Abstract
Phascolosoma esculenta, an economically important species inhabiting the high tide areas of the intertidal zone, is particularly sensitive to water pollution. Considering its potential as a bioindicator, studies on the ecotoxicology of P. esculenta are imperative. The toxic effects of cadmium (Cd) were analyzed by exposing P. esculenta to different concentrations of Cd (6, 24, 96 mg/L). In this study, the changes in the antioxidative indexes of total superoxide dismutase (T-SOD), glutathione s-transferase (GST), reduced glutathione (GSH), and microscale malondialdehyde (MDA) were recorded. Copper/zinc superoxide dismutase (Cu/Zn SOD) is one of the most important free radical scavenging members. To reveal the antioxidative function of P. esculenta, an important member of the antioxidative system, designated Pe-Cu/Zn SOD, was cloned and analyzed. Phylogenic analysis revealed that Pe-Cu/Zn SOD was located in the invertebrate evolutionary branch of intracellular Cu/Zn SOD (icCu/Zn SOD). The quantitative real-time polymerase chain reaction results showed that Pe-Cu/Zn SOD messenger ribonucleic acid was widely expressed in all tissues examined. The highest expression levels in coelomic fluid after Cd exposure indicated its function in the stress response. Using a prokaryotic expression system, we obtained a Pe-Cu/Zn SOD recombinant protein, which enhanced the heavy metal tolerance of Escherichia coli. In vivo assays also confirmed that the Pe-Cu/Zn SOD recombinant protein had an antioxidative and free radical scavenging ability. A Cd toxicity experiment, in which purified Pe-Cu/Zn SOD protein was injected into the body cavities of P. esculenta, showed that the reactive oxygen species content in the coelomic fluid of the experimental group was significantly lower compared with the control group. These results suggest that Pe-Cu/Zn SOD played a role in Cd detoxification by chelating heavy metal ions and scavenging reactive oxygen free radicals, and that P. esculenta could be used as a bioindicator to evaluate heavy metal pollution.
1. Introduction
Cadmium (Cd) is a nonessential element that mainly exists in the form of Cd2+ in organisms. Its biological toxicity is mainly because of its interference with the various metabolic processes of cells, especially energy metabolism, membrane transport, and protein synthesis. Cd may interfere with the genetic control and repair mechanisms of deoxyribose nucleic acid (DNA) either directly or indirectly. Recently, the mechanisms by which Cd induces oxidative stress have been investigated.
Cadmium has been shown to change the permeability of the mitochondrial membrane, inhibit mitochondrial ATP synthesis, and disturb the mitochondrial electron respiratory transport chain. During this period, Cd induces the accumulation of reactive oxygen species (ROS) [1,2], which refers to the oxygen-containing derivatives of oxygen radicals, including superoxide anions (O2−) and hydroxyl radicals (OH) [3]. Under normal circumstances, the production and elimination of ROS in organisms is in dynamic equilibrium [4], and moderate amounts of ROS are used as signal transduction molecules [5]. When organisms face heavy metal stress, an overdose of ROS accumulation destroys regulation of the antioxidative system, ultimately causing a series of oxidative damage. In other aspects, Cd can damage the organisms’ antioxidative defense system; for example, Cd could bind with sulfhydryl groups in oxidative enzymes and antioxidative molecules, thereby reducing its ability to scavenge ROS. Cd2+ can also replace Zn2+, Fe2+, and other metal ions in proteins, resulting in an increase in intracellular free metals, which produce excessive ROS through Fenton and Haber–Weiss reactions, and eventually cause lipid peroxidation.
When organisms are in a state of oxidative stress, the antioxidative system is activated to remove excessive ROS [6,7]. Superoxide dismutases (SODs) are important antioxidative enzymes that are widely present in eukaryotes and prokaryotes; they can scavenge excess oxygen free radicals and protect the organism from oxidative damage [8]. According to the different metal auxiliary groups, Cu, Zn, Mn, Fe, and Ni, SODs can be divided into four types: Cu/Zn SOD, Mn-SOD, Fe-SOD, and Ni-SOD [9]. Among these types, Cu/Zn SOD is one of the most important free radical scavenging members and exists primarily in the cytoplasm and intercellular matrix. The Cu/Zn SOD family can be divided into intracellular Cu/Zn SOD (icCu/Zn SOD), encoded by the SOD1 gene, and extracellular Cu/Zn SOD (ecCu/Zn SOD), encoded by SOD3 [10]. icCu/Zn SOD is most widely distributed in organisms, mainly in the eukaryotic cytoplasm and also in the chloroplast matrix, peroxisome, and mitochondria [11] while ecCu/Zn SOD is found in the extracellular and cytoplasmic matrices [12]. When organisms are exposed to heavy metals, the expression of Cu/Zn SOD is upregulated to maintain normal physiological functions and scavenge free radicals. For example, after exposing clams to Cd, Fang et al. [13] reported that the expression of Cu/Zn SOD messenger ribonucleic acid (mRNA) was significantly increased in Mactra veneriformis, indicating that Cu/Zn SOD protected clams from Cd toxicity. When Euplotes crassus was exposed to Cd, antioxidation due to Cu/Zn SOD was observed [14].
Phascolosoma esculenta (Sipuncula: Phascolosomatidea) is a Sipuncula that lives in an intertidal flat, and its habitat is easily affected by heavy metal pollution of coastal regions. P. esculenta are sensitive to changes in the environmental conditions, which could reflect the pollution status. In addition, the relative stable life cycle of P. esculenta makes this species countable. Thus, P. esculenta are a potential bioindicator for monitoring heavy metal pollution in marine mudflats. In order to understand the toxic effect of Cd on P. esculenta’s response under Cd stress (Pe-Cu/Zn SOD), the acute toxicity of Cd on P. esculenta was determined. The effects of Cd on oxidative stress in P. esculenta were analyzed by measuring the content or activity of malondialdehyde (MDA) and several important antioxidants, such as SOD, CAT, and reduced glutathione (GSH), in coelomic fluid. Gene cloning, quantitative polymerase chain reaction (qPCR), enzyme activity determination, prokaryotic expression, and flow cytometry were used to analyze the expression and antioxidant function of Pe-Cu/Zn SOD under Cd stress. This study provides basic data for the molecular toxicology research of P. esculenta and lays a foundation for further studies on Pe-Cu/Zn SOD function.
2. Results
2.1. Response of Antioxidative Indexes in the Supernatants after Cd Exposure
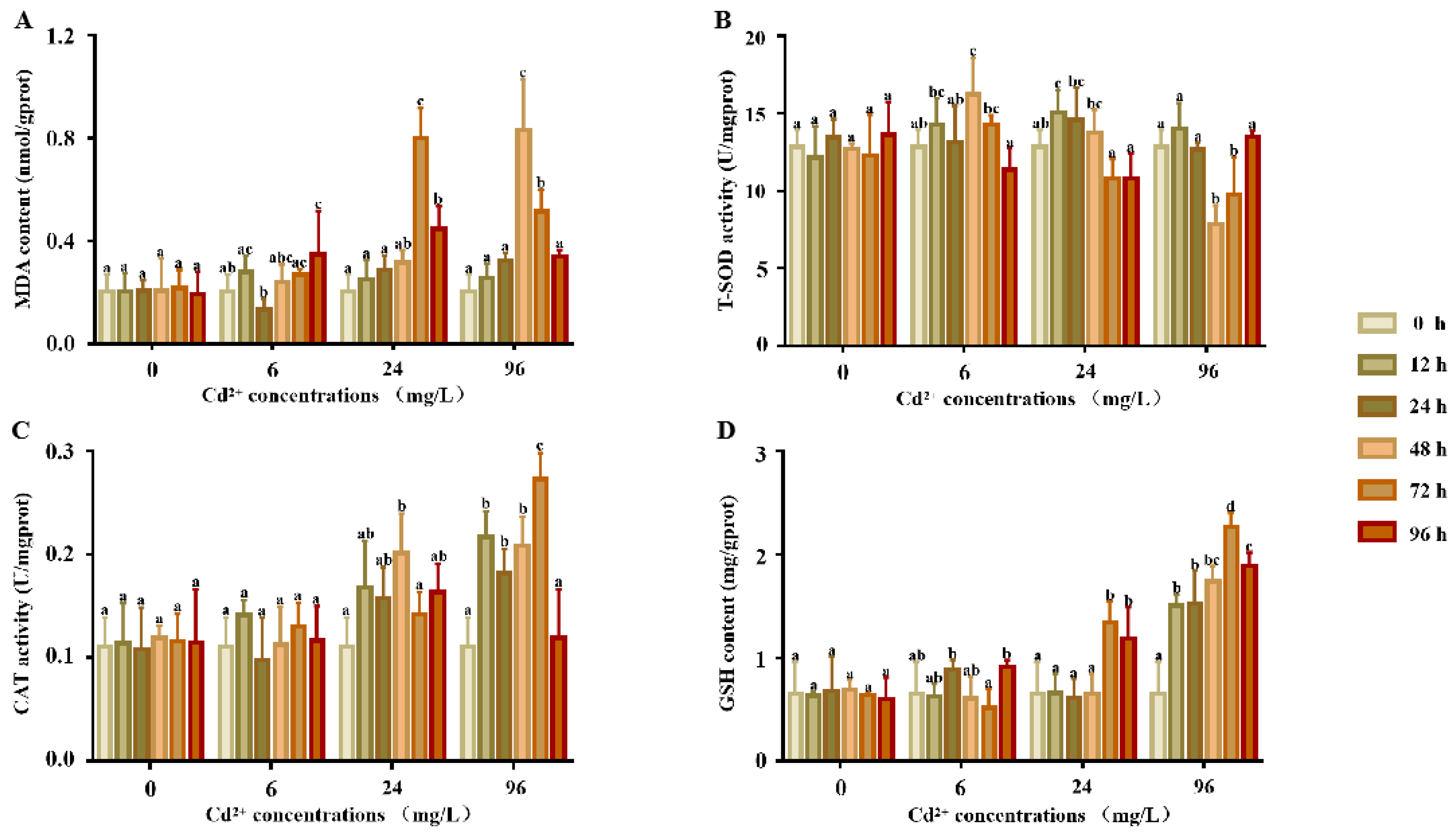
There were no significant differences in the MDA content (Figure 1A) between each time point in the experimental and control groups (p > 0.05). In the 6 mg/L group, MDA was significantly increased 96 h after exposure, but no significant differences were noted at any other points in time. When compared to the control groups, the MDA contents of the 24 and 96 mg/L groups were significantly increased. The MDA content reached a peak at 72 h in the 24 mg/L group and 48 h in the 96 mg/L group and then decreased.
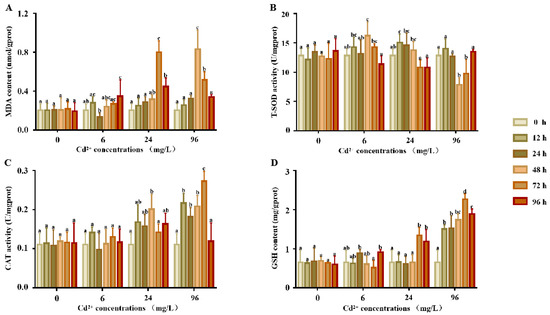
Figure 1.
T-SOD, GST activity, and GSH and MDA contents in the supernatants of P. esculenta after Cd exposure. (A) The content of MDA in supernatants after Cd exposure. (B) The activity of total superoxide dismutase (T-SOD) in the supernatants after Cd exposure. (C) The activity of CAT in the supernatants after Cd exposure. (D) The content of GSH in the supernatants after Cd exposure. The color of the columns shows the different experimental times and the abscissa shows the Cd2+ concentrations. Lowercase letters indicate the significant differences (p < 0.05) of the different concentration groups at the same time (mean ± sd, n = 6).
The response of T-SOD activity (Figure 1B) showed no significant differences at any point in time in the control groups (p > 0.05). In comparison, the SOD activity of the 6 and 24 mg/L groups first showed a significant increase and then decreased, with peaks at 48 and 12 h, respectively. In the 96 mg/L group, SOD activity first decreased from 0 to 48 h and then increased from 48 to 96 h.
The response of the CAT activity is shown in Figure 1C. There were no significant differences in the CAT activity between each time point in the control and 6 mg/L groups. Compared to the control groups, the CAT activity of the 24 and 96 mg/L groups showed a significant increase, the CAT activity first increased and then decreased, and the activity reached a peak at 48 and 72 h, respectively.
The response of the GSH content is shown in Figure 1D. The GSH content of all treatment groups was significantly higher than that of the control group. There were no significant differences in the GSH content between each time point in the control and 6 mg/L groups. Compared to the control groups, the CAT activity of the 24 and 96 mg/L groups was significantly higher, the CAT activity increased first and then decreased, and the activity reached a peak at 48 and 72 h, respectively.
2.2. Pe-Cu/Zn SOD Sequence Analysis and Protein Structure
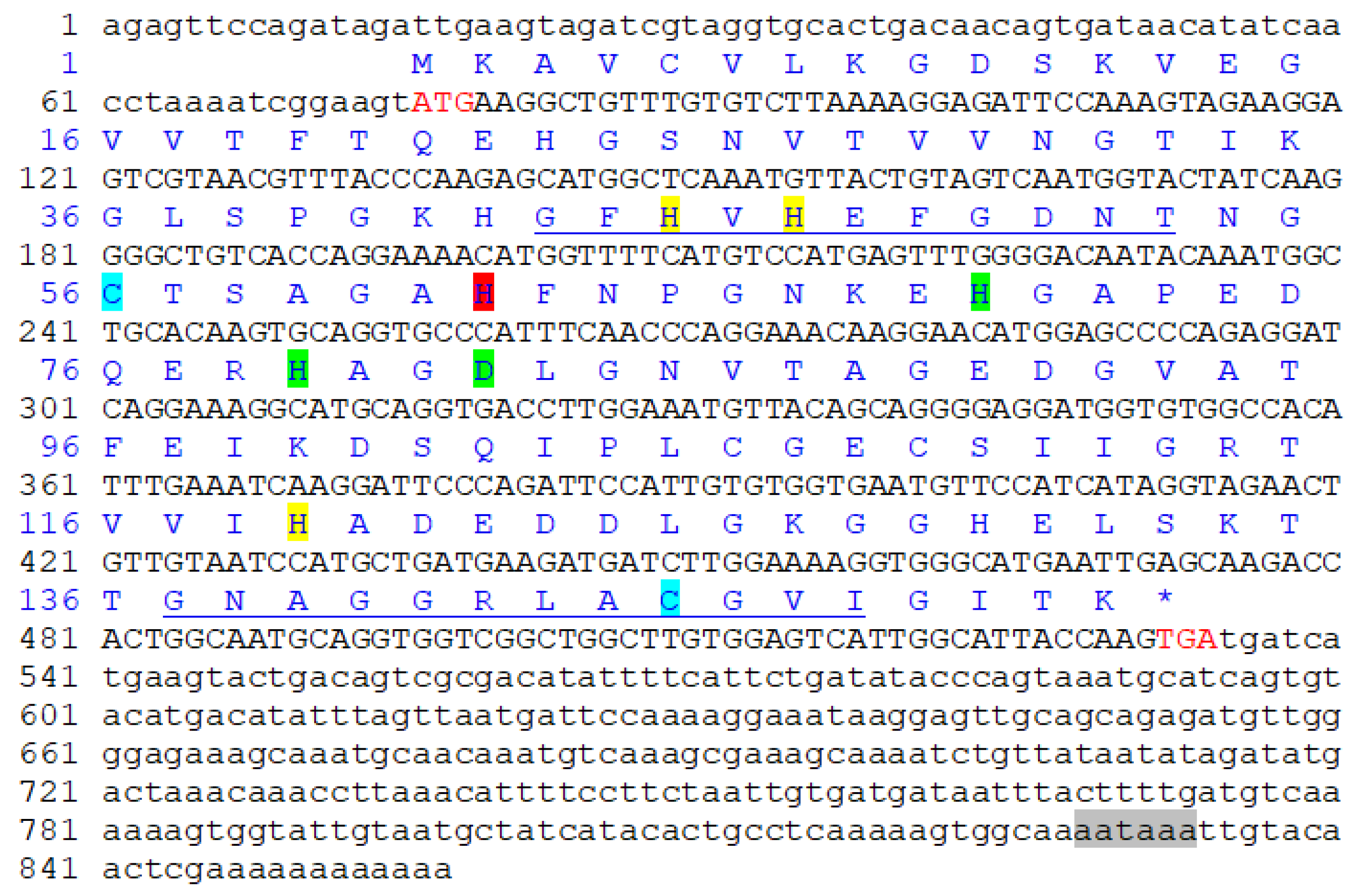
The obtained total length of Pe-Cu/Zn SOD cDNA was 857 bp, including 75 bp 5 ‘UTR, 323 bp 3’ UTR, and 459 bp open reading frame. There was a tailed signal sequence AATAAA upstream of the Polya tail (Figure 2). The open reading frame encoded 152 amino acids. The predicted molecular weight of the Pe-Cu/Zn SOD protein was approximately 15.6 KD and the theoretical isoelectric point was 5.65.
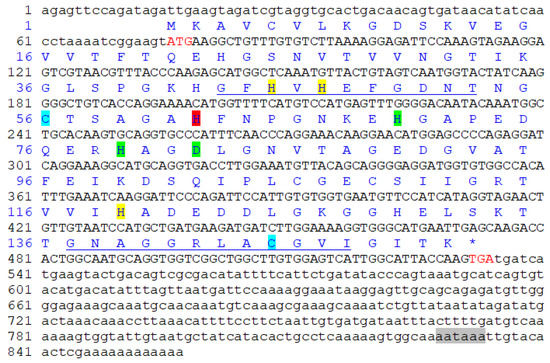
Figure 2.
Pe-Cu/Zn SOD full-length cDNA and amino acid sequence. The red letters ATG and TGA indicate the start and stop codons, respectively. The highlights represent different binding sites: yellow = Cu2+-binding site; green = Zn2+ binding site; red = Cu2+- and Zn2+-binding site; blue = cysteine site; the underline represents the Cu/Zn SOD family tag sequence; and the gray shade represents the 3′ terminal tailing signal. * represents the termination codon.
2.3. Sequence Alignment and Phylogenetic Analysis
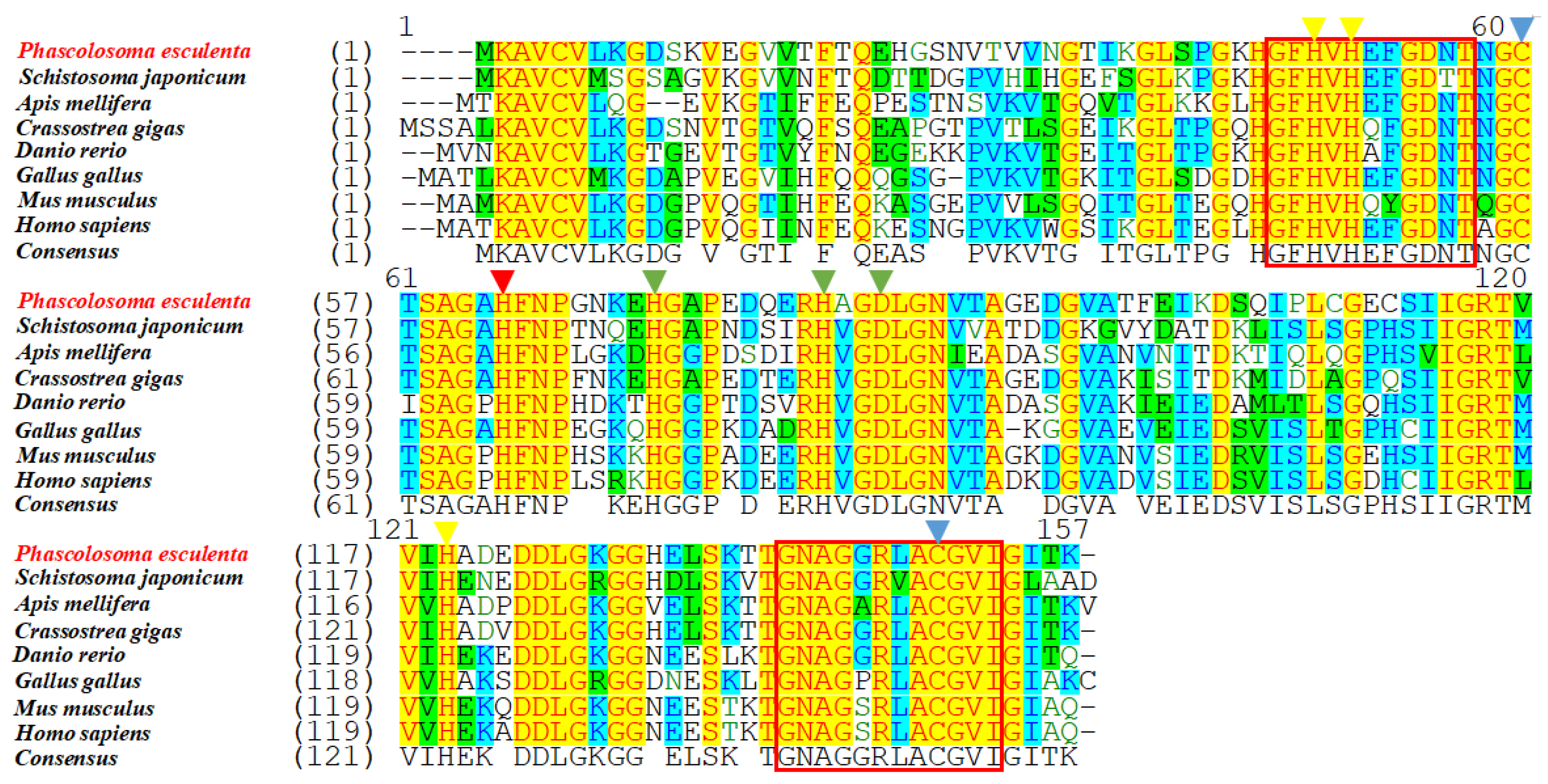
The predicted aa sequences of the Pe-Cu/Zn SOD proteins were compared and aligned with their homologs in other species. The results showed that the Cu/Zn SOD sequence was highly conserved, and the similarity of Pe-Cu/Zn SOD with Crassostrea gigas and Schistosoma japonicum was 78.8% and 72.5%, respectively. In addition, Pe-Cu/Zn SOD conserved the family characteristic sequences GFHVHEFGDNT and GNAGGRLACGVI (Figure 3).
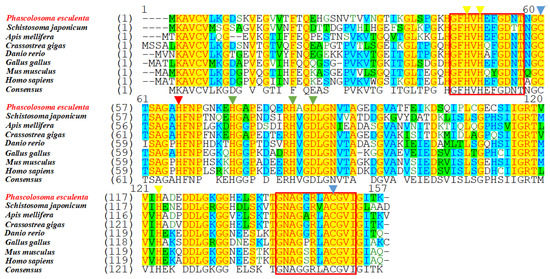
Figure 3.
Multiple sequence alignment of Cu/Zn SOD homologous proteins. The yellow triangles indicate the Cu2+-binding sites, the green triangles indicate the Zn2+-binding sites, the red triangle indicates the Cu2+- and Zn2+-binding sites, the blue triangles indicate the cysteine sites, and the red box indicates the Cu/Zn SOD family tag sequence. The consensus and identity positions of the Pe-Cu/Zn SOD sequence with H. sapiens, M. musculus, G. gallus, D. rerio, C. gigas, A. mellifera, and S. japonicum were 68.8% and 63.0%, 68.8% and 63.0%, 71.8% and 66%, 68.8% and 65.6%, 78.8% and 76.3%, 70.8% and 63.6%, and 72.5% and 64.1%, respectively.
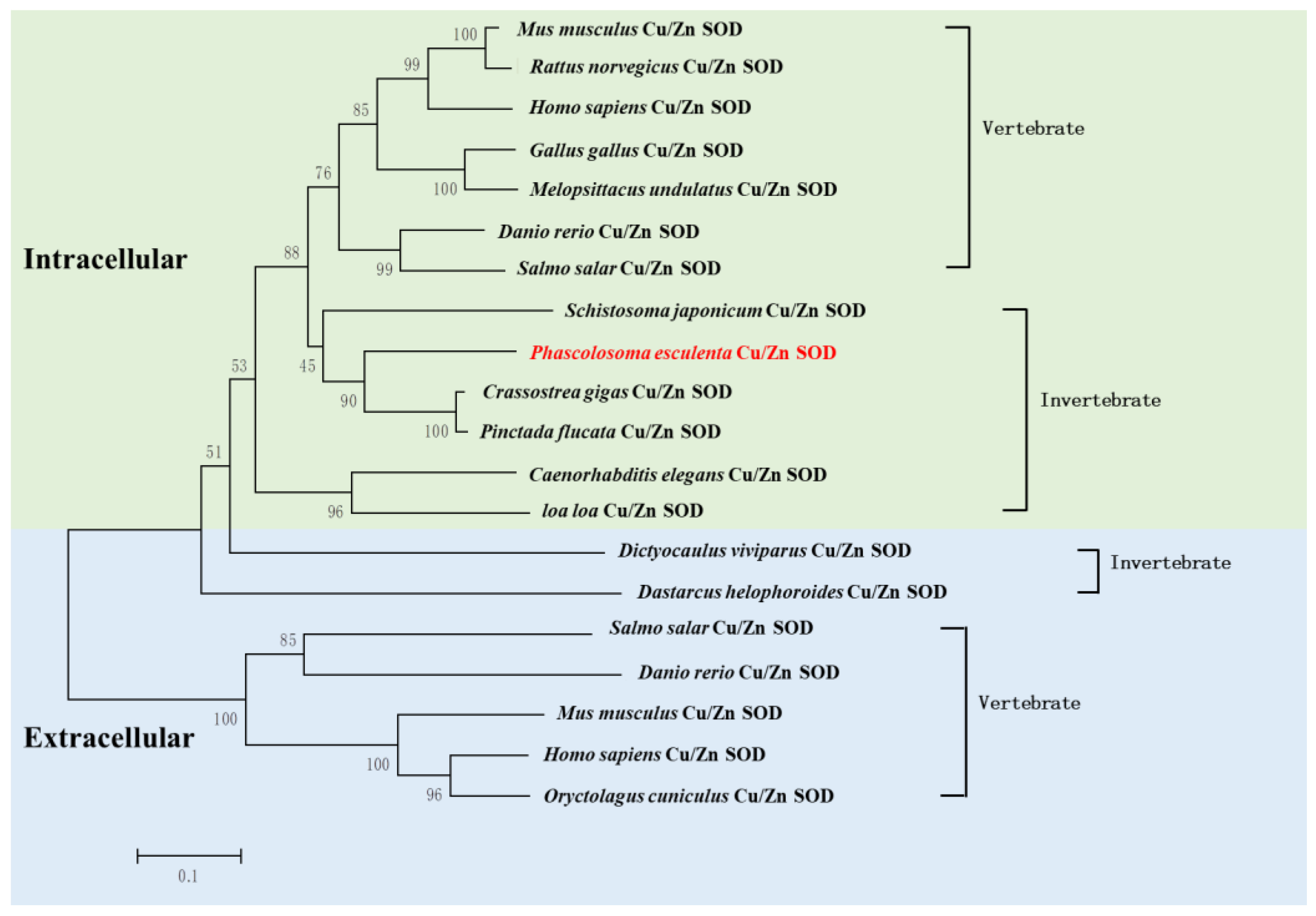
Additionally, we analyzed the phylogenetic relationships of Cu/Zn SOD using a neighbor-joining tree. The results showed that Pe-Cu/Zn SOD was located in the invertebrate evolutionary branch of icCu/Zn SOD and was far away from the evolution of ecCu/Zn SOD and icCu/Zn SOD in vertebrates (Figure 4).
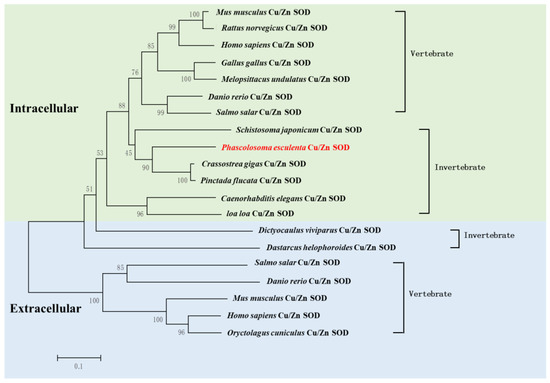
Figure 4.
Phylogenic analysis of Pe-Cu/Zn SOD. The evolutionary tree was constructed using MEGA5.1 software. P. esculenta is shown in red font. Pe-Cu/Zn SOD is located in the invertebrate clade of intracellular Cu/Zn SOD. Scale bar: 0.1 of the branch length value.
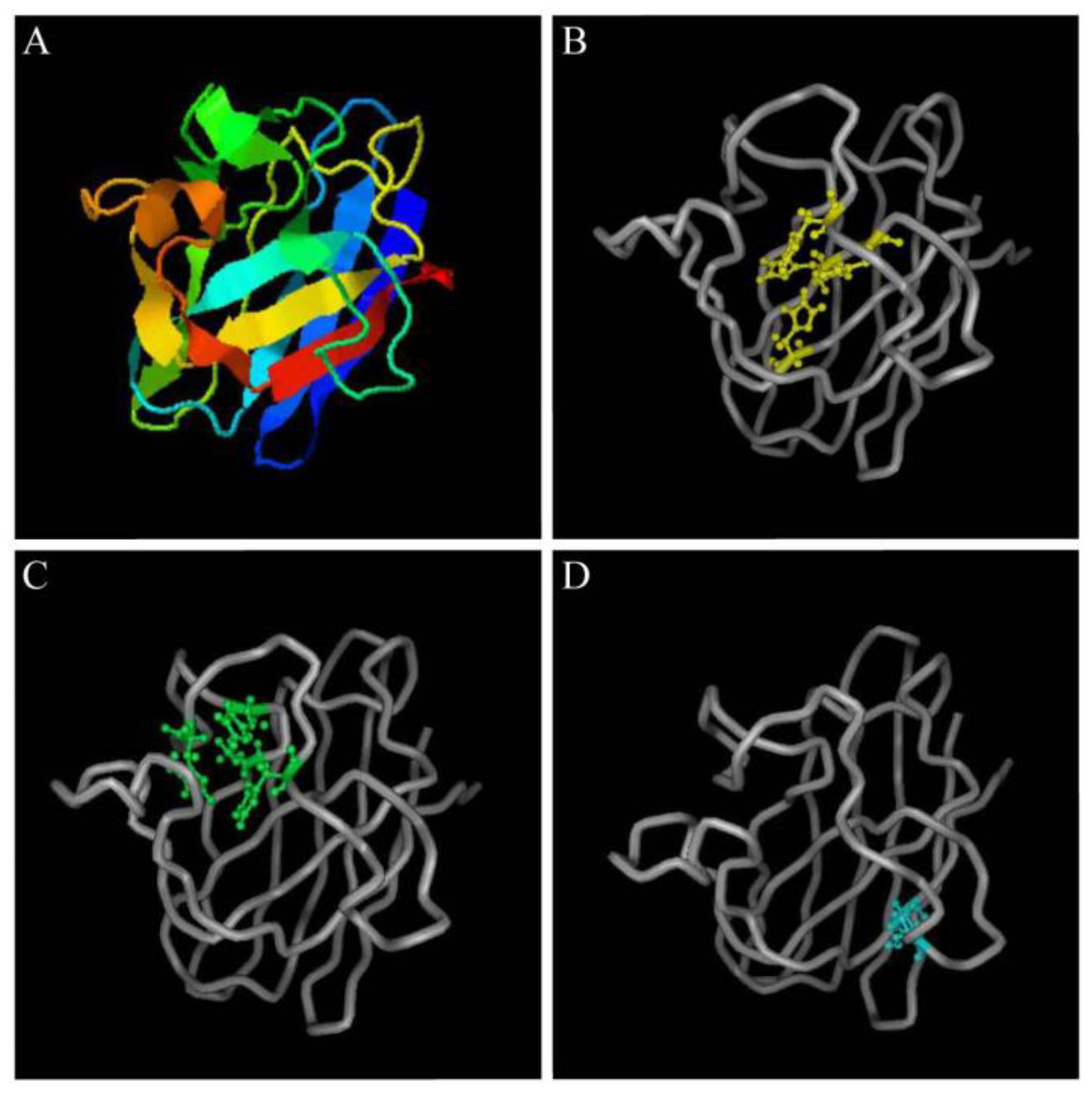
2.4. Structural Characteristics of the Pe-Cu/Zn SOD Protein
The predicted Pe-Cu/Zn SOD protein had conserved Cu2+- and Zn2+-binding sites, in which Cu2+ coordinated with His-45, -47, -62, and -119 while Zn2+ coordinated with His-62, -70, -79, and Asp 82. A pair of intrachain disulfide bonds stabilizing the enzyme structure was formed between cysteine Cys 56 and Cys 145–SH (Figure 5).
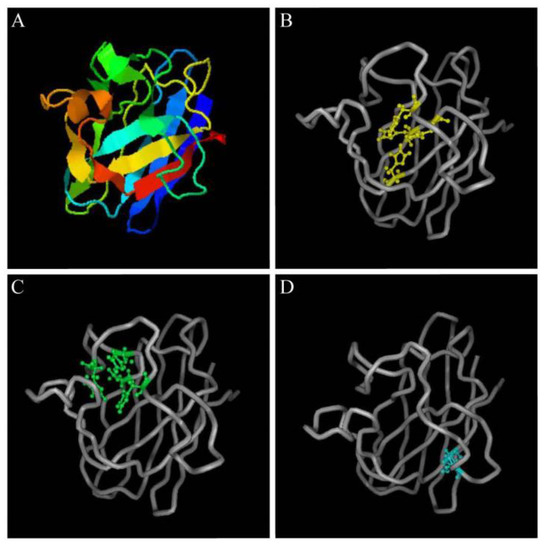
Figure 5.
Structural prediction of Pe-Cu/Zn SOD proteins. (A) Predicted tertiary structure of Pe-Cu/Zn SOD; (B) Cu2+-binding sites; (C) Zn2+-binding sites; and (D) two cysteine sites.
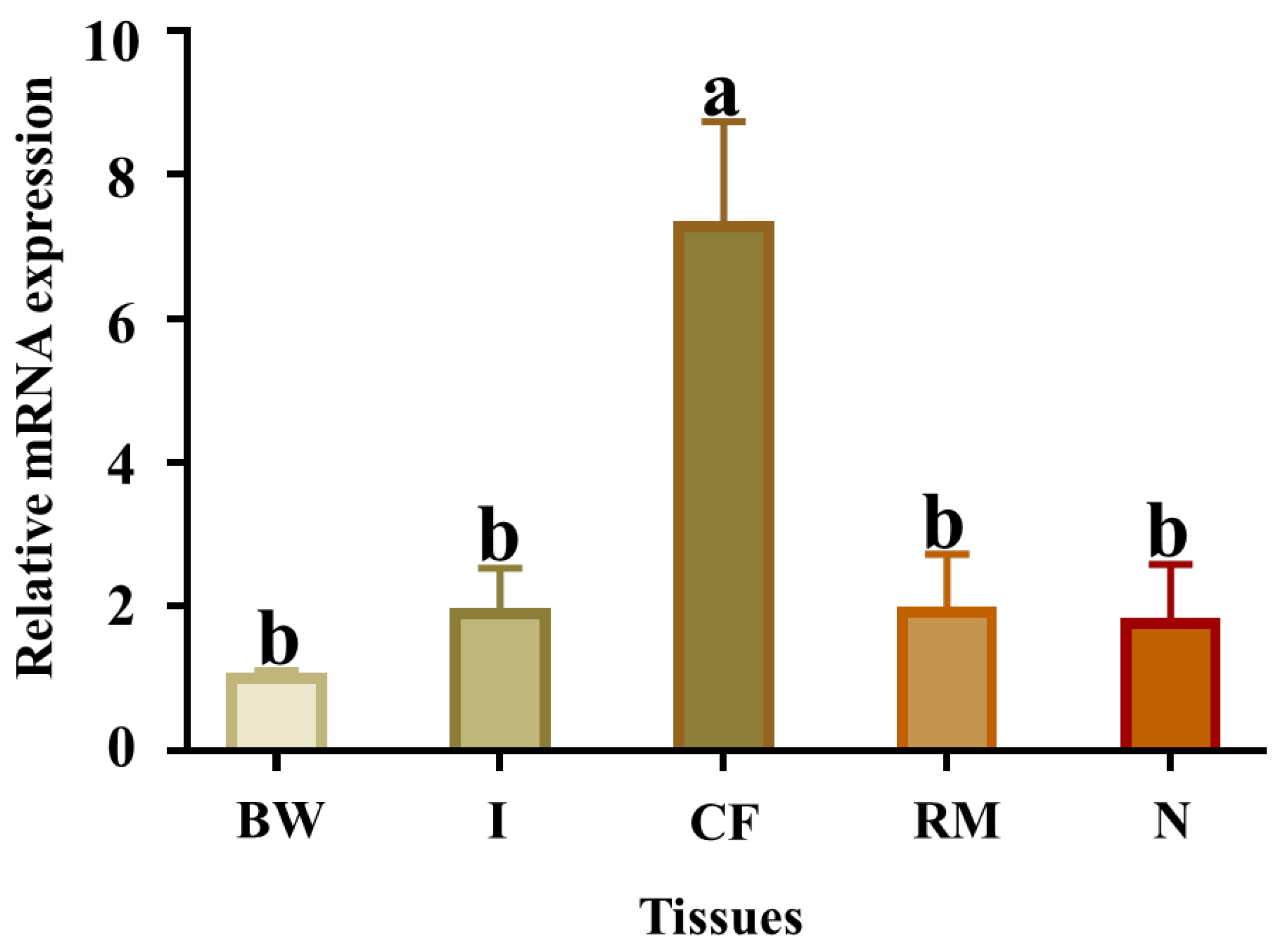
2.5. Tissue Expression Patterns of Pe-Cu/Zn SOD mRNA
The qPCR results showed that Pe-Cu/Zn SOD mRNA was widely expressed in all tissues examined, with the highest levels in the coelomic fluid tissue (Figure 6).
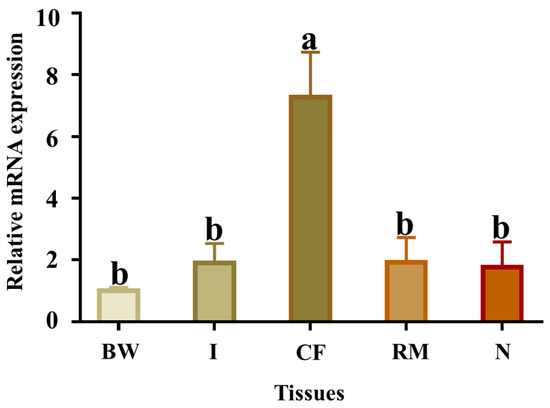
Figure 6.
Relative abundance of Pe-Cu/Zn SOD mRNA detected by qPCR in different tissues. GAPDH was used as a positive control. BW, body wall; CF, coelom fluid; I, intestine; N, nephridium; RM, retractor muscle. Lowercase letters indicate a significant difference (p < 0.05) between different tissues (mean ± standard deviation, n = 6).
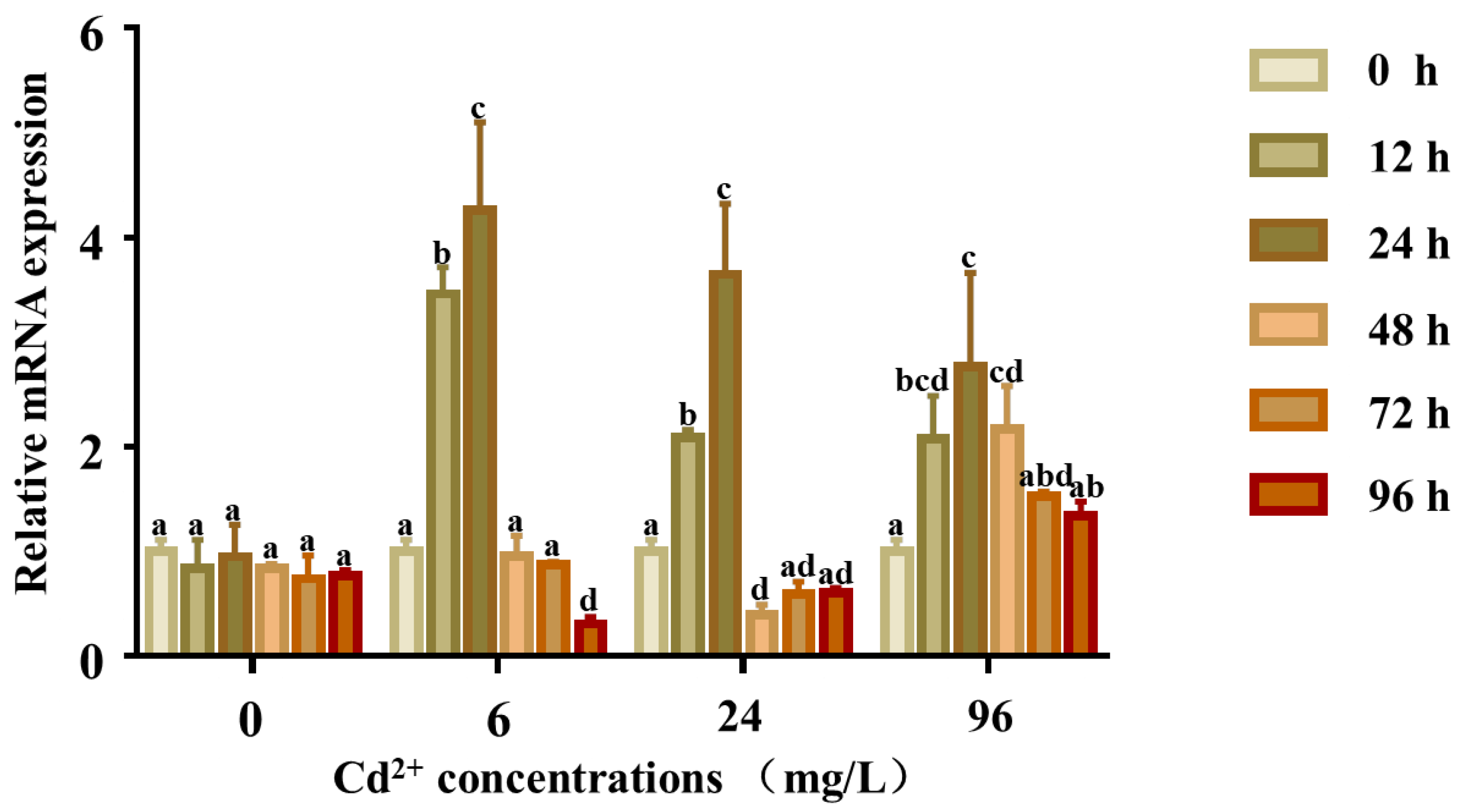
2.6. Expression Characteristics of Pe-Cu/Zn SOD under Cd Stress
The expression levels of Pe-Cu/Zn SOD mRNA were all increased after exposure to Cd (6, 24, and 96 mg/L) compared with the control group. In all treatment groups, the expression levels of Pe-Cu/Zn SOD mRNA showed a consistent trend, which increased from 0 to 24 h. In the 6 and 96 mg/L groups, the expression levels of Pe-Cu/Zn SOD mRNA decreased from 24 to 96 h. The expression levels fluctuated in the 24 mg/L group, increasing from 48 to 72 h and then decreasing from 72 to 96 h (Figure 7).
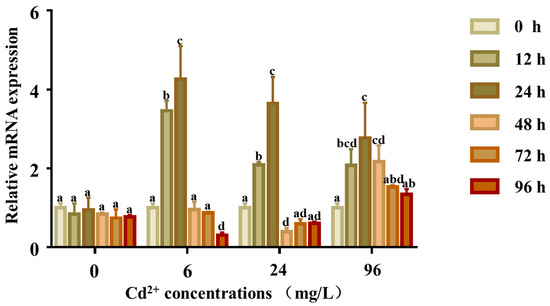
Figure 7.
Expression of the Pe-Cu/Zn SOD gene in coelom fluid under Cd2+ stress. The expression level of Pe-Cu/Zn SOD mRNA in the coelom fluid after Cd exposure. GAPDH served as an internal control, and each data point represents the average fold change relative to the Pe-Cu/Zn SOD mRNA expression in the 0 mg/L group at 24 h, and the lowercase letters indicate significant differences (p < 0.05) between the data of different concentration groups at the same time (mean ± SD, n = 6).
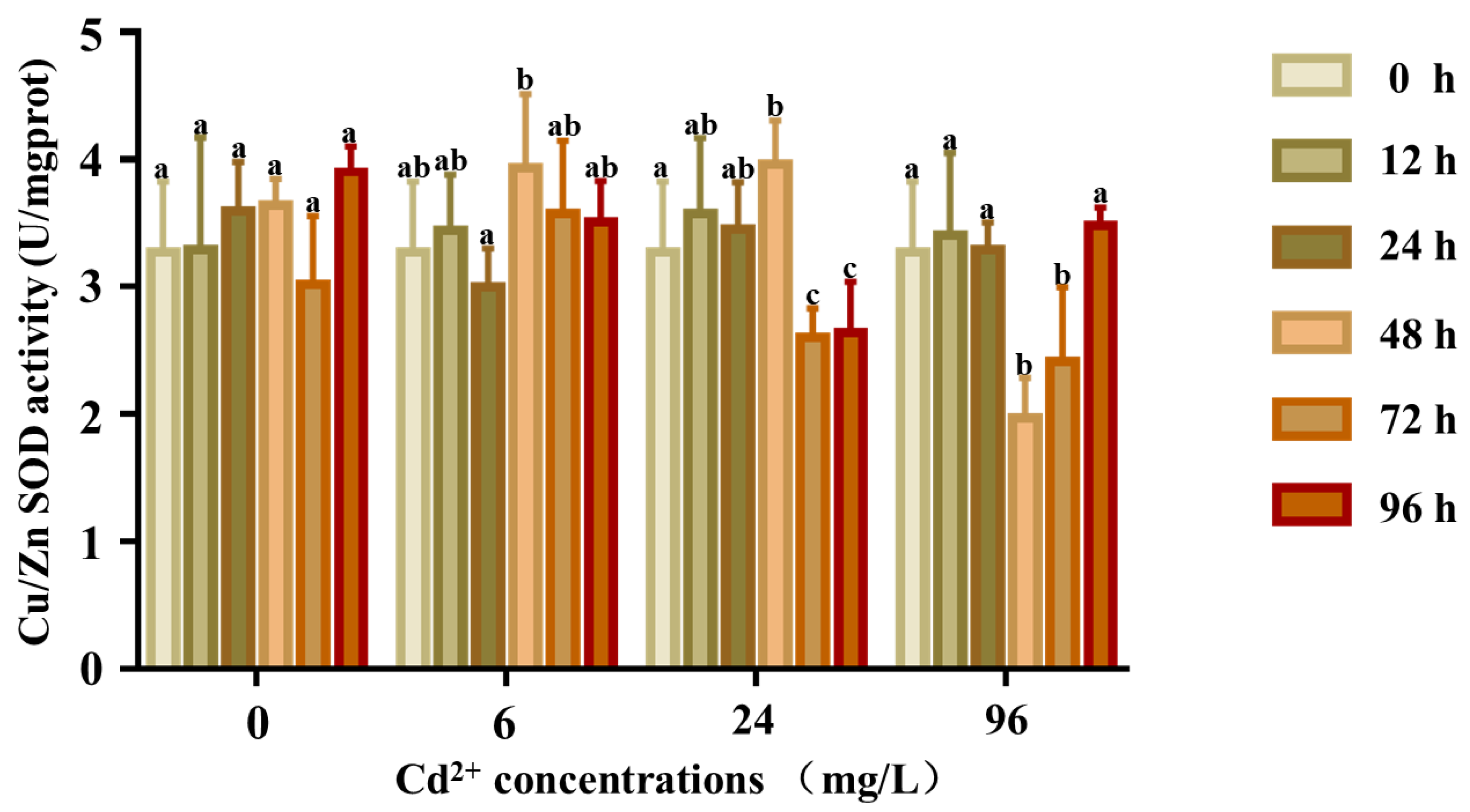
The response of the SOD activity is shown in Figure 8. There were no significant differences in the SOD activity at any time in the control groups (p > 0.05), whereas the SOD activity of the 6 and 24 mg/L groups was significantly higher at 12 and 24 h, respectively. The activity first increased and reached a peak at 24 h and then decreased to lower than that of the control group. In the 96 mg/L group, the SOD activity first increased till 48 h and then returned to normal levels.
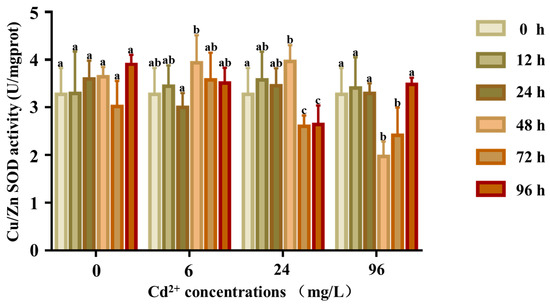
Figure 8.
Cu/Zn SOD activity in the coelom fluid of P. esculenta under Cd2+ stress. The activity of Cu/Zn SOD in coelom fluid after Cd exposure. The color of the columns shows the different experimental times, and the abscissa shows the Cd2+ concentration. Lowercase letters indicate significant differences (p < 0.05) in different concentration groups at the same time (mean ± SD, n = 6).
2.7. Expression and Purification of Pe-Cu/Zn SOD Protein
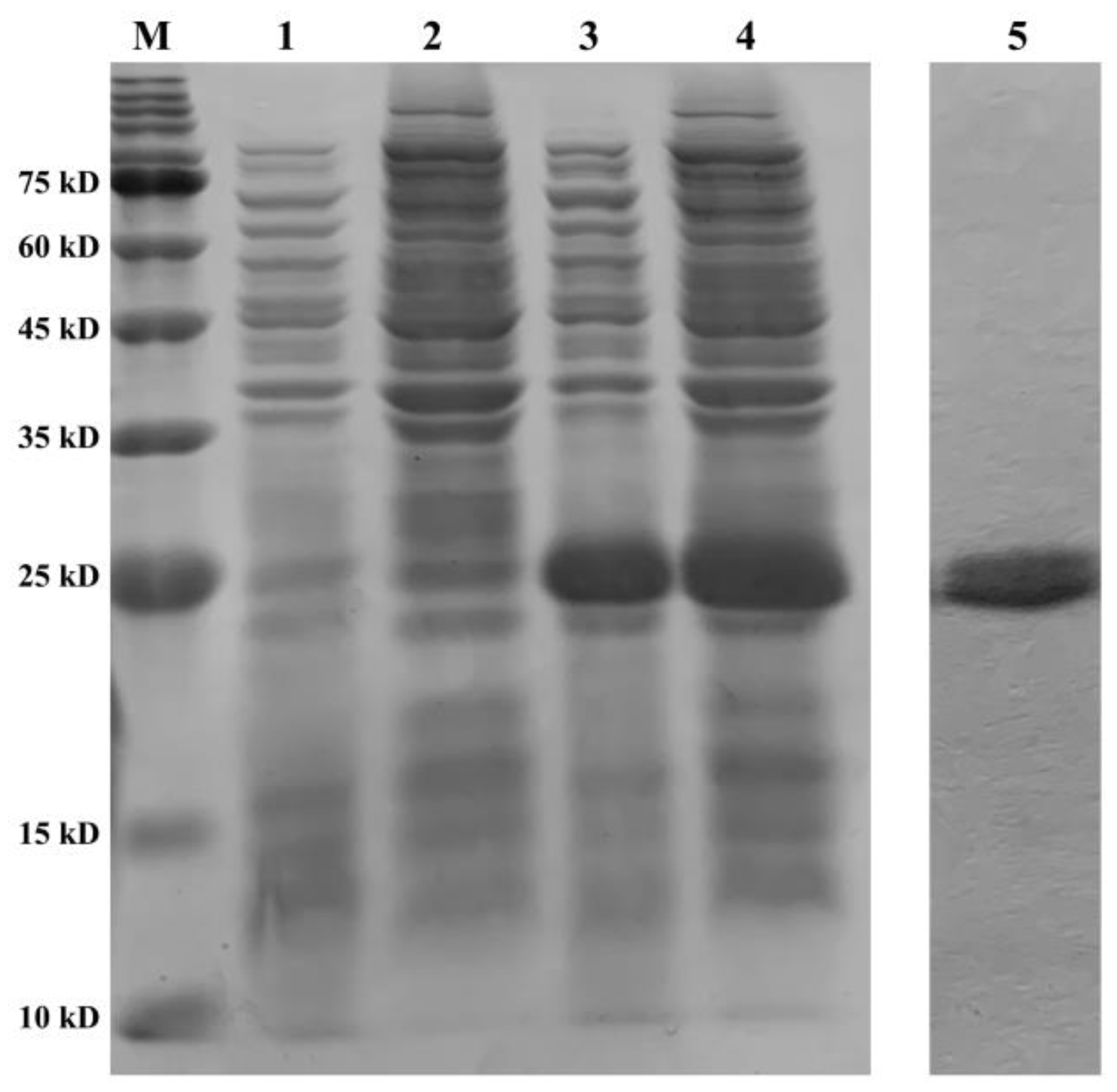
The recombinant plasmid of PeCu/Zn SOD was constructed, and the recombinant protein was induced by IPTG. The recombinant protein was expressed in the precipitate and supernatant of the broken E. coli cells (Figure 9). After purification, a single band with a molecular weight of approximately 21 kDa was obtained (Figure 9).
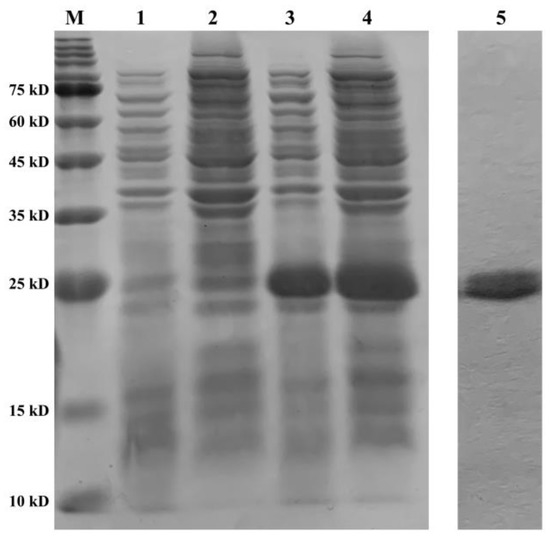
Figure 9.
Purification and validation of the recombinant protein. Line M shows the protein marker; line 1 shows the supernatant of BL21 (pET28a) at 8 h; line 2 shows the precipitate of BL21 (pET28a) at 8 h; line 3 shows the supernatant of BL21 (pET28a-Pe-Cu/Zn SOD) at 8 h; line 4 shows the precipitate of BL21 (pET28a-Pe-Cu/Zn SOD) at 8 h; and line 5 shows the purified protein Pe-Cu/Zn SOD.
2.8. Cd Tolerance of Recombinant E. coli
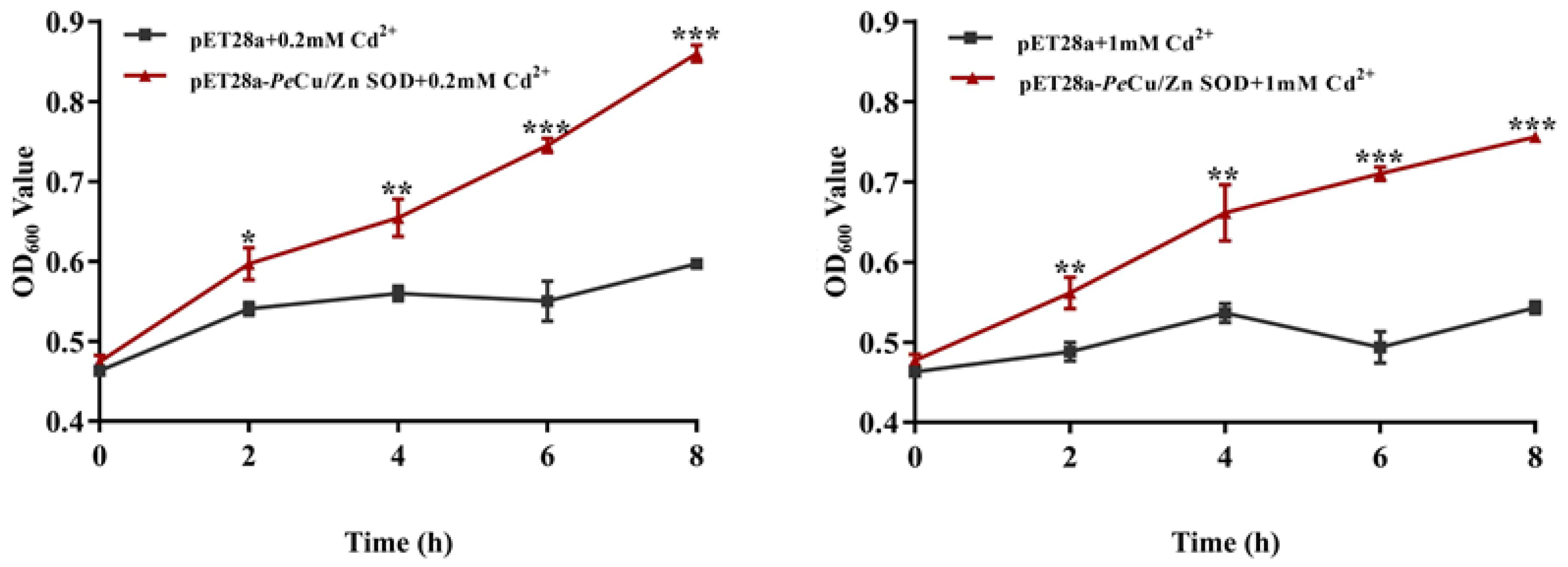
The transformed bacteria BL21 (pET28a) and BL21 (pET28a-Pe-Cu/Zn SOD) were exposed to 0.2 and 1 mM Cd2+. The results showed that OD600 of the two groups was significantly different, and OD600 of the recombinant bacteria was significantly higher than that of the control group. In the 0.2 mM Cd treatment group, there was a significant difference in the growth status between the control group and the recombinant bacteria after IPTG induction for 2 h (p < 0.05), and the growth status of the recombinant bacteria was further enhanced after IPTG induction for 4 h (p < 0.01) while the growth advantage of the recombinant bacteria was more significant after IPTG induction for 6 and 8 h (p < 0.001). In the 1 mM Cd-treated group, there was a significant difference between the control group and the recombinant bacteria after 2 and 4 h of IPTG induction (p < 0.01). At 6 and 8 h of induction, the growth advantage of recombinant bacteria was further enhanced (p < 0.001), indicating that Pe-Cu/Zn SOD significantly improved the Cd tolerance of E. coli (Figure 10).
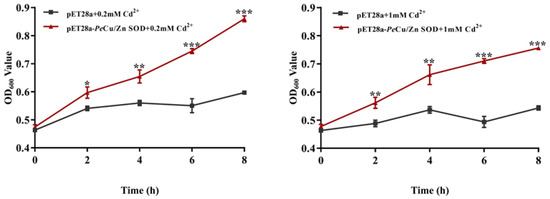
Figure 10.
Effects of metal ions on the growth of E. coli. This indicates that the growth status of pET28a-Pe-Cu/Zn SOD E. coli was significantly higher than that of pET28a-DE3 E. coli (p < 0.5). All data is expressed as the mean ± standard deviation (n = 3). *: p < 0.05, **: p < 0.01, and ***: p < 0.001.
2.9. Regulation of Pe-Cu/Zn SOD of the ROS Content Induced by Cd in Coelomocytes
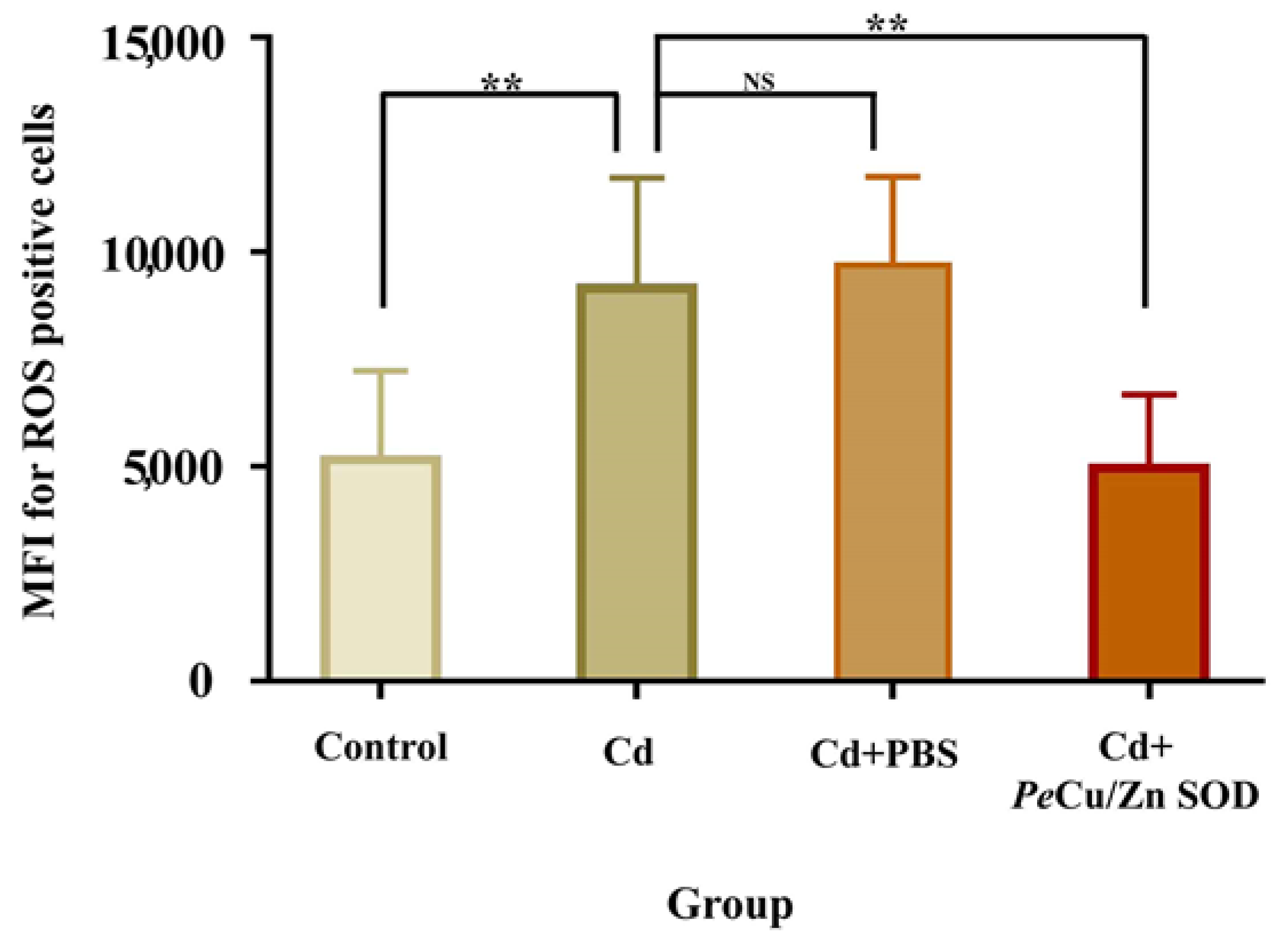
The results showed that the content of ROS in coelomocytes of P. esculenta was significantly increased after 96 mg/L Cd stress (p < 0.01), and the content of ROS in the Cd + Pe-Cu/Zn SOD group was significantly lower than that in the Cd group alone (p < 0.01), but there was no significant difference compared with that in the control group (Figure 11), indicating that Pe-Cu/Zn SOD had the ability to scavenge ROS, with a protective effect against Cd-induced oxidative damage. In addition, there was no significant difference in the ROS content between the Cd and Cd + PBS groups (p > 0.05), indicating that the injection treatment had little effect on the content of ROS in coelomocytes (Figure 11).
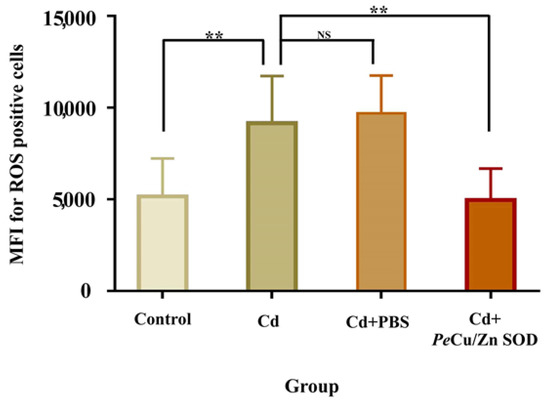
Figure 11.
Regulation of Pe-Cu/Zn SOD of the ROS content induced by cadmium in coelomocytes. The ROS content in the coelomic fluid cells of P. esculenta was significantly increased under Cd stress; the ROS content in the Cd + Pe-Cu/Zn SOD group was significantly decreased compared with that in the Cd group alone and showed no significant difference with the control group. There was no significant difference between the Cd and Cd + PBS groups. All data are expressed as the mean ± standard deviation (n = 6). **: p < 0.01, NS: no significant difference (p > 0.05).
3. Discussion
3.1. Sequence and Protein Structure of Pe-Cu/Zn SOD
In this study, we cloned the full-length Cu/Zn SOD cDNA sequence of P. esculenta, which contained an 857 bp nucleotide and encoded 152 amino acids. The unstable signal ATTTA appeared in the 3′ untranslated region, which was also found in Meretrix meretrix [15], Argopecten irradians, and other marine invertebrates [16]. It has been reported that the unstable signal ATTTA may play an important role in the degradation of excess mRNA. Cu/Zn SOD can be divided into two types: exCu/Zn SOD with a signal peptide at the N-terminal and icCu/Zn SOD without a signal peptide [10]. ExCu/Zn SOD is generally located in the extracellular matrix, with a length of 176–251 amino acids; icCu/Zn SOD mainly exists in the cytoplasm and nucleus, with a length of 147–167 amino acids [17]. In this study, Pe-Cu/Zn SOD contained 152 amino acids without a signal peptide at the N-terminal. Phylogenetic tree analysis showed that Pe-Cu/Zn SOD was located in the intracellular branch of Cu/Zn SOD.
The amino acid sequence of Pe-Cu/Zn SOD was compared with that of homologous proteins of other species. Indeed, Kim et al. [18] also found the conserved tag sequences GNAGGRAACGVI and GFHIHQFGDNT of the Cu/Zn SOD family in Pe-Cu/Zn SOD. As predicted, the residue Cu2+-binding sites (His-45, -47, -62, and -119) and Zn2+-binding sites (His-62, -70, -79, and Asp-82) were also found in Pe-Cu/Zn SOD. Cys-56 and Cys-145 form disulfide bonds in the Pe-Cu/Zn SOD protein, which plays an important role in maintaining the structural stability of the enzyme [19]. These conserved amino acids are essential for the structure and function of Cu/Zn SOD and may be involved in the stabilization of the SOD conformation under adverse environmental conditions.
3.2. Physiological and Biochemical Changes of P. esculenta under Cd Stress
The main toxic effect of Cd on organisms is oxidative damage [20]. As an inducer of peroxide, Cd can stimulate the production of excessive free radicals, which eventually leads to oxidative damage [21]. MDA is a product of lipid peroxidation induced by oxygen free radicals and is a recognized biomarker in marine invertebrates [22]; for example, the change in the MDA content in the serum of Palaemon carincauda reflects the degree of heavy metal stress [23]. It has been reported that MDA is sensitive to Cd exposure; it typically increases slowly in organisms at low Cd concentrations while under high Cd concentrations, it increases sharply for a short time and then decreases [24]. We found the same expression pattern in this study. The MDA content increased slowly in the low concentration group but increased sharply in the high concentration group, indicating that the high concentration of Cd ions is toxic to P. esculenta. We suggest that MDA is related to antioxidation.
SOD is also an important antioxidant enzyme, and its activity often reflects the degree of oxidative stress. Sun et al. [25] found that a low dose of Cd induced an increase in SOD enzyme activity in the visceral mass and gills of Tegillarca granosa Linnaeus while a high dose of Cd inhibited it. Wang [26] found that under Cd stress, the SOD activity of Mizuhopecten yessoensis increased and the enzyme activity decreased. Under different concentrations of Cd stress, the SOD activity in the gills of Sinanodonta woodiana demonstrated the rule of “low concentration induction, high concentration inhibition” [27]. Studies have shown that Cd can change the original molecular conformation of SOD by occupying Zn or Mn structural sites in the SOD protein, thus inhibiting enzyme activity [28,29]. In summary, in the early stages of Cd exposure, SOD activity increased to eliminate excessive ROS. Under high concentrations of Cd stress, a large amount of ROS accumulated in the cells and the SOD activity decreased due to the inhibition by toxic substances. Our results are consistent with these findings. The change in SOD in the coelomic fluid of P. esculenta showed the same pattern of hormesis at different concentrations of Cd. At low (6 mg/L) and medium (24 mg/L) Cd stress, SOD enzyme activity first increased and then decreased, indicating that a lower concentration of Cd causes the body to produce oxygen free radicals, and the body regulates this balance by producing the SOD enzyme. In the high concentration group (96 mg/L), the activity of SOD was inhibited, which indicated that when the concentration of Cd exceeded a certain threshold, the activity of SOD decreased, resulting in tissue damage.
CAT is known to disproportionate H2O2 into water and oxygen molecules. Livingstone et al. [30] found that CAT functions in the antioxidative system of marine invertebrates. The CAT activity in hepatopancreas of Haliotis discus hannai significantly increased after Cd stress, indicating its role in resisting ROS [31]. After Cd exposure, CAT activity in the tissues of Mactra veneriformis and Ruditapes philippinarum was significantly increased [32,33]. In this study, CAT activity in the coelomic fluid of P. esculenta did not change significantly in the control group or the 6 mg/L experimental group but increased significantly in the 24 and 9–6 mg/L Cd groups. It can be inferred that a large amount of H2O2 was produced in the coelomic fluid of P. esculenta under high Cd stress, which induced an increase in CAT activity.
GSH is a non-enzymatic antioxidant that scavenges ROS [34,35]. The change in the GSH content reflects the redox state of the cells [21]. An increase in the GSH content after Cd stress has been found in many marine invertebrates, for example, Neomysis awatschensis [36]. In this study, the GSH content in coelomic fluid increased significantly after Cd stress at 24 and 96 mg/L Cd, which indicates that GSH plays an important role in resistance to Cd-induced oxidative stress.
In conclusion, we found that Cd stress changes the physiological and biochemical indices of coelomic fluid. When exposed to Cd stress, the antioxidant enzymes and antioxidative molecules of P. esculenta were activated to resist oxidative damage.
3.3. Response of Cu/Zn SOD to Cd Stress
Cadmium can induce the formation of ROS and ultimately lead to oxidative damage [37,38]. As a member of the antioxidant system, Cu/Zn SOD is sensitive to Cd exposure. Cd can combine with the sulfhydryl group of SOD, disrupting the structure of SOD, and lead to a decrease in and inactivation of SOD [39]. When organisms are exposed to Cd stress, the activities of SOD and other antioxidant enzymes are enhanced [40,41]. Therefore, Cu/Zn SOD is a biomarker of early Cd exposure [42,43,44]. In marine invertebrates, Cu/Zn SOD has been reported to be involved in the defense against Cd stress. After exposure to Cd, the expression of Cu/Zn SOD in the digestive gland of M. veneriformis increased sharply, indicating that Cu/Zn SOD plays a role in maintaining cellular metabolic homeostasis and protecting clams from Cd toxicity [13].
Kim et al. [14] found that the relative expression of Cu/Zn SOD mRNA in Euplotes crassus increased after 0.025, 0.05, and 0.1 mg/L Cd treatment, indicating that Cu/Zn SOD may participate in the protection of cells against metal and mediated oxidative stress. For example, Zheng et al. [20] found that the Cu/Zn SOD mRNA expression and enzyme activity in Cristaria plicata were significantly increased after Cd stress, indicating that Cu/Zn SOD may play a role in scavenging free radicals. Xie et al. [45] reported that the Cu/Zn SOD activity of Corbicula fluminea was significantly increased after Cd stress and then decreased, which may be related to the elimination of free radicals and inhibition of enzyme activity.
In this study, the activity of Pe-Cu/Zn SOD mRNA and enzyme was detected after Cd stress. The results showed that Pe-Cu/Zn SOD mRNA was significantly induced after exposure to different concentrations of Cd. The activity of the enzyme increased under low concentrations (6 and 24 mg/L) of Cd but significantly decreased at high concentrations (96 mg/L). The activity of Cu/Zn SOD mRNA and enzyme changed significantly after Cd stress, indicating that Pe-Cu/Zn SOD is induced in response to the oxidative stress induced by Cd.
3.4. Antioxidant Function of Cu/Zn SOD
Cu/Zn SOD is an enzyme that can scavenge oxygen free radicals and has strong antioxidative and immunity capacity. To analyze its functions, an in vitro experiment with purified protein was performed using P. esculenta. Hwang et al. [46] found that Cu/Zn SOD improved the antioxidant capacity of Candida albicans cells and Liu et al. [47] found that Glyphodes pyloalis-Cu/Zn SOD improved the tolerance of hydrogen peroxide in E. coli. In a similar study using Apostichopus japonicus [3], the recombinant E. coli that expressed Aj-Cu/Zn SOD had higher viability than the control bacteria. Perera et al. [48] detected the antioxidant activity of purified Cu/Zn SOD protein of Hippocampus abdominalis. The results showed that Ha-Cu/Zn SOD could eliminate superoxide free radicals. In our study, the Cd tolerance of E. coli expressing Pe-Cu/Zn SOD was significantly increased when compared to the control, which indicated that Pe-Cu/Zn SOD had a protective effect against Cd stress. These results suggest that the purified Pe-Cu/Zn SOD protein has an antioxidative capacity.
In order to reveal the natural functions of Cu/Zn SOD, in vivo experiments were performed. Petkau et al. [49,50] found that intravenous injection of bovine SOD into mice can significantly repair the damage caused by X-rays in red and white blood cells. Similarly, Oda et al. [51] reported that the survival rate of mice infected with the influenza virus was significantly improved by injecting SOD protein, which indicated that SOD could improve the immunity of mice. In this study, we injected purified Pe-Cu/Zn SOD into the body cavities of P. esculenta. In contrast to the control groups, Pe-Cu/Zn SOD significantly reduced ROS induced by Cd in coelomocytes. Therefore, we suggest that Pe-Cu/Zn SOD plays an important role in the response to Cd stress and oxidative stress in P. esculenta.
4. Materials and Methods
4.1. Samples
P. esculenta was obtained from Xiangshan County (121.681777 N°, 29.48704 E°), Ningbo (Zhejiang, China). The samples were kept indoors after collection. Healthy and vital samples were selected (average weight 4.5 ± 1.0 g), kept in clean natural seawater for 24 h, and inflated continuously during temporary maintenance. All experimental procedures were approved by the Animal Care and Use Committee of the Ningbo University.
4.2. Chemical Exposure and Sampling
The water used in this experiment was clean natural seawater with a temperature of 22 ± 5 °C and a salinity of 28‰. The experiment was carried out in a 32 × 21 × 20 cm plastic water tank, with 12 L of continuously aerated water in each tank. According to our previous experiment, the half-lethal concentration of Cd for 96 h was 192 mg/L [52]. In this experiment, according to the 96 h LC50 concentration gradient (0, 1/32 of 96 h LC50, 1/8 of 96 h LC50, ½ h of LC50), a total of 126 P. esculenta were used. Six P. esculenta were dissected at 12, 24, 48, 72, and 96 h in each group, and the coelomic fluid was collected in 2 mL RNase-free tubes. In addition, six P. esculenta were collected from the control group (0 mg/L) at 0 h. Body cavity fluid was stratified and stored in liquid nitrogen and then transferred to a −80 °C refrigerator for subsequent experiments.
4.3. Total Superoxide Dismutase, Glutathione S-Transferase Activity, and GSH and MDA Content Determination in the Supernatants
The samples were homogenized in cold physiological saline and centrifuged at 12,000 rpm for 10 min (4 °C). Afterwards, the supernatants were collected and stored at −80 °C for further experiments. The total protein content was measured using BCA protein assay kits (CW Biotech, Beijing, China) according to the manufacturer’s instructions. A total superoxide dismutase (T-SOD) assay kit (hydroxylamine method), glutathione S-transferase (GST) assay kit, reduced glutathione (GSH) assay kit, and microscale malondialdehyde (MDA) assay kit (TBA method) from Nanjing Jiancheng Bioengineering Institute were used to test the supernatant according to the manufacturer’s instructions.
4.4. Full-Length Complimentary DNA Cloning of Cu/Zn SOD
Total RNA was extracted using TRIzol Reagent (Invitrogen, USA). Primer Premier v5.0 was used to select appropriate primers for Pe-Cu/Zn SOD (Table S1) based on the transcriptome data of our previous study (GenBank accession No. OL757513). The HiFiScript first-strand cDNA synthesis kit (Cwbio, China) was used to obtain cDNA for intermediate segment sequence cloning. A 2 × Power Taq PCR MasterMix kit (BioTeke, China) was used for the PCR reaction. The PCR procedure was as follows: 94 °C, 5 min; 30 cycles (94 °C, 30 s; 58 °C, 30 s; 72 °C, 50 s); 72 °C, 10 min.
Based on the cloned Cu/Zn SOD cDNA intermediate fragment sequence, primers for 5 rapid amplification of cDNA ends (5 RACE) and 3′ RACE were designed (Table S1). The 5 RACE reverse transcription assay was performed using the Smart RACE cDNA amplification kit (CloneTech, USA) while the 3′ RACE reverse transcription assay was performed using the 3′-Full RACE Core Set with a PrimeScript RTase kit (Takara, China). Both assays were conducted according to our previous research [52]. The products obtained were stored at −20 °C until further analysis.
4.5. Sequence Alignment, Structure Prediction, and Phylogenetic Analysis
The Pe-Cu/Zn SOD protein primary structures were predicted using online tools (http://www.bio-soft.net/sms/ (accessed on 3 July 2020)). The molecular weights of Pe-Cu/Zn SOD proteins were predicted using the ExPASy ProtParam tool (http://web.expasy.org/protparam/ (accessed on 3 July 2020)). The protein sequence was aligned using Vector NT110 (Invitrogen, CA, USA). Secondary and 3-D structures were generated and analyzed using ProtParam (http://web.expasy.org/protparam/ (accessed on 3 July 2020)) and I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER (accessed on 4 July 2020)). Cu/Zn SOD homologues in various species were used for comparison, and a phylogenetic tree was constructed using MEGA v5.0. The GenBank accession numbers of the Cu/Zn SOD proteins are shown in Table S2.
4.6. mRNA Expression and Enzyme Activity of Pe-Cu/Zn SOD
The expression profile of Pe-Cu/Zn SOD was monitored using qPCR. Total RNA was extracted and reverse-transcribed into cDNA using the PrimeScript RT reagent kit (Takara, Japan). The primers used for qPCR are listed in Table S1, and GAPDH primers were used as a positive control. qPCR amplification was performed using SYBR Premix Ex Taq II (Takara, Japan). qPCR was conducted at 95 °C for 4 min, followed by 40 amplification cycles (10 s at 95 °C, 15 s at 60 °C, and 15 s at 72 °C). The comparative ΔΔCt method was used to analyze the relative expression levels of Pe-Cu/Zn SOD. The relative mRNA expression levels are presented as mean ± standard deviation (n = 6). The data were analyzed using one-way analysis of variance with SPSS v20.0, and statistical significance was defined as p < 0.05. The supernatants mentioned in Section 2.2 were used to determine SOD activity. Pe-Cu/Zn SOD activity was detected using an SOD assay kit purchased from Jiancheng Bioengineering (Nanjing, China) and calculated using the formula based on the absorbance values. SOD activity was expressed as U/mg protein.
4.7. Recombinant Protein Expression and Purification
Based on the cloned Pe-Cu/Zn SOD cDNA ORF sequence, the forward primer Cu/Zn SOD-F with a BamH I restriction site and the reverse primer Cu/Zn SOD-R with a Xho I restriction site were used to amplify the coding region of Pe-Cu/Zn SOD (Table S1). The Pe-Cu/Zn SOD ORF was amplified using 2 × Super Pfx MasterMix (CWBIO). The PeCu/Zn SOD ORF and pET-28a (+) plasmids (Novagen) were double-digested and then ligated using T4 DNA ligase (TaKaRa) to obtain the pET28a-MT recombinant plasmid, which was then transferred to Trans5a Chemically Competent Cell (TransGen Biotech) and sequenced. The pET28a-MT recombinant plasmid was extracted using the Plasmid Extraction Mini Kit (Solarbio) and then transferred to Transetta (DE3) Chemically Competent Cell (TransGen Biotech) to obtain pET28a-PeCu/Zn SOD -DE3 recombinant E. coli.
pET28a-PeCu/Zn SOD-DE E. coli was expanded in liquid LB medium (+kanamycin) at 37 °C and 200 rpm until OD600 reached 0.4–0.6. Isopropyl-β-d-1-thiogalactopyranoside (IPTG) was added to the final concentration of 1 mmol/L, and then incubated for 8 h to induce protein expression. The recombinant protein was found to be expressed mainly in inclusion bodies by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis and purified using a His-tagged protein purification kit (Inclusion Body Protein, CWBIO). Using dialysis membranes MD44 (3500D, Solarbio), the purified proteins were sequentially dialyzed once in 50 mM PBS containing 6, 4, 2, and 1 M urea and, finally, twice in 50 mM PBS without urea for 12 h each.
4.8. Cd Tolerance of Recombinant E. coli
The control (pET28a) and recombinant BL21-expressing bacteria (pET28a-Pe-Cu/Zn SOD) were cultured at 37 °C and 200 rpm until OD600 reached approximately 0.4. IPTG at a final concentration of 1 mM was added to induce the expression of Pe-Cu/Zn SOD. At the same time, 0.2 and 1 mM CdCl2 were added. The shaking table culture was continued for 8 h and the OD600 value was determined every 2 h for each of the 3 parallel experiments conducted.
4.9. Analysis of the Pe-Cu/Zn SOD Protection Function In Vivo
Three treatment groups (Cd, Cd + PeCu/Zn SOD, and Cd + PBS) were set up. Six P. esculenta samples were treated with 96 mg/L Cd2+ for 24 h in each group. In the Cd + Pe-Cu/Zn SOD group, 100 µL of soluble Pe-Cu/Zn SOD purified protein was injected into the body cavity of P. esculenta before Cd treatment, whereas in the Cd + PBS group, it was injected with 100 µL of 1 × PBS. In the control group, P. esculenta was placed in clean natural seawater without Cd treatment.
4.10. Data Analysis
SPSS 20.0 (IBM company, Armonk, NYC, USA)and Excel software (Microsoft company, Redmond, WA, USA) were used for statistical analysis. The experimental groups were compared using one-way ANOVA and Duncan’s tests. The differences between the groups were analyzed and plotted using Graphpad software 7.0 (Graphpad software company, San Diego, CA, USA).
5. Conclusions
We report that the toxic effects of different concentrations of Cd (6, 24, 96 mg/L) on P. esculenta caused significant changes in the antioxidative indexes, T-SOD, GST, GSH, and MDA. These results indicated that Cd induced oxidative stress in P. esculenta. We cloned the full-length Pe-Cu/Zn SOD and identified it as an icCu/Zn SOD. The qPCR results showed that Pe-Cu/Zn SOD mRNA was expressed widely at the highest levels in the coelomic fluid. This significant increase after Cd exposure indicated that Pe-Cu/Zn SOD featured in the stress response. We obtained Pe-Cu/Zn SOD recombinant protein and found that it enhanced the heavy metal tolerance of E. coli. In vivo assays confirmed that Pe-Cu/Zn SOD recombinant protein exhibited an antioxidative activity and free radical scavenging ability, suggesting that Pe-Cu/Zn SOD could chelate heavy metal ions and scavenge reactive oxygen free radicals. We suggest that P. esculenta can be used as a bioindicator to evaluate heavy metal pollution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012136/s1.
Author Contributions
Y.L., validation, formal analysis, writing—review, supervision, and editing. C.D., validation, formal analysis, writing—review and editing. C.L., methodology writing—original draft, and investigation. X.G., visualization and supervision. J.Z., conceptualization, methodology, supervision, funding acquisition. C.Z., conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to all members of the Fish Histology Laboratory in Ningbo University for providing direct assistance and constructive discussion for this research. This project was supported by the Ningbo Science and Technology Plan Projects (2019B10016, 2016C10004): the Major Science and Technology Projects in Zhejiang Province (2011C12013): Natural Science Foundation of Zhejiang Province (LY18C190007): National Natural Science Foundation of China (No. 31272642): and the Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, and Sponsored K.C. Wong Magna Fund in Ningbo University.
Institutional Review Board Statement
The experimental animal of this study is P. esculenta, a species of invertebrate and belonging to Sipuncula. In China, this species does not require ethical approval for experiments. All experiments comply with the requirements of the governing regulation for the use of experimental animals in Zhejiang Province (Zhejiang provincial government order No. 263, released on 17 August 2009, effective from 1 October 2010) and the animal care and use Committee of Ningbo University.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within this article.
Acknowledgments
The authors wish to thank all the members from the Fish Reproduction Physiology Laboratory at Ningbo University for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, W.; Guan, X.; Han, Y.; Guo, C.; Rong, J.H.; Su, W.H.; Zha, S.J.; Wang, Y.C.; Liu, G.X. Waterborne Cd2+ weakens the immune responses of blood clam through impacting Ca2+ signaling and Ca2+ related apoptosis pathways. Fish Shellfish. Immunol. 2018, 77, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Dorta, D.J.; Leite, S.; DeMarco, K.C.; Prado, I.M.R.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Li, Y.; Zhou, X.; Zhang, X.T.; Liu, T.T.; Liu, B.G.; Wang, L.; Li, L.; Li, C. The distribution, expression of the Cu/Zn superoxide dismutase in Apostichopus japonicus and its function for sea cucumber immunity. Fish Shellfish. Immunol. 2019, 89, 745–752. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F. Cadmium and cellular signaling cascades: To be or not to be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef]
- Xiang, N.; Zhao, C.; Diao, X.; Han, Q.; Zhou, H.L. Dynamic responses of antioxidant enzymes in pearl oyster Pinctada martensii exposed to di (2-ethylhexyl) phthalate (DEHP). Environ. Toxicol. Pharmacol. 2017, 54, 184–190. [Google Scholar] [CrossRef]
- Nardi, A.; Mincarelli, L.F.; Benedetti, M.; Fattorini, D.; Errico, G.; Regoli, F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 2017, 169, 493–502. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, S.Y.; Cho, Y.S.; Bang, I.C.; Kim, K.H.; Kim, D.S.; Nam, Y.K. Molecular characterization and mRNA expression during metal exposure and thermal stress of copper/zinc- and manganese-superoxide dismutases in disk abalone, Haliotis discus discus. Fish Shellfish. Immunol. 2007, 23, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Anne-Frances, M. Superoxide dismutases: Ancient enzyme and new insights. Fed. Eur. Biochem. Soc. 2012, 586, 585–595. [Google Scholar]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free. Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Valentine, J.S.; Doucette, P.A.; Potter, S.Z. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005, 74, 563–593. [Google Scholar] [CrossRef] [PubMed]
- Oury, T.D.; Crapo, J.Z.; Enghild, J.J. Human extracellular superoxide dismutase is a tetramer composed of two disulphide-linked dimers: A simplified, high-yield purification of extracellular superoxide dismutase. Biochem. J. 1996, 317, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, H.S.; Wang, T.M.; Liu, B.Z.; Zhao, H.L.; Chen, M.Y. Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2010, 151, 325–333. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.; Yim, B.; Rhee, J.S.; Won, E.J.; Lee, Y.M. Identification and molecular characterization of two Cu/Zn-SODs and Mn-SOD in the marine ciliate Euplotes crassus: Modulation of enzyme activity and transcripts in response to copper and cadmium. Aquat. Toxicol. 2018, 199, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.; Li, H.; Gang, X.L.; Hao, S.; Bo, H.C. Molecular Cloning and Sequence Analysis of an Intracellular Cu/Zn-superoxide Dismutase Gene from Hard Clam (Meretrix meretrix). Biotechnol. Bull. 2010, 11, 123–128, 133. [Google Scholar]
- Bao, Y.; Li, L.; Xu, F.; Zhang, G.F. Intracellular copper/zinc superoxide dismutase from bay scallop Argopecten irradians: Its gene structure, mRNA expression and recombinant protein. Fish Shellfish. Immunol. 2009, 27, 210–220. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef]
- Kim, B.M.; Lee, J.W.; Seo, J.S.; Shin, K.H.; Rhee, J.S.; Lee, J.S. Modulated expression and enzymatic activity of the monogonont rotifer Brachionus koreanus Cu/Zn- and Mn-superoxide dismutase (SOD) in response to environmental biocides. Chemosphere 2015, 120, 470–478. [Google Scholar] [CrossRef]
- Ni, D.; Song, L.; Gao, Q.; Wu, L.T.; Yu, T.D.; Zhao, J.M.; Qiu, L.M.; Zhang, H.; Shi, F.F. The cDNA cloning and m RNA expression of cytoplasmic Cu, Zn superoxide dismutase (SOD) gene in scallop Chlamys farreri. Fish Shellfish. Immunol. 2007, 23, 1032–1042. [Google Scholar] [CrossRef]
- Zheng, J.L.; Yuan, S.S.; Wu, C.W.; Lv, Z.M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquatictoxicology 2016, 180, 36–44. [Google Scholar] [CrossRef]
- Xia, L.; Chen, S.; Dahms, H.U.; Ying, X.P.; Peng, X. Cadmium induced oxidative damage and apoptosis in the hepatopancreas of Meretrix meretrix. Ecotoxicology 2016, 25, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, L.; Tao, Y. Antioxidant responses in clam Venerupis philippinarum exposed to environmental pollutant hexabromocyclododecane. Environ. Sci. Pollut. Res. 2014, 21, 8206–8215. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, P.; Li, Z.H.; Zhao, L.; Lai, X.F.; Chen, J.H.; Gao, H.; Yan, B.L. Toxicity effects of cadmium on the ridgetail white prawn Exopalaemon carinicauda. Asian J. Ecotoxicol. 2016, 11, 207–213. (In Chinese) [Google Scholar]
- Deng, S.P.; Zhao, Y.T.; Zhu, C.H.; Zeng, M.; Fu, S.; Li, G.L. Effects of Cadmium on the antioxidant enzyme activity and lipid peroxidation in Sanguinolaria acuta. Acta Hydrobiol. Sin. 2012, 36, 7. (In Chinese) [Google Scholar]
- Sun, B.; Ge, Q.W.; Lu, H.X.; Xu, Y.J. Effects of Cadmium exposure on antioxidant enzyme system of Tegillarca granosa Linnaeus. Ecol. Sci. 2014, 30, 383–388. (In Chinese) [Google Scholar]
- Wang, L.L.; Xia, B.; Chen, B.J.; Li, C.H.; Tang, X.X. Effects of cadmium stress on antioxidant defense system of Patinopecten yessoensis. Mar. Environ. Sci. 2012, 31, 39–42. (In Chinese) [Google Scholar]
- Xing, H.F.; Li, Y.Q.; Yang, H.Z.; Wang, L. Effects of Cadmium on the antioxidant enzyme activity and lipid peroxidation in the mantle and gill of freshwater bivalbe A. woodiana woodiana. Acta Sci. Circumstantiae 2013, 33, 856–860. (In Chinese) [Google Scholar]
- Bierrum, M.J.; Bauer, R.; Danielsen, E.; Kofpd, P. The Zn-site in bovine copper, zinc superoxide dismutase studied by 111Cd PAC. Free. Radic. Res. 1991, 12, 297–303. [Google Scholar]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Livingstone, D.R.; Lips, F.; Martinez, P.G.; Pipe, R.K. Antioxidant enzymes in the digestive gland of the common mussel (Mytilus edulis). Mar. Biol. 1992, 112, 265–276. [Google Scholar] [CrossRef]
- Lei, Y.J. The Toxicity Effects of Copper and Cadmium and the Possible Protective Effects of Selenium and α-Lipoic Acid on juvenile Abalone Haliotis Discus Hannai Ino; Ocean University of China: Qingdao, China, 2014. [Google Scholar]
- Wang, X.Y. Physiological Responses of the Four-Horned Clam Mactra Veneriformis to Cadmium and Mercury Pollution Stress; Graduate School of Chinese Academy of Sciences: Beijing, China, 2009. [Google Scholar]
- Lu, Z.; Wang, S.; Shan, X.; Ji, C.L.; Wu, H.F. Differential biological effects in two pedigrees of clam Ruditapes philippinarum exposed to cadmium using iTRAQ-based proteomics. Environ. Toxicol. Pharmacol. 2019, 65, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yan, B.; Li, Y.Q.; Wang, Q.; Wang, L. Effects of Cd2+ on glutathione system of hepatopancreas and gills in freshwater crab Sinopotamon yangtsekiense. Environ. Sci. 2008, 29, 2302–2307. (In Chinese) [Google Scholar]
- Loro, V.L.; Jorge, M.B.; Silva, K.R.; Wood, C.M. Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus: Protective effects of salinity. Aquat. Toxicol. 2012, 110–111, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Lee, D.; Kim, B.; Nam, S.E.; Rhee, J.S. Dose- and age-specific antioxidant responses of the mysid crustacean Neomysis awatschensis to metal exposure. Aquat. Toxicol. 2018, 201, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Pretto, A.; Loro, V.L.; Morsch, V.M.; Moraes, B.S.; Menezes, C.; Clasen, B.; Hoehne, L.; Dressler, V. Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium. Arch. Environ. Contam. Toxicol. 2010, 58, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Song, P.; Gu, W.B.; Yang, Z. Effects of heavy metals on production of thiol compounds and antioxidant enzymes in Agaricus bisporus. Ecotoxicol. Environ. Saf. 2011, 74, 1685–1692. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Peskin, A.V.; Parsons-Mair, H.N. Thiol oxidase activity of copper, zinc superoxide dismutase. J. Biol. Chem. 2002, 277, 1906–1911. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.F.; Liu, C.F. Effects of Cu2+ on the antioxidative defence in muscle of Paralichthys olivaceus. Freshw. Fish. 2007, 2, 27–29. (In Chinese) [Google Scholar]
- Liu, H.F.; Wang, F. Advanced research on pollution of heavy metals on biomarkers in aquatic animals. Freshw. Fish. 2009, 5, 299–302. (In Chinese) [Google Scholar]
- Kim, B.M.; Rhee, J.S.; Park, G.S.; Lee, J.; Lee, Y.M.; Lee, J.S. Cu/Zn- and Mn-superoxide dismutase (SOD) from the copepod Tigriopus japonicus: Molecular cloning and expression in response to environmental pollutants. Chemosphere 2011, 84, 1467–1475. [Google Scholar] [CrossRef]
- Rhee, J.S.; Won, E.J.; Kim, R.O.; Lee, J.; Shin, K.H.; Lee, J.S. Expression of superoxide dismutase (SOD) genes from the copper-exposed polychaete, Neanthes succinea. Mar. Pollut. Bull. 2011, 63, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Krishnani, K.K.; Meena, K.K.; Gupta, S.K.; Singh, N.P. Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 2017, 171, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, H.; Zheng, S.; Zhang, X.L.; Mu, S.N. Molecular characterization of Cu/Zn SOD gene in Asian clam Corbicula fluminea and mRNA expression and enzymatic activity modulation induced by metals. Gene 2018, 663, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Rhie, G.; Oh, J.H.; Huh, W.K.; Yim, H.S.; Kang, S.O. Copper- and zinc- containing superoxide dismutase (Cu/Zn SOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 2002, 148, 3705–3713. [Google Scholar] [CrossRef]
- Liu, Y.C.; Su, H.; Wang, P.C.; Xu, Y.L.; Wei, T.C.; Wang, H.; Xu, Y. Cloning and fusion expression of extracellular Cu-Zn superoxide dismutase gene and detection of recombinant protease activity in Somalis borer. Acta Sericologica Sin. 2018, 44, 188–195. [Google Scholar]
- Perera, N.; Godahewa, G.; Lee, J. Copper-zinc-superoxide dismutase (CuZnSOD), an antioxidant gene from seahorse (Hippocampus abdominalis); molecular cloning, sequence characterization, antioxidant activity and potential peroxidation function of its recombinant protein. Fish Shellfish. Immunol. 2016, 57, 386–399. [Google Scholar] [CrossRef]
- Petkau, A.; Kelly, K.; Chelack, W.S.; Barefoot, C. Protective effect of superoxide dismutase on erythrocytes of X-irradiated mice. Biochem. Biophys. Res. Commun. 1976, 70, 452–458. [Google Scholar] [CrossRef]
- Petkau, A.; Chelack, W.S.; Pleskach, S.D. Protection by superoxide dismutase of white blood cells in X-irradiated mice. Life Sci. 1978, 22, 867–882. [Google Scholar] [CrossRef]
- Oda, T.; Akaike, T.T.; Hamamoto, T.; Hirano, T.; Maeda, H. Oxygen radicals in infuenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science 1989, 244, 974–976. [Google Scholar] [CrossRef]
- Meng, J.; Gao, X.; Luo, S.; Lin, C.; Du, C.; Hou, C.; Wang, J.P.; Jin, S.; Tang, D.J.; Zhang, C.; et al. Cloning, Functional Characterization and Response to Cadmium Stress of the Thioredoxin-like Protein 1 Gene from Phascolosoma esculenta. Int. J. Mol. Sci. 2022, 23, 332. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).