Representative Tissue in Prostate Cancer Biobanking: A Feasibility Study" International Journal of Molecular Sciences 23, no. 20: 12103. https://doi.org/10.3390/ijms232012103
APA StyleTitze, U., Sommerkamp, J., Stege, C., Schneider, F., Brochhausen, C., Schulz, B., Titze, B., Abd Ali, F., Pokupic, S., Sievert, K.-D., & Hansen, T. (2022). Ex Vivo Fluorescence Confocal Microscopy (FCM) Ensures Representative Tissue in Prostate Cancer Biobanking: A Feasibility Study. International Journal of Molecular Sciences, 23(20), 12103. https://doi.org/10.3390/ijms232012103






