Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model
Abstract
:1. Introduction
2. Results
2.1. Quantification of Glutaraldehyde (GA) Aerosol Deposition
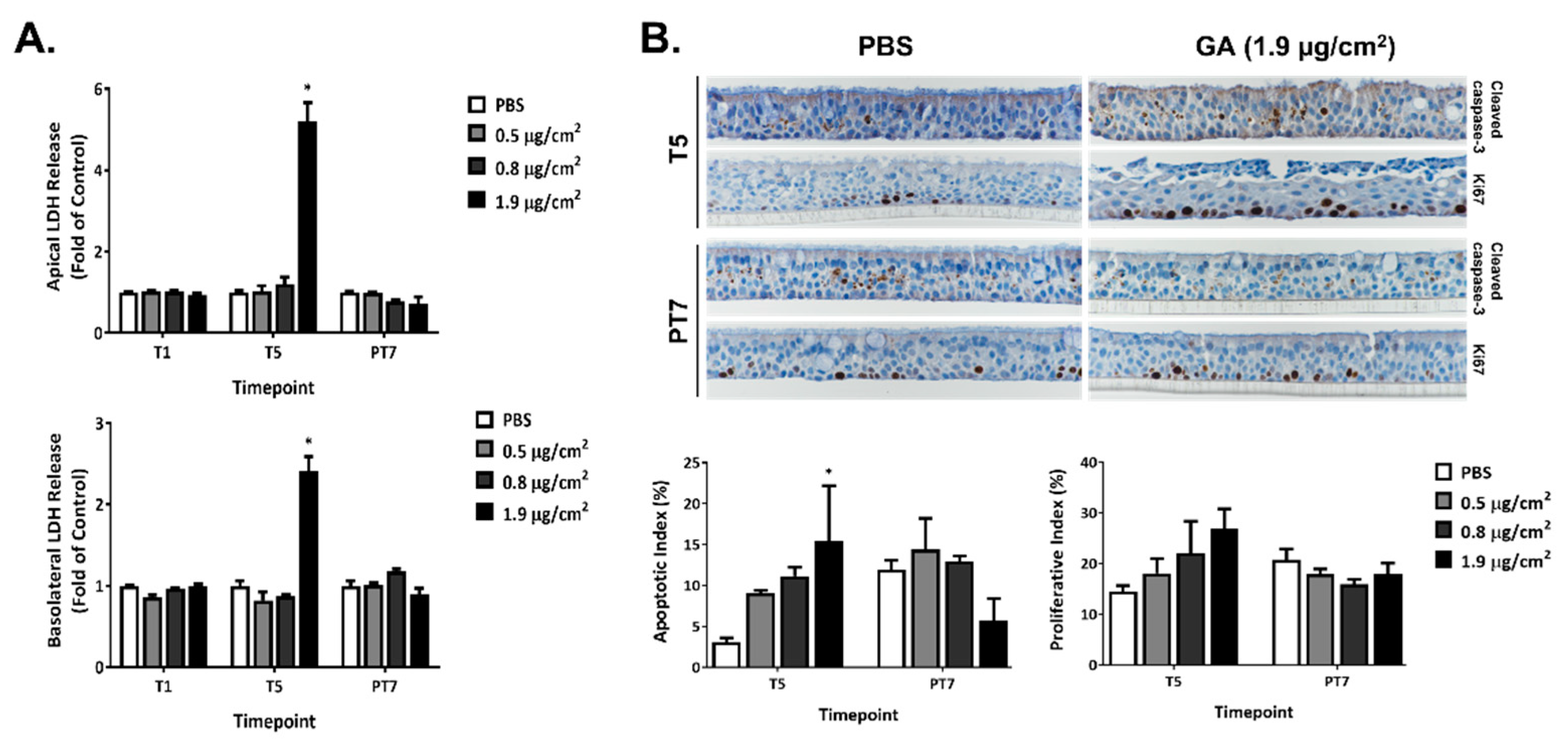
2.2. Cytotoxicity of GA Aerosols in ALI Cultures
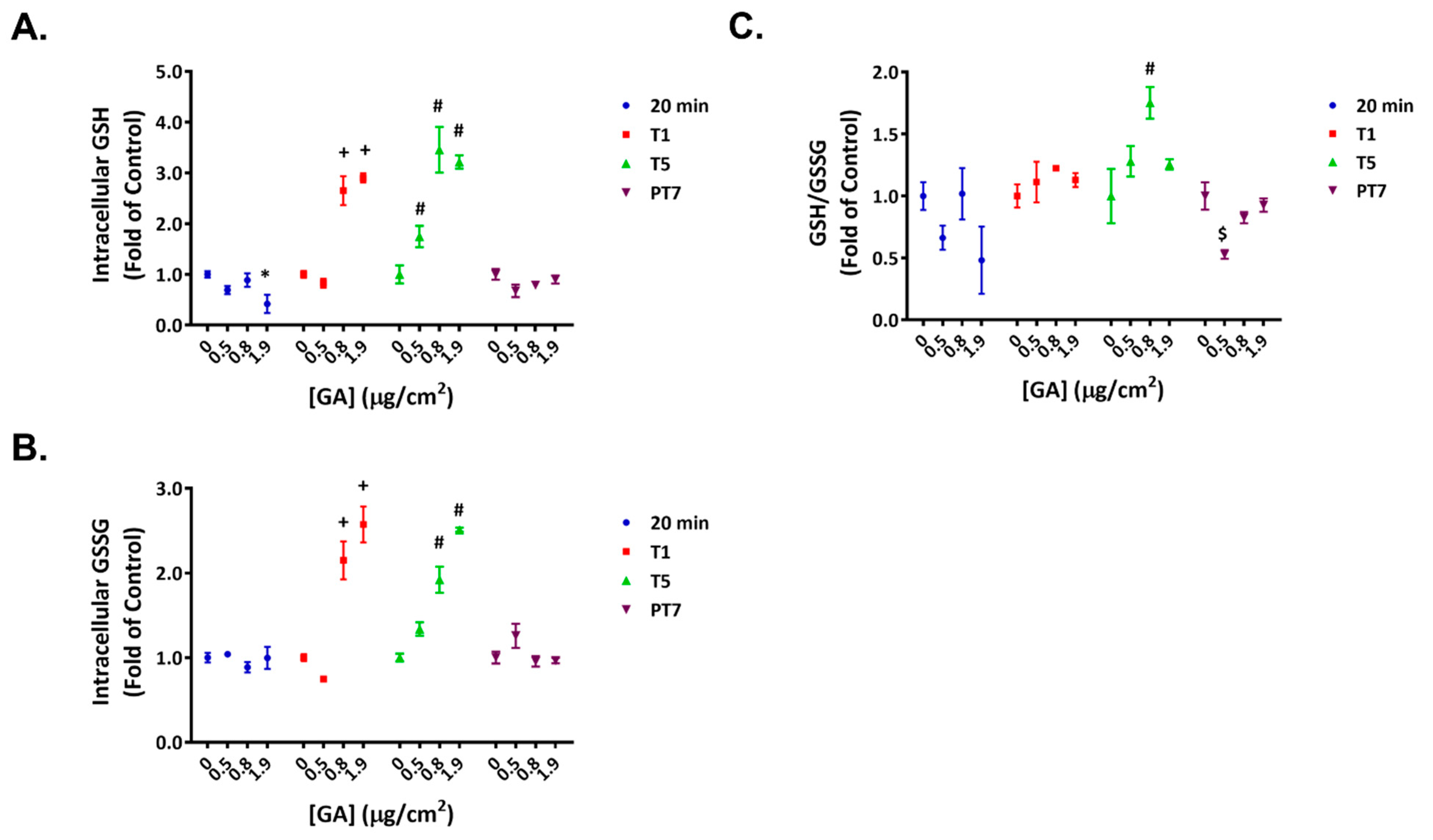
2.3. Reversible Disruption of Glutathione Homeostasis by GA Aerosols
2.4. Modulation of Select Oxidative Stress Markers by GA Aerosols
2.5. Modulation of Cytokine and Matrix Metalloproteinases (MMP) Secretion by GA Aerosols
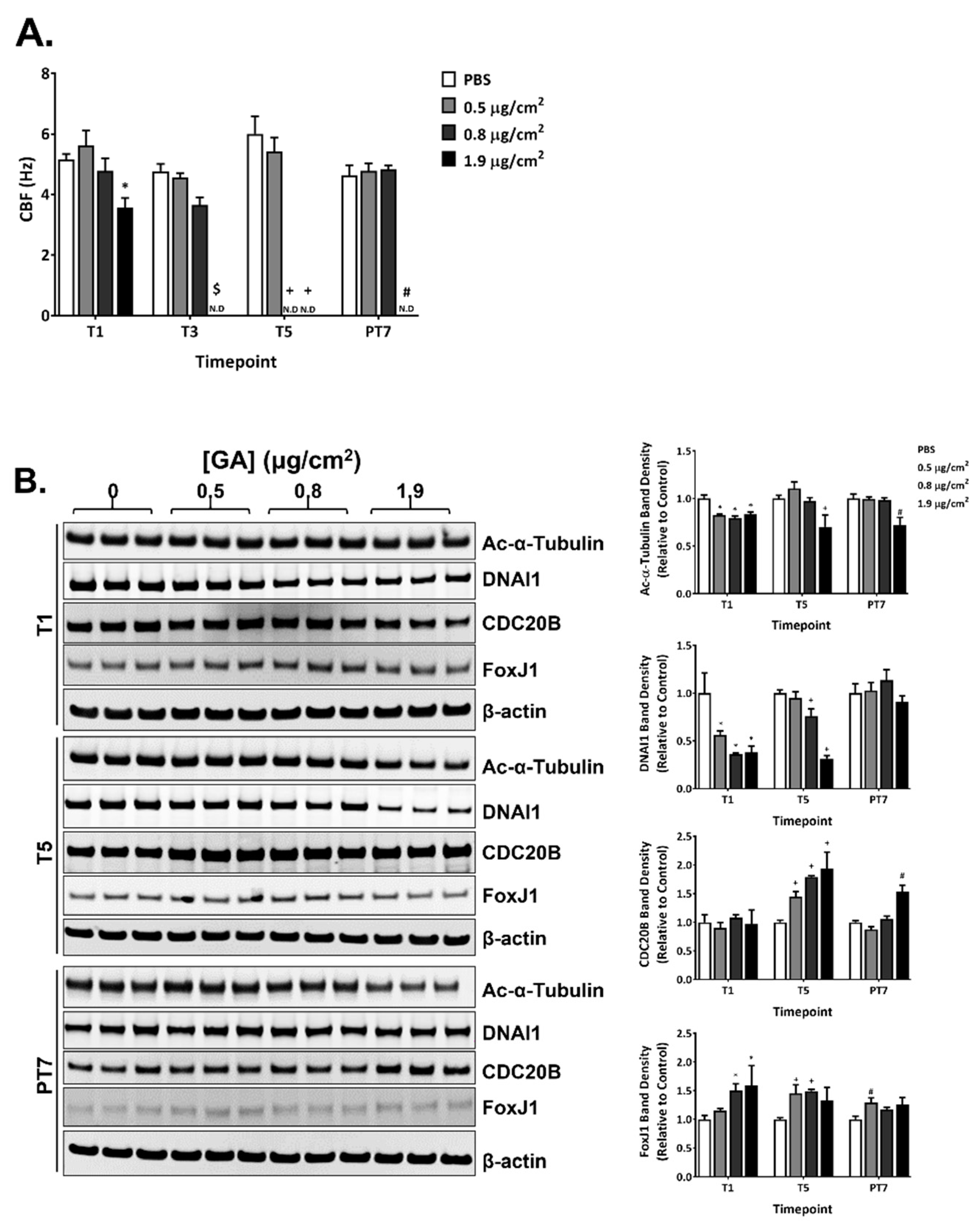
2.6. Effects of GA Aerosols on Ciliary Cells in ALI Cultures
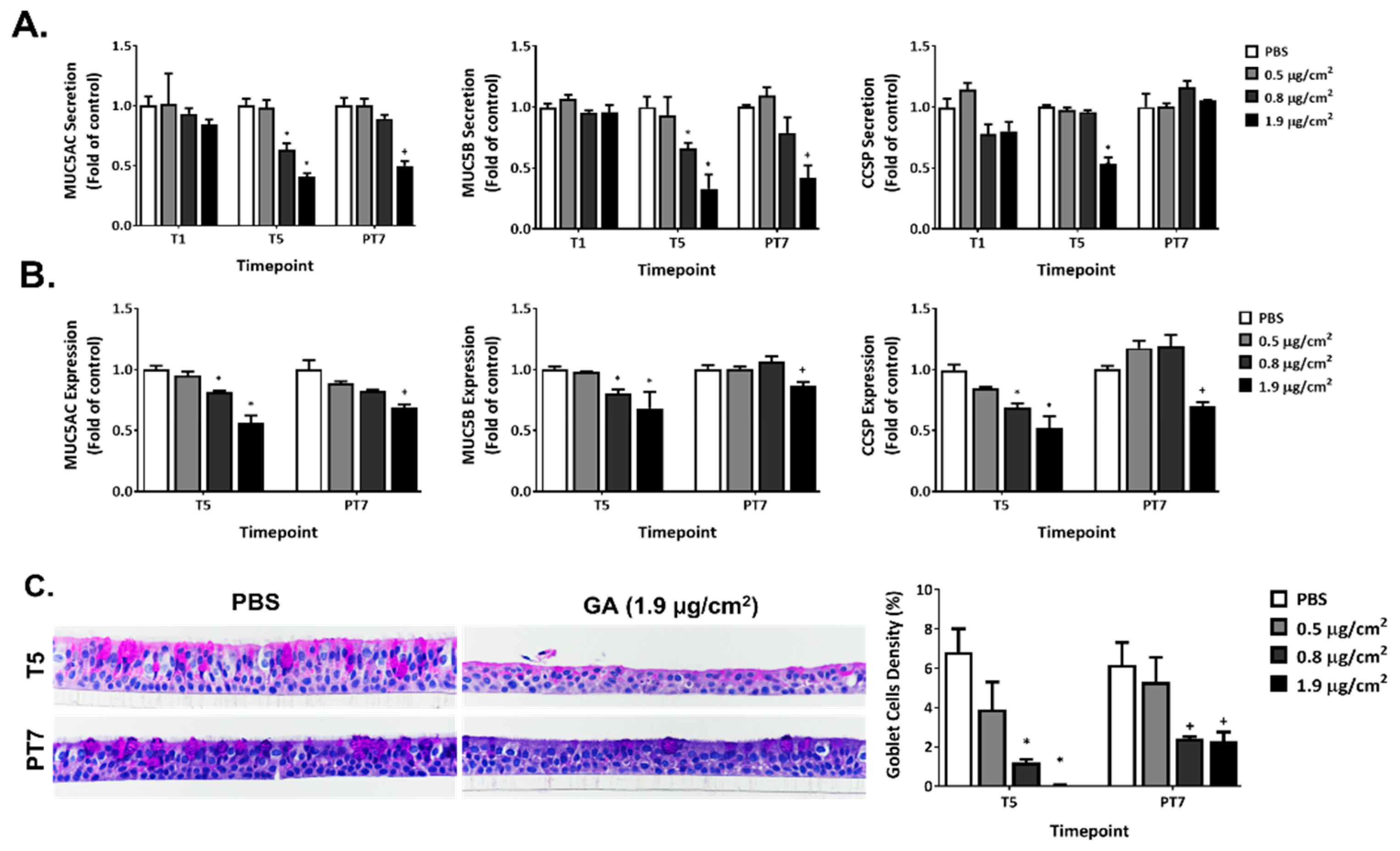
2.7. Disruption of Mucin Homeostasis by GA Aerosols in ALI Cultures
2.8. Effect of GA Aerosols on General Morphology in ALI Cultures
3. Discussion
4. Materials and Methods
4.1. Glutaraldehyde (GA) Aerosol Deposition Quantification and Exposures in Air-Liquid-Interface (ALI) Cultures
4.2. Human ALI Airway Tissue Model
4.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
4.4. Immunohistochemistry and Histology
4.5. Glutathione Homeostasis
4.6. Immunoblotting
4.7. Bio-Plex Cytokine and Matrix Metalloproteinases (MMPs) Assays
4.8. Cilia Beating Frequency (CBF)
4.9. Mucin ELISA
4.10. Trans-Epithelial Electrical Resistance (TEER)
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. FDA-Cleared Sterilants and High-Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices. 2019. Available online: https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices-information-manufacturers/fda-cleared-sterilants-and-high-level-disinfectants-general-claims-processing-reusable-medical-and (accessed on 9 September 2022).
- National Toxicology Program. NTP Toxicology and Carcinogenesis studies of glutaraldehyde (CAS NO. 111-30-8) in F344/N rats and B6C3F1 mice (Inhalation Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1999, 490, 1–234. [Google Scholar]
- Rideout, K.; Teschke, K.; Dimich-Ward, H.; Kennedy, S.M. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Glutaraldehyde; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2017. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1467&tid=284 (accessed on 9 September 2022).
- Houtmeyers, E.; Gosselink, R.; Gayan-Ramirez, G.; Decramer, M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999, 13, 1177–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atamas, S.P.; Chapoval, S.P.; Keegan, A.D. Cytokines in chronic respiratory diseases. F1000 Biol. Rep. 2013, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.F. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18 (Suppl. 34), 50s–59s. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 104–132. [Google Scholar]
- Cao, X.; Wang, Y.; Xiong, R.; Muskhelishvili, L.; Davis, K.; Richter, P.A.; Heflich, R.H. Cigarette whole smoke solutions disturb mucin homeostasis in a human in vitro airway tissue model. Toxicology 2018, 409, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Bryant, M.; Cao, X. Assessing the respiratory toxicity of dihydroxyacetone using an in vitro human airway epithelial tissue model. Toxicol. In Vitro 2019, 59, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Shemansky, J.M.; Bryant, M.; Rosenfeldt, H.; Healy, S.M.; Cao, X. Evaluating mode of action of acrolein toxicity in an in vitro human airway tissue model. Toxicol. Sci. 2018, 166, 451–464. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Wynne, R.; Tripathi, P.; Bryant, M.S.; Rua, D.; Cao, X. Toxicity of ortho-phthalaldehyde aerosols in a human in vitro airway tissue model. Chem. Res. Toxicol. 2021, 34, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wu, Q.; Muskhelishvili, L.; Davis, K.; Wang, Y.; Rua, D.; Cao, X. Evaluating the sub-acute toxicity of formaldehyde fumes in an in vitro human airway epithelial tissue model. Int. J. Mol. Sci. 2022, 23, 2593. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Marczak, A.; Jóźwiak, Z. Damage to the cell antioxidative system in human erythrocytes incubated with idarubicin and glutaraldehyde. Toxicol. In Vitro 2009, 23, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.; Siegel, D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.L.; Midwinter, R.G.; Ni, J.; Hamid, H.A.; Parish, C.R.; Stocker, R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1723–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Halatek, T.; Opalska, B.; Swiercz, R.; Palczynski, C.; Gorski, P.; Rydzynski, K.; Bernard, A. Glutaraldehyde inhalation exposure of rats: Effects on lung morphology, clara-cell protein, and hyaluronic acid levels in BAL. Inhal. Toxicol. 2003, 15, 85–97. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury Maxence, V.; Toso Daniel, B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser James, S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horani, A.; Ustione, A.; Huang, T.; Firth Amy, L.; Pan, J.; Gunsten Sean, P.; Haspel Jeffrey, A.; Piston David, W.; Brody Steven, L. Establishment of the early cilia preassembly protein complex during motile ciliogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E1221–E1228. [Google Scholar] [CrossRef]
- Mukherjee, I.; Roy, S.; Chakrabarti, S. Identification of important effector proteins in the FOXJ1 transcriptional network associated with ciliogenesis and ciliary function. Front. Genet 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wu, T.; Kirschner, M.W. The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. Elife 2014, 3, e03083. [Google Scholar] [CrossRef] [PubMed]
- Copeland, S.; Nugent, K. Persistent and unusual respiratory findings after prolonged glutaraldehyde exposure. Int. J. Occup. Environ. Med. 2015, 6, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Romero Starke, K.; Friedrich, S.; Schubert, M.; Kämpf, D.; Girbig, M.; Pretzsch, A.; Nienhaus, A.; Seidler, A. Are healthcare workers at an increased risk for obstructive respiratory diseases due to cleaning and disinfection agents? a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 5159. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O.; St Clair, M.B.G.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Karl, F.W. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Chan-Yeung, M.; McMurren, T.; Catonio-Begley, F.; Lam, S. Occupational asthma in a technologist exposed to glutaraldehyde. J. Allergy Clin. Immunol. 1993, 91, 974–978. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dearman; Basketter; Evans; Kimber. Comparison of cytokine secretion profiles provoked in mice by glutaraldehyde and formaldehyde. Clin. Exp. Allergy 1999, 29, 124–132. [Google Scholar] [CrossRef]
- Takeda, N.; Maghni, K.; Daigle, S.; L’Archevêque, J.; Castellanos, L.; Al-Ramli, W.; Malo, J.-L.; Hamid, Q. Long-term pathologic consequences of acute irritant-induced asthma. J. Allergy Clin. Immunol. 2009, 124, 975–981.e1. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Klink, K.J.; Meade, B.J. Divergent immunological responses following glutaraldehyde exposure. Toxicol. Appl. Pharmacol. 2004, 197, 1–8. [Google Scholar] [CrossRef]
- Kari, F. NTP technical report on the toxicity studies of glutaraldehyde (CAS No. 111-30-8) Adminstered by inhalation to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 1993, 25, 1–E10. [Google Scholar] [PubMed]
- van Birgelen, A.P.J.M.; Chou, B.J.; Renne, R.A.; Grumbein, S.L.; Roycroft, J.H.; Hailey, J.R.; Bucher, J.R. Effects of glutaraldehyde in a 2-year inhalation study in rats and mice. Toxicol. Sci. 2000, 55, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González Jara, M.A.; Mora Hidalgo, A.; Avalos Gulin, J.C.; López Albiach, M.; Muñoz Ortiz, L.; Torán Monserrat, P.; Esteva Ollé, X. Exposure of health workers in primary health care to glutaraldehyde. J. Occup. Med. Toxicol. 2013, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Gray, A.C.; McLeod, J.D.; Clothier, R.H. A Review of in vitro modelling approaches to the identification and modulation of squamous metaplasia in the human tracheobronchial epithelium. Altern. Lab. Anim. 2007, 35, 493–504. [Google Scholar] [CrossRef]
- Vanhoutteghem, A.; Djian, P.; Green, H. Ancient origin of the gene encoding involucrin, a precursor of the cross-linked envelope of epidermis and related epithelia. Proc. Natl. Acad. Sci. USA 2008, 105, 15481–15486. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D. Ortho-phthalaldehyde: A possible alternative to glutaraldehyde for high level disinfection. J. Appl. Microbiol. 1999, 86, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Catlin, N.R.; Willson, C.J.; Stout, M.; Kissling, G.E.; Waidyanatha, S.; Baker, G.L.; Hayden, B.K.; Wyde, M. Evaluation of the respiratory tract toxicity of ortho-phthalaldehyde, a proposed alternative for the chemical disinfectant glutaraldehyde. Inhal. Toxicol. 2017, 29, 414–427. [Google Scholar] [CrossRef]
- FDA. Medical Device Development Tools (MDDT). 2022. Available online: https://www.fda.gov/medical-devices/science-and-research-medical-devices/medical-device-development-tools-mddt (accessed on 9 September 2022).



| Analytes (pg/mL) | T1 | T5 | PT7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS | 0.5 µg/cm2 | 0.8 µg/cm2 | 1.9 µg/cm2 | PBS | 0.5 µg/cm2 | 0.8 µg/cm2 | 1.9 µg/cm2 | PBS | 0.5 µg/cm2 | 0.8 µg/cm2 | 1.9 µg/cm2 | |
| IL-1RA | 49.2 | 48.5 | 44.2 | 47.4 | 151.8 | 170.5 | 165.4 | 194.5 | 195.6 | 206.0 | 299.1 | 345.0 * |
| (8.0) | (5.1) | (8.1) | (5.2) | (17.9) | (13.9) | (6.0) | (24.3) | (19.8) | (22.0) | (62.60 | (61.9) | |
| IL-6 | 3.4 | 2.7 | 2.7 | 5.2 * | 4.4 | 3.2 | 6.0 | 6.4 | 18.8 | 8.5 | 5.7 | 4.0 * |
| (0.2) | (0.4) | (0.4) | (0.6) | (0.7) | (1.1) | (1.5) | (3.7) | (5.9) | (1.2) | (0.5) | (1.1) | |
| IL-8 | 4911.2 | 3784.9 | 3734.8 | 8713.9 * | 6822.0 | 5743.5 | 6669.1 | 11,908.0 * | 14,452.8 | 11,649.5 | 8074.2 * | 8825.2 * |
| (772.8) | (442.6) | (178.7) | (1324.2) | (977.6) | (308.7) | (473.6) | (816.9) | (1856.3) | (591.1) | (2185.7) | (2354.5) | |
| IL-9 | 34.6 | 30.3 | 31.9 | 44.0 * | 38.3 | 35.9 | 38.6 | 50.4 * | 56.8 | 51.5 | 42.9 * | 43.8 * |
| (2.1) | (2.8) | (3.0) | (1.9) | (2.2) | (2.2) | (0.9) | (0.9) | (2.1) | (2.1) | (6.7) | (3.7) | |
| FGF basic | 264.6 | 247.7 | 265.1 | 208.1 | 260.3 | 257.0 | 202.0 | 218.7 | 293.5 | 277.9 | 230.0 | 95.5 * |
| (28.1) | (38.4) | (47.3) | (16.8) | (29.3) | (53.0) | (51.6) | (11.9) | (75.8) | (27.6) | (52.7) | (49.3) | |
| G-CSF | 67.0 | 52.8 | 47.0 | 71.4 | 76.2 | 61.0 | 92.3 | 83.2 | 268.7 | 172.8 | 114.6 * | 142.3 * |
| (3.7) | (18.1) | (17.4) | (31.4) | (24.3) | (15.2) | (12.7) | (38.7) | (54.4) | (52.0) | (25.3) | (35.2) | |
| GM-CSF | 6.0 | 5.1 | 4.3 | 4.3 | 8.3 | 6.3 | 6.7 | 8.9 | 17.1 | 12.1 * | 8.6 * | 6.2 * |
| (0.9) | (1.3) | (0.2) | (0.8) | (1.9) | (0.7) | (1.2) | (0.8) | (1.4) | (1.4) | (0.5) | (0.8) | |
| MCP-1 | 27.1 | 26.5 | 23.5 | 22.9 | 43.4 | 35.2 | 46.3 | 44.8 | 131.4 | 90.1 | 69.3 * | 53.8 * |
| (3.5) | (5.1) | (0.8) | (4.5) | (7.0) | (3.9) | (11.7) | (6.9) | (16.0) | (28.5) | (11.6) | (6.9) | |
| PDGF-BB | 986.6 | 1080.7 | 958.6 | 651.9 * | 1473.7 | 1502.4 | 1457.9 | 1625.9 | 2425.6 | 2510.1 | 2339.1 | 2131.5 * |
| (48.5) | (22.5) | (48.5) | (66.6) | (50.9) | (114.4) | (66.7) | (63.5) | (98.4) | (90.1) | (94.0) | (66.5) | |
| TNF-α | 46.3 | 47.2 | 39.6 | 43.5 | 73.8 | 68.6 | 63.9 | 79.8 | 180.0 | 146.8 | 102.2 * | 84.7 * |
| (1.6) | (4.3) | (5.7) | (1.6) | (9.0) | (3.4) | (7.7) | (5.8) | (5.9) | (7.3) | (3.8) | (7.9) | |
| VEGF | 628.0 | 584.8 | 578.1 | 706.1 | 637.8 | 632.6 | 571.7 | 489.6 * | 1301.7 | 1266.8 | 1224.2 | 1126.9 * |
| (35.6) | (32.6) | (26.8) | (48.0) | (32.4) | (47.6) | (21.5) | (72.2) | (35.6) | (92.7) | (29.7) | (66.3) | |
| MMP-1 | 919.4 | 959.5 | 939.8 | 1000.1 | 1146.6 | 1059.1 | 1117.6 | 1242.0 | 1868.1 | 1559.8 | 1416.6 * | 1295.8 * |
| (35.4) | (70.2) | (0.0) | (0.0) | (33.3) | (48.1) | (54.8) | (234.1) | (233.0) | (91.5) | (83.0) | (168.7) | |
| MMP-7 | 767.8 | 696.8 | 713.4 | 882.5 | 1493.0 | 1120.4 | 1696.3 | 1885.8 | 8043.5 | 4890.5 * | 2744.9 * | 2110.0 * |
| (74.4) | (51.1) | (45.3) | (205.9) | (330.3) | (67.2) | (689.1) | (564.2) | (1977.4) | (731.8) | (465.6) | (618.8) | |
| MMP-12 | 56.9 | 50.4 | 77.4 * | 63.9 | 122.5 | 117.0 | 175.7 * | 180.1 * | 608.8 | 299.9 * | 191.9 * | 160.5 * |
| (2.1) | (5.8) | (14.0) | (6.7) | (16.4) | (10.2) | (18.9) | (23.9) | (80.3) | (37.9) | (21.1) | (49.1) | |
| T5 | PT7 | |||||||
|---|---|---|---|---|---|---|---|---|
| DPBS | 0.5 µg/cm2 | 0.8 µg/cm2 | 1.9 µg/cm2 | DPBS | 0.5 µg/cm2 | 0.8 µg/cm2 | 1.9 µg/cm2 | |
| Atrophy | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 2/3 |
| (0.0) | (0.0) | (0.0) | (1.3) | (0.0) | (0.0) | (0.0) | (1.0) | |
| [0%] | [0%] | [0%] | [100%] | [0%] | [0%] | [0%] | [67%] | |
| Ciliation, Decreased | 0/3 | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 1/3 | 3/3 |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| [0%] | [0%] | [100%] | [100%] | [0%] | [0%] | [33%] | [100%] | |
| Depletion, Goblet Cell | 0/3 | 2/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 1/3 |
| (0.0) | (1.0) | (1.7) | (2.3) | (0.0) | (0.0) | (0.0) | (1.0) | |
| [0%] | [67%] | [100%] | [100%] | [0%] | [0%] | [0%] | [33%] | |
| Differentiation, Squamous | 0/3 | 0/3 | 0/3 | 2/3 | N/D | N/D | N/D | N/D |
| (0.0) | (0.0) | (0.0) | (1.5) | |||||
| [0%] | [0%] | [0%] | [67%] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Q.; Ren, B.; Muskhelishvili, L.; Davis, K.; Wynne, R.; Rua, D.; Cao, X. Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model. Int. J. Mol. Sci. 2022, 23, 12118. https://doi.org/10.3390/ijms232012118
Wang Y, Wu Q, Ren B, Muskhelishvili L, Davis K, Wynne R, Rua D, Cao X. Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model. International Journal of Molecular Sciences. 2022; 23(20):12118. https://doi.org/10.3390/ijms232012118
Chicago/Turabian StyleWang, Yiying, Qiangen Wu, Baiping Ren, Levan Muskhelishvili, Kelly Davis, Rebecca Wynne, Diego Rua, and Xuefei Cao. 2022. "Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model" International Journal of Molecular Sciences 23, no. 20: 12118. https://doi.org/10.3390/ijms232012118
APA StyleWang, Y., Wu, Q., Ren, B., Muskhelishvili, L., Davis, K., Wynne, R., Rua, D., & Cao, X. (2022). Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model. International Journal of Molecular Sciences, 23(20), 12118. https://doi.org/10.3390/ijms232012118






