Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review
Abstract
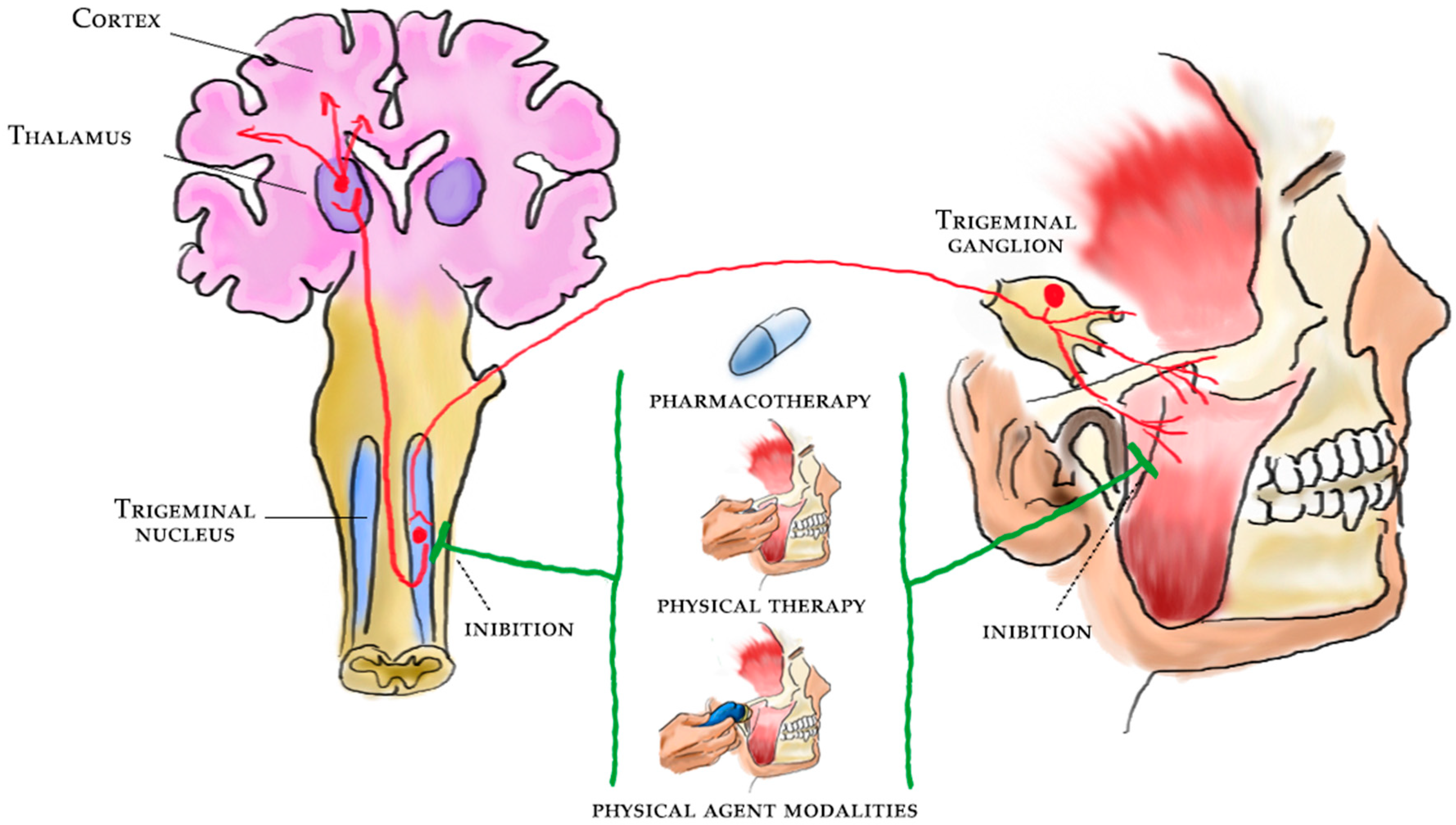
1. Introduction
2. Central Sensitization
3. Diagnosis of Central Sensitization in TMD
3.1. Algometry and Pressure Pain Thresholds
3.2. Assessment of Orofacial Somatosensory Function
3.3. Temporomandibular Disorders, Primary Headaches, and Cervical Pain
3.4. Evaluation Tools for Central Sensitization
4. Evidence for Central Sensitization in TMD
5. Pharmacological Therapy and Central Sensitization in TMD
5.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
5.2. Beta-Blockers
5.3. Antidepressants
5.4. Antiseizure Medications
5.5. Opioids
5.6. Other Therapies and New Perspectives
6. Physical Therapy and Rehabilitation for the Central Sensitization in TMD
6.1. Physical Therapy
6.2. Occlusal Splints
6.3. Extracorporeal Shockwave Therapy
6.4. Laser Therapy
6.5. Transcutaneous Electrical Nerve Stimulation
6.6. Biofeedback
7. Interventional Therapies and Central Sensitization in TMD
7.1. Acupuncture and Dry Needling
7.2. Botulinum Toxin
7.3. Oxygen–Ozone Therapy
8. Study Limitations and Strengths
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Bueno, C.H.; Pereira, D.D.; Pattussi, M.P.; Grossi, P.K.; Grossi, M.L. Gender differences in temporomandibular disorders in adult populational studies: A systematic review and meta-analysis. J. Oral Rehabil. 2018, 45, 720–729. [Google Scholar] [CrossRef]
- Simoen, L.; Van den Berghe, L.; Jacquet, W.; Marks, L. Depression and anxiety levels in patients with temporomandibular disorders: Comparison with the general population. Clin. Oral Investig. 2020, 24, 3939–3945. [Google Scholar] [CrossRef]
- Bitiniene, D.; Zamaliauskiene, R.; Kubilius, R.; Leketas, M.; Gailius, T.; Smirnovaite, K. Quality of life in patients with temporomandibular disorders. A systematic review. Stomatologija 2018, 20, 3–9. [Google Scholar]
- Castro-Calderón, A.; Roccuzzo, A.; Ferrillo, M.; Gada, S.; González-Serrano, J.; Fonseca, M.; Molinero-Mourelle, P. Hyaluronic acid injection to restore the lost interproximal papilla: A systematic review. Acta Odontol Scand. 2022, 80, 295–307. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; Benoliel, R. Neuropathic orofacial pain: Facts and fiction. Cephalalgia 2017, 37, 670–679. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Moreno-Fernández, A.M.; Jiménez-Castellanos, E.; Iglesias-Linares, A.; Bueso-Madrid, D.; Fernández-Rodríguez, A.; de Miguel, M. Fibromyalgia syndrome and temporomandibular disorders with muscular pain. A review. Mod. Rheumatol. 2017, 27, 210–216. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Fortunato, F.; Bindi, M.; Pezzotti, F.; Ammendolia, A.; Giudice, A.; Foglio Bonda, P.L.; de Sire, A. Temporomandibular disorders and neck pain in primary headache patients: A retrospective machine learning study. Acta Odontol. Scand. 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Chaves, T.C.; Dach, F.; Florencio, L.L.; Carvalho, G.F.; Gonçalves, M.C.; Bigal, M.E.; Speciali, J.G.; Bevilaqua-Grossi, D. Concomitant Migraine and Temporomandibular Disorders are Associated With Higher Heat Pain Hyperalgesia and Cephalic Cutaneous Allodynia. Clin. J. Pain 2016, 32, 882–888. [Google Scholar] [CrossRef]
- Greenspan, J.D.; Slade, G.D.; Bair, E.; Dubner, R.; Fillingim, R.B.; Ohrbach, R.; Knott, C.; Diatchenko, L.; Liu, Q.; Maixner, W. Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. J. Pain 2013, 14, T63–T74.e746. [Google Scholar] [CrossRef]
- Paolucci, T.; de Sire, A.; Ferrillo, M.; di Fabio, D.; Molluso, A.; Patruno, A.; Pesce, M.; Lai, C.; Ciacchella, C.; Saggino, A.; et al. Telerehabilitation proposal of mind-body technique for physical and psychological outcomes in patients with fibromyalgia. Front. Physiol. 2022, 13, 917956. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Adams, L.M.; Turk, D.C. Central sensitization and the biopsychosocial approach to understanding pain. J. Appl. Behav. Res. 2018, 23, e12125. [Google Scholar] [CrossRef]
- Merrill, R.L. Central mechanisms of orofacial pain. Dent. Clin. N. Am. 2007, 51, 45–59. [Google Scholar] [CrossRef]
- Craig, A.D. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003, 26, 303–307. [Google Scholar] [CrossRef]
- Sessle, B.J. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar]
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; International Association for the Study of Pain Press: Seattle, WA, USA, 1994; p. 210. [Google Scholar]
- Lorduy, K.M.; Liegey-Dougall, A.; Haggard, R.; Sanders, C.N.; Gatchel, R.J. The prevalence of comorbid symptoms of central sensitization syndrome among three different groups of temporomandibular disorder patients. Pain Pract. 2013, 13, 604–613. [Google Scholar] [CrossRef]
- Dahan, H.; Shir, Y.; Velly, A.; Allison, P. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J. Headache Pain 2015, 16, 528. [Google Scholar] [CrossRef]
- Sanzarello, I.; Merlini, L.; Rosa, M.A.; Perrone, M.; Frugiuele, J.; Borghi, R.; Faldini, C. Central sensitization in chronic low back pain: A narrative review. J. Back Musculoskelet Rehabil. 2016, 29, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Cattaneo, R.; Marci, M.C.; Pietropaoli, D.; Ortu, E. Central Sensitization-Based Classification for Temporomandibular Disorders: A Pathogenetic Hypothesis. Pain Res. Manag. 2017, 2017, 5957076. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.F.; Lovato da Silva, C.H.; Nasser, M.; Fedorowicz, Z.; Al-Muharraqi, M.A. Interventions for the management of temporomandibular joint osteoarthritis. Cochrane Database Syst. Rev. 2012, 2012, CD007261. [Google Scholar]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: A systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet Rehabil. 2022, 18, 1–16. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; d’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022, 23, 1–17. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef]
- Florjanski, W.; Malysa, A.; Orzeszek, S.; Smardz, J.; Olchowy, A.; Paradowska-Stolarz, A.; Wieckiewicz, M. Evaluation of Biofeedback Usefulness in Masticatory Muscle Activity Management—A Systematic Review. J. Clin. Med. 2019, 8, 766. [Google Scholar] [CrossRef]
- Andre, A.; Kang, J.; Dym, H. Pharmacologic Treatment for Temporomandibular and Temporomandibular Joint Disorders. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 49–59. [Google Scholar] [CrossRef]
- Harba, A.N.; Harfoush, M. Evaluation of the participation of hyaluronic acid with platelet-rich plasma in the treatment of temporomandibular joint disorders. Dent. Med. Probl. 2021, 58, 81–88. [Google Scholar] [CrossRef]
- Deregibus, A.; Ferrillo, M.; Grazia Piancino, M.; Chiara Domini, M.; de Sire, A.; Castroflorio, T. Are occlusal splints effective in reducing myofascial pain in patients with muscle-related temporomandibular disorders? A randomized-controlled trial. Turk. J. Phys. Med. Rehabil. 2021, 67, 32–40. [Google Scholar] [CrossRef]
- Kuzmanovic Pficer, J.; Dodic, S.; Lazic, V.; Trajkovic, G.; Milic, N.; Milicic, B. Occlusal stabilization splint for patients with temporomandibular disorders: Meta-analysis of short and long term effects. PLoS ONE 2017, 12, e0171296. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Z.; Jia, J.; Jin, L.; Li, J.H.; Wang, Z.Y.; Cao, D.Y. Low-Level Laser Therapy for Temporomandibular Disorders: A Systematic Review with Meta-Analysis. Pain Res. Manag. 2018, 2018, 4230583. [Google Scholar] [CrossRef]
- Marotta, N.; Ferrillo, M.; Demeco, A.; Drago Ferrante, V.; Inzitari, M.T.; Pellegrino, R.; Pino, I.; Russo, I.; de Sire, A.; Ammendolia, A. Effects of Radial Extracorporeal Shock Wave Therapy in Reducing Pain in Patients with Temporomandibular Disorders: A Pilot Randomized Controlled Trial. Appl. Sci. 2022, 12, 3821. [Google Scholar] [CrossRef]
- Fertout, A.; Manière-Ezvan, A.; Lupi, L.; Ehrmann, E. Management of temporomandibular disorders with transcutaneous electrical nerve stimulation: A systematic review. Cranio 2022, 40, 217–228. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Ferrillo, M.; Agostini, F.; Sconza, C.; Lippi, L.; Respizzi, S.; Giudice, A.; Invernizzi, M.; Ammendolia, A. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 2528. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; He, S.; Xu, J.; You, W.; Li, Q.; Long, J.; Luo, L.; Kemp, G.J.; Sweeney, J.A.; Li, F.; et al. The neuro-pathophysiology of temporomandibular disorders-related pain: A systematic review of structural and functional MRI studies. J. Headache Pain 2020, 19, 21–78. [Google Scholar] [CrossRef]
- Bender, S.D. Orofacial pain and headache: A review and look at the commonalities. Curr Pain Headache Rep. 2014, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.E.; Sessle, B.J.; Hu, J.W. Activation of peripheral GABAA receptors inhibits temporomandibular joint-evoked jaw muscle activity. J. Neurophysiol. 1999, 81, 1966–1969. [Google Scholar] [CrossRef][Green Version]
- Tashiro, A.; Bereiter, D.A.; Thompson, R.; Nishida, Y. GABAergic influence on temporomandibular joint-responsive spinomedullary neurons depends on estrogen status. Neuroscience 2014, 259, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sperry, M.M.; Granquist, E.J.; Winkelstein, B.A. Increased substance P and synaptic remodeling occur in the trigeminal sensory system with sustained osteoarthritic temporomandibular joint sensitivity. Pain Rep. 2021, 6, e911. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.M.; Conti, P.C.; de Faria, F.A.; Bonjardim, L.R. Temporomandibular disorders and painful comorbidities: Clinical association and underlying mechanisms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.C.; Costa, Y.M.; Gonçalves, D.A.; Svensson, P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J. Oral Rehabil. 2016, 43, 702–715. [Google Scholar] [CrossRef]
- Campi, L.B.; Visscher, C.M.; Ongaro, P.C.J.; do Vale Braido, G.V.; Fernandes, G.; Gonçalves, D.A.G. Widespread Pain and Central Sensitization in Adolescents with Signs of Painful Temporomandibular Disorders. J. Oral Facial. Pain Headache 2020, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.F.; Smith, S.; Bhalang, K.; Slade, G.D.; Maixner, W. Development of temporomandibular disorders is associated with greater bodily pain experience. Clin. J. Pain 2010, 26, 116–120. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Kiczmer, P.; Węgrzyn, K.; Pałka, Ł.; Janda, K.; Sobolewska, E. Pressure Algometry Evaluation of Two Occlusal Splint Designs in Bruxism Management-Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 2342. [Google Scholar] [CrossRef]
- Gomes, M.B.; Guimarães, J.P.; Guimarães, F.C.; Neves, A.C. Palpationand pressure pain threshold: Reliability and validity in patientswith temporomandibular disorders. Cranio 2008, 26, 202–210. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Galán-del-Río, F.; Ortega-Santiago, R.; Jiménez-García, R.; Arendt-Nielsen, L.; Svensson, P. Bilateral thermal hyperalgesia in trigeminal and extra-trigeminal regions in patients with myofascial temporomandibular disorders. Exp. Brain Res. 2010, 202, 171–179. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans; using multiple raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef]
- Bernhardt, O.; Schiffman, E.L.; Look, J.O. Reliability and validity of a new fingertip-shaped pressure algometer for assessing pressure pain thresholds in the temporomandibular joint and masticatory muscles. J. Orofac. Pain. 2007, 21, 29–38. [Google Scholar]
- Treede, R.D.; Rolke, R.; Andrews, K.; Magerl, W. Pain elicited byblunt pressure: Neurobiological basis and clinical relevance. Pain 2002, 98, 235–240. [Google Scholar] [CrossRef]
- List, T.; Helkimo, M.; Karlsson, R. Influence of pressure rates onthe reliability of a pressure threshold meter. J. Craniomandib. Disord. 1991, 5, 173–178. [Google Scholar] [PubMed]
- Farella, M.; Michelotti, A.; Cimino, R.; Martina, R. An investigation of central and peripheral factors affecting pressure pain thresholds of the human jaw muscles. J. Musculoskel. Pain 1999, 7, 253–259. [Google Scholar] [CrossRef]
- da Silva, M.M.; Albertini, R.; Leal-Junior, E.C.; de Tarso Camillo de Carvalho, P.; Silva, J.A., Jr.; Bussadori, S.K.; de Oliveira, L.V.; Casarin, C.A.; Andrade, E.L.; Bocalini, D.S.; et al. Effects of exercise training and photobiomodulation therapy (EXTRAPHOTO) on pain in women with fibromyalgia and temporomandibular disorder: Study protocol for a randomized controlled trial. Trials 2015, 16, 252. [Google Scholar] [CrossRef]
- Herpich, C.M.; Leal-Junior, E.C.P.; Amaral, A.P.; Tosato, J.; Glória, I.P.; Garcia, M.B.; Barbosa, B.R.; El Hage, Y.; Arruda, É.E.; Gomes, C.Á.; et al. Effects of phototherapy on muscle activity and pain in individuals with temporomandibular disorder: A study protocol for a randomized controlled trial. Trials 2014, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.; Baad-Hansen, L.; Pigg, M.; List, T.; Eliav, E.; Ettlin, D.; Michelotti, A.; Tsukiyama, Y.; Matsuka, Y.; Jääskeläinen, S.K.; et al. Guidelines and recommendations for assessment of somatosensory function in oro-facial pain conditions—A taskforce report. J. Oral Rehabil. 2011, 38, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.; Backonja, M.; Bouhassira, D. Usefulness andlimitations of quantitative sensory testing: Clinical andresearch application in neuropathic pain states. Pain 2007, 129, 256–259. [Google Scholar] [CrossRef]
- Cruccu, G.; Sommer, C.; Anand, P.; Attal, N.; Baron, R.; Garcia-Larrea, L.; Haanpaa, M.; Jensen, T.S.; Serra, J.; Treede, R.D. EFNS guidelines on neuropathic pain assessment: Revised 2009. Eur. J. Neurol. 2010, 17, 1010–1018. [Google Scholar] [CrossRef]
- Maier, C.; Baron, R.; Tolle, T.R.; Binder, A.; Birbaumer, N.; Birklein, F.; Gierthmühlen, J.; Flor, H.; Geber, C.; Huge, V. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathicpain syndromes. Pain 2010, 150, 439–450. [Google Scholar] [CrossRef]
- Akinci, A.; Al Shaker, M.; Chang, M.H.; Cheung, C.W.; Danilov, A.; José Dueñas, H.; Kim, Y.C.; Guillen, R.; Tassanawipas, W.; Treuer, T.; et al. Predictive factors and clinical biomarkers for treatment in patients with chronic pain caused by osteoarthritis with a central sensitisation component. Int. J. Clin. Pract. 2016, 70, 31–44. [Google Scholar] [CrossRef]
- Pigg, M.; Baad-Hansen, L.; Svensson, P.; Drangsholt, M.; List, T. Reliability of intraoral quantitative sensory testing (QST). Pain 2010, 148, 220–226. [Google Scholar] [CrossRef]
- Plesh, O.; Adams, S.H.; Gansky, S.A. Temporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sample. J. Orofac. Pain 2011, 25, 190–198. [Google Scholar]
- Botros, J.; Gornitsky, M.; Samim, F.; der Khatchadourian, Z.; Velly, A.M. Back and neck pain: A comparison between acute and chronic pain-related Temporomandibular Disorders. Can. J. Pain 2022, 6, 112–120. [Google Scholar] [CrossRef]
- Ohrbach, R.; Fillingim, R.B.; Mulkey, F.; Gonzalez, Y.; Gordon, S.; Gremillion, H.; Lim, P.-F.; Ribeiro-Dasilva, M.; Greenspan, J.D.; Knott, C. Clinical findings and pain symptoms as potential risk factors for chronic tmd: Descriptive data and empirically identified domains from the opera case-control study. J. Pain 2011, 12, T27–T45. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders; 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Matre, D.; Knardahl, S. ‘Central sensitization’ in chronic neck/shoulder pain. Scand. J. Pain 2012, 3, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, S. Chronic migraine: A process of dysmodulation and sensitization. Mol. Pain 2018, 14, 1744806918767697. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; Camparis, C.M.; Speciali, J.G.; Franco, A.L.; Castanharo, S.M.; Bigal, M.E. Temporomandibular disorders are differentially associated with headache diagnoses: A controlled study. Clin. J. Pain 2011, 27, 611–615. [Google Scholar] [CrossRef]
- Furquim, B.D.; Flamengui, L.M.; Conti, P.C. TMD and chronic pain: A current view. Dental Press J. Orthod. 2015, 20, 127–133. [Google Scholar] [CrossRef]
- Bevilaqua-Grossi, D.; Lipton, R.B.; Napchan, U.; Grosberg, B.; Ashina, S.; Bigal, M.E. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia 2010, 30, 425–432. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Torres-Cueco, R.; van Wilgen, C.P.; Girbes, E.L.; Struyf, F.; Roussel, N.; van Oosterwijck, J.; Daenen, L.; Kuppens, K.; Vanwerweeen, L.; et al. Applying modern pain neuroscience in clinical practice: Criteria for the classification of central sensitization pain. Pain Physician 2014, 17, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Vandyken, B.; Keizer, A.; Vandyken, C.; Macedo, L.G.; Kuspinar, A.; Dufour, S. Pelvic floor muscle tenderness on digital palpation among women: Convergent validity with central sensitization. Braz. J. Phys. Ther. 2020, 25, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoon, K.B.; Yoon, D.M.; Yoo, J.H.; Ahn, K.R. Influence of centrally mediated symptoms on postoperative pain in osteoarthritis patients undergoing total knee arthroplasty: A prospective observational evaluation. Pain Pract. 2015, 15, E46–E53. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.M.; Williams, M.; Bevers, K.R.; Mayer, T.G.; Gatchel, R.J. Use of the Central Sensitization Inventory (CSI) as a treatment outcome measure for patients with chronic spinal pain disorder in a functional restoration program. Spine J. 2017, 17, 819–829. [Google Scholar] [CrossRef]
- Beales, D.; Fary, R.; Little, C.; Nambiar, S.; Sveinall, H.; Yee, Y.L.; Tampin, B.; Mitchell, T. Characterisation of pain in people with hereditary neuropathy with liability to pressure palsy. J. Neurol. 2017, 264, 2464–2471. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Marziniak, M.; Stumpenhorst, F.; Reinholz, J.; Knecht, S. Pain sensitivity can be assessed by self-rating: Development and validation of the Pain Sensitivity Questionnaire. Pain 2009, 146, 65–74. [Google Scholar] [CrossRef]
- Coronado, R.A.; George, S.Z. The Central Sensitization Inventory and Pain Sensitivity Questionnaire: An exploration of construct validity and associations with widespread pain sensitivity among individuals with shoulder pain. Musculoskelet Sci. Pract. 2018, 36, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Verneuer, B.; Dany, K.; Marziniak, M.; Wolowski, A.; Colak-Ekici, R.; Schulte, T.L.; Bullmann, V.; Grewe, S.; Gralow, I.; et al. Validation of the pain sensitivity questionnaire in chronic pain patients. Pain 2012, 153, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- den Boer, C.; Dries, L.; Terluin, B.; van der Wouden, J.C.; Blankenstein, A.H.; van Wilgen, C.P.; Lucassen, P.; van der Horst, H.E. Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions; operationalizations and measurement instruments. J. Psychosom. Res. 2019, 117, 32–40. [Google Scholar] [CrossRef]
- Dixon, E.A.; Benham, G.; Sturgeon, J.A.; Mackey, S.; Johnson, K.A.; Younger, J. Development of the Sensory Hypersensitivity Scale (SHS): A self-report tool for assessing sensitivity to sensory stimuli. J. Behav. Med. 2016, 39, 537–550. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Paris-Alemany, A.; Hidalgo-Pérez, A.; López-de-Uralde-Villanueva, I.; Angulo-Diaz-Parreño, S.; Muñoz-García, D. Evidence for Central Sensitization in Patients with Temporomandibular Disorders: A Systematic Review and Meta-analysis of Observational Studies. Pain Pract. 2018, 18, 388–409. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Dai, J.; Li, Y. Quantitative sensory testing in patients with the muscle pain subtype of temporomandibular disorder: A systemic review and meta-analysis. Clin. Oral Investig. 2021, 25, 6547–6559. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Galán-del-Río, F.; Fernández-Carnero, J.; Pesquera, J.; Arendt-Nielsen, L.; Svensson, P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: Evidence of impairment in central nociceptive processing. J. Pain 2009, 10, 1170–1178. [Google Scholar] [CrossRef]
- Daemen, M.; Kurvers, H.; Kitslaar, P.; Slaaf, D.W.; Bullens, P.H.; Van den Wildenberg, F.A. Neurogenic inflammation in an animal model of neuropathic pain. Neurol. Res. 1998, 20, 41–45. [Google Scholar] [CrossRef]
- Svensson, P.; List, T.; Hector, G. Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain 2001, 92, 399–409. [Google Scholar] [CrossRef]
- Sarlani, E.; Grace, E.G.; Reynolds, M.A.; Greenspan, J.D. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J. Orofac. Pain 2004, 18, 41–55. [Google Scholar] [PubMed]
- Fernández-de-Las-Peñas, C.; Von Piekartz, H. Clinical Reasoning for the Examination and Physical Therapy Treatment of Temporomandibular Disorders (TMD): A Narrative Literature Review. J. Clin. Med. 2020, 9, 3686. [Google Scholar] [CrossRef]
- Campi, L.B.; Jordani, P.C.; Tenan, H.L.; Camparis, C.M.; Gonçalves, D.A. Painful temporomandibular disorders and central sensitization: Implications for management-a pilot study. Int. J. Oral Maxillofac. Surg. 2017, 46, 104–110. [Google Scholar] [CrossRef]
- Asquini, G.; Bianchi, A.E.; Borromeo, G.; Locatelli, M.; Falla, D. The impact of COVID-19-related distress on general health, oral behaviour, psychosocial features, disability and pain intensity in a cohort of Italian patients with temporomandibular disorders. PLoS ONE 2021, 16, e0245999. [Google Scholar] [CrossRef]
- Ghurye, S.; McMillan, R. Pain-Related Temporomandibular Disorder-Current Perspectives and Evidence-Based Management. Dent. Update 2015, 42, 533–546. [Google Scholar] [CrossRef]
- Dworkin, S.F.; Huggins, K.H.; LeResche, L.; Von Korff, M.; Howard, J.; Truelove, E.; Sommers, E. Epidemiology of signs and symptoms in temporomandibular disorders: Clinical signs in cases and controls. J. Am. Dent. Assoc. 1990, 120, 273–281. [Google Scholar] [CrossRef]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Sugiyo, S.; Moritani, M.; Kobayashi, M.; Yonehara, N. Mechanisms of orofacial pain control in the central nervous system. Arch. Histol. Cytol. 2006, 69, 79–100. [Google Scholar] [CrossRef]
- Mujakperuo, H.R.; Watson, M.; Morrison, R.; Macfarlane, T.V. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database Syst. Rev. 2010, Cd004715. [Google Scholar] [CrossRef]
- Haas, D.A. Pharmacologic considerations in the management of temporomandibular disorders. J. Can. Dent. Assoc. 1995, 61, 105–114. [Google Scholar] [PubMed]
- Wright, E.F. Manual of Temporomandibular Disorders, 2nd ed.; Wiley-Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Ta, L.E.; Dionne, R.A. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: A randomized placebo-controlled comparison of celecoxib to naproxen. Pain 2004, 111, 13–21. [Google Scholar] [CrossRef] [PubMed]
- de Carli, M.L.; Guerra, M.B.; Nunes, T.B.; di Matteo, R.C.; de Luca, C.E.; Aranha, A.C.; Bolzan, M.C.; Witzel, A.L. Piroxicam and laser phototherapy in the treatment of TMJ arthralgia: A double-blind randomised controlled trial. J. Oral Rehabil. 2013, 40, 171–178. [Google Scholar] [CrossRef]
- Di Rienzo Businco, L.; Di Rienzo Businco, A.; D’Emilia, M.; Lauriello, M.; Coen Tirelli, G. Topical versus systemic diclofenac in the treatment of temporo-mandibular joint dysfunction symptoms. Acta Otorhinolaryngol. Ital. 2004, 24, 279–283. [Google Scholar] [PubMed]
- Dong, X.D.; Svensson, P.; Cairns, B.E. The analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonism. Pain 2009, 147, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Thambar, S.; Arora, H. Evaluating the effectiveness of nonsteroidal anti-inflammatory drug(s) for relief of pain associated with temporomandibular joint disorders: A systematic review. Clin. Exp. Dent. Res. 2020, 6, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.L.; Kuriyama, A.; Kuwatsuka, Y.; Nickoloff, S.; Storch, D.; Jackson, W.; Zhang, Z.J.; Hayashino, Y. Beta-blockers for the prevention of headache in adults; a systematic review and meta-analysis. PLoS ONE 2019, 14, e0212785. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.L.; Oliveira, M.C.; Pelegrini-da-Silva, A.; de Arruda Veiga, M.C.; Parada, C.A.; Tambeli, C.H. Peripheral sympathetic component of the temporomandibular joint inflammatory pain in rats. J. Pain 2006, 7, 929–936. [Google Scholar] [CrossRef]
- Oliveira-Fusaro, M.C.; Clemente-Napimoga, J.T.; Teixeira, J.M.; Torres-Chávez, K.E.; Parada, C.A.; Tambeli, C.H. 5-HT induces temporomandibular joint nociception in rats through the local release of inflammatory mediators and activation of local β adrenoceptors. Pharmacol. Biochem. Behav. 2012, 102, 458–464. [Google Scholar] [CrossRef]
- Tchivileva, I.E.; Hadgraft, H.; Lim, P.F.; Di Giosia, M.; Ribeiro-Dasilva, M.; Campbell, J.H.; Willis, J.; James, R.; Herman-Giddens, M.; Fillingim, R.B.; et al. Efficacy and safety of propranolol for treatment of temporomandibular disorder pain: A randomized; placebo-controlled clinical trial. Pain 2020, 161, 1755–1767. [Google Scholar] [CrossRef]
- Burch, R. Antidepressants for Preventive Treatment of Migraine. Curr. Treat.Options Neurol. 2019, 21, 18. [Google Scholar] [CrossRef]
- Haviv, Y.; Rettman, A.; Aframian, D.; Sharav, Y.; Benoliel, R. Myofascial pain: An open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J. Oral Facial. Pain Headache 2015, 29, 144–151. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar]
- Rizzatti-Barbosa, C.M.; Nogueira, M.T.; de Andrade, E.D.; Ambrosano, G.M.; de Barbosa, J.R. Clinical evaluation of amitriptyline for the control of chronic pain caused by temporomandibular joint disorders. Cranio 2003, 21, 221–225. [Google Scholar] [CrossRef]
- Cascos-Romero, J.; Vázquez-Delgado, E.; Vázquez-Rodríguez, E.; Gay-Escoda, C. The use of tricyclic antidepressants in the treatment of temporomandibular joint disorders: Systematic review of the literature of the last 20 years. Med. Oral Patol. Oral Cir. Bucal 2009, 14, E3–E7. [Google Scholar] [PubMed]
- Goyal, P.; Singh, R.K.; Gangwar, S.; Mohammad, S.; Pal, U.S.; Singh, G. Effect of duloxetine in temporomandibular joint disorders: A comparison with arthrocentesis. Natl. J. Maxillofac. Surg. 2020, 11, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kimos, P.; Biggs, C.; Mah, J.; Heo, G.; Rashiq, S.; Thie, N.M.; Major, P.W. Analgesic action of gabapentin on chronic pain in the masticatory muscles: A randomized controlled trial. Pain 2007, 127, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Harkins, S.; Linford, J.; Cohen, J.; Kramer, T.; Cueva, L. Administration of clonazepam in the treatment of TMD and associated myofascial pain: A double-blind pilot study. J. Craniomandib. Disord. 1991, 5, 179–186. [Google Scholar]
- Herman, C.R.; Schiffman, E.L.; Look, J.O.; Rindal, D.B. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: A randomized clinical trial. J. Orofac. Pain 2002, 16, 64–70. [Google Scholar] [PubMed]
- Kunjur, J.; Anand, R.; Brennan, P.A.; Ilankovan, V. An audit of 405 temporomandibular joint arthrocentesis with intra-articular morphine infusion. Br. J. Oral Maxillofac. Surg. 2003, 41, 29–31. [Google Scholar] [CrossRef]
- List, T.; Tegelberg, Å.; Haraldson, T.; Isacsson, G. Intra-articular morphine as analgesic in temporomandibular joint arthralgia/osteoarthritis. Pain 2001, 94, 275–282. [Google Scholar] [CrossRef]
- List, T.; Axelsson, S.; Leijon, G. Pharmacologic interventions in the treatment of temporomandibular disorders; atypical facial pain; and burning mouth syndrome. A qualitative systematic review. J. Orofac. Pain 2003, 17, 301–310. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Häggman-Henrikson, B.; Alstergren, P.; Davidson, T.; Högestätt, E.D.; Östlund, P.; Tranaeus, S.; Vitols, S.; List, T. Pharmacological treatment of oro-facial pain-health technology assessment including a systematic review with network meta-analysis. J. Oral Rehabil. 2017, 44, 800–826. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, J.; Yu, Y.; Hu, S.; Wang, Y.; Wu, M. Therapeutic Agents for the Treatment of Temporomandibular Joint Disorders: Progress and Perspective. Front. Pharmacol. 2020, 11, 596099. [Google Scholar] [CrossRef]
- Gil-Martínez, A.; Paris-Alemany, A.; López-de-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. J. Pain Res. 2018, 11, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular disorders: A review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef] [PubMed]
- List, T.; Jensen, R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 2017, 37, 692–704. [Google Scholar] [CrossRef]
- Ratnayake, J.; Guan, G.; Polonowita, A.; Gray, A.R.; Loch, C.; Li, K.C.; Waddell, J.N.; Lyons, K.; Brunton, P.A. Measuring Changes in Jaw Opening Forces to Assess the Degree of Improvement in Patients with Temporomandibular Disorders. Appl. Sci. 2022, 12, 1224. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106. [Google Scholar] [CrossRef]
- Dinsdale, A.; Costin, B.; Dharamdasani, S.; Page, R.; Purs, N.; Treleaven, J. What conservative interventions improve bite function in those with temporomandibular disorders? A systematic review using self-reported and physical measures. J. Oral Rehabil. 2022, 49, 456–475. [Google Scholar] [CrossRef] [PubMed]
- Storm Mienna, C.; Glas, L.; Magnusson, M.; Ilgunas, A.; Häggman-Henrikson, B.; Wänman, A. Patients’ experiences of supervised jaw-neck exercise among patients with localized TMD pain or TMD pain associated with generalized pain. Acta Odontol. Scand. 2019, 77, 495–501. [Google Scholar] [CrossRef]
- Contento, V.S.; Dalton, B.H.; Power, G.A. The inhibitory tendon-evoked reflex is increased in the torque-enhanced state following active lengthening compared to a purely isometric contraction. Brain Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- Shimada, A.; Ishigaki, S.; Matsuka, Y.; Komiyama, O.; Torisu, T.; Oono, Y.; Sasaki, K. Effects of exercise therapy on painful temporomandibular disorders. J. Oral Rehabil. 2019, 46, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.J.; Gresty, M.; Keshner, E.; Shupert, C. The control of head movements during human balance corrections. J. Vestib. Res. 1997, 7, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, X.; Xu, X. The difficult relationship between occlusal interferences and temporomandibular disorder—Insights from animal and human experimental studies. J. Oral Rehabil. 2013, 40, 279–295. [Google Scholar] [CrossRef]
- Alhilou, A.M.; Shimada, A.; Svensson, C.I.; Svensson, P.; Ernberg, M.; Cairns, B.E.; Christidis, N. Nerve growth factor and glutamate increase the density and expression of substance P-containing nerve fibers in healthy human masseter muscles. Sci. Rep. 2021, 11, 15673. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, M.; Chen, Y.J.; Li, Q.; Wu, A.Z. Psychological stress induces temporary masticatory muscle mechanical sensitivity in rats. J. Biomed. Biotechnol. 2011, 2011, 720603. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Lombardo, L.; Siciliani, G. Temporomandibular disorders and dental occlusion. A systematic review of association studies: End of an era? J. Oral Rehabil. 2017, 44, 908–923. [Google Scholar] [CrossRef]
- Ferrillo, M.; Marotta, N.; Giudice, A.; Calafiore, D.; Curci, C.; Fortunato, L.; Ammendolia, A.; de Sire, A. Effects of Occlusal Splints on Spinal Posture in Patients with Temporomandibular Disorders: A Systematic Review. Healthcare 2022, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Mattyasovszky, S.G.; Langendorf, E.K.; Ritz, U.; Schmitz, C.; Schmidtmann, I.; Nowak, T.E.; Wagner, D.; Hofmann, A.; Rommens, P.M.; Drees, P. Exposure to radial extracorporeal shock waves modulates viability and gene expression of human skeletal muscle cells: A controlled in vitro study. J. Orthop. Surg. Res. 2018, 13, 75. [Google Scholar] [CrossRef]
- Ammendolia, A.; Marotta, N.; Demeco, A.; Marinaro, C.; Moggio, L.; Barletta, M.; Costantino, C. Effectiveness of radial shockwave therapy in calcific and non-calcific tendinopathy of the shoulder: A systematic review and meta-analysis. Muscles Ligaments Tendons J. 2020, 10, 40–47. [Google Scholar] [CrossRef]
- Ramon, S.; Gleitz, M.; Hernandez, L.; Romero, L.D. Update on the efficacy of extracorporeal shockwave treatment for myofascial pain syndrome and fibromyalgia. Int. J. Surg. 2015, 24, 201–206. [Google Scholar] [CrossRef]
- Hausdorf, J.; Lemmens, M.A.; Heck, K.D.; Grolms, N.; Korr, H.; Kertschanska, S.; Steinbusch, H.W.; Schmitz, C.; Maier, M. Selective loss of unmyelinated nerve fibers after extracorporeal shockwave application to the musculoskeletal system. Neuroscience 2008, 155, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Ge, M.; Gao, M. Efficacy of low-level laser therapy in the treatment of TMDs: A meta-analysis of 14 randomised controlled trials. J. Oral Rehabil. 2015, 42, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Douglas De Oliveira, D.W.; Lages, F.S.; Guimarães, R.C.; Pereira, T.S.; Botelho, A.M.; Glória, J.C.R.; Tavano, K.T.A.; Gonçalves, P.F.; Flecha, O.D. Do TMJ symptoms improve and last across time after treatment with red (660 nm) and infrared (790 nm) low level laser treatment (LLLT)? A survival analysis. Cranio 2017, 35, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, A.; Marotta, N.; Marinaro, C.; Demeco, A.; Mondardini, P.; Costantino, C. The synergic use of the High Power Laser Therapy and Glucosamine sulfate in Knee osteoarthritis: A Randomized Controlled Trial. Acta Biomed. 2021, 92, e2021237. [Google Scholar] [PubMed]
- Johnson, J.F. Laser therapy and pain management. In Laser Therapy in Veterinary Medicine: Photobiomodulation; Wiley-Blackwell: Ames, IA, USA, 2017; pp. 75–87. [Google Scholar]
- Ammendolia, A.; Cespites, M.; Iocco, M. Topical use of aloe gel and low-level laser therapy in overuse tendinitis of elite volleyball players: A randomized controlled trial. Sport Sci. Health 2016, 12, 209–213. [Google Scholar] [CrossRef]
- Yasmeen, S. Low Level Laser Therapy Review. Int. J. Curr. Res. 2020, 12, 144. [Google Scholar]
- Shanavas, M.; Chatra, L.; Shenai, P.; Rao, P.K.; Jagathish, V.; Kumar, S.P.; Naduvakkattu, B. Transcutaneous electrical nerve stimulation therapy: An adjuvant pain controlling modality in TMD patients—A clinical study. Dent. Res. J. 2014, 11, 676–679. [Google Scholar]
- Vance, C.G.; Dailey, D.L.; Rakel, B.A.; Sluka, K.A. Using TENS for pain control: The state of the evidence. Pain Manag. 2014, 4, 197–209. [Google Scholar] [CrossRef]
- Bergeron-Vézina, K.; Corriveau, H.; Martel, M.; Harvey, M.P.; Léonard, G. High-and low-frequency transcutaneous electrical nerve stimulation does not reduce experimental pain in elderly individuals. Pain 2015, 156, 2093. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Nota, A.; Tecco, S.; Caruso, S.; Marchetti, E.; Marzo, G.; Cutilli, T. Ultra-low-frequency transcutaneous electric nerve stimulation (ULF-TENS) in subjects with craniofacial pain: A retrospective study. Cranio 2020, 38, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ewall, G.; Parkins, S.; Lin, A.; Jaoui, Y.; Lee, H.K. Cortical and Subcortical Circuits for Cross-Modal Plasticity Induced by Loss of Vision. Front. Neural Circuits 2021, 25, 665009. [Google Scholar] [CrossRef] [PubMed]
- Hallman, D.; Lyskov, E. Autonomic Regulation in Musculoskeletal Pain; IntechOpen: London, UK, 2012. [Google Scholar]
- Naviaux, R.K. Metabolic features and regulation of the healing cycle—A new model for chronic disease pathogenesis and treatment. Mitochondrion 2019, 46, 278–297. [Google Scholar] [CrossRef]
- Zhu, H. Acupoints Initiate the Healing Process. Med. Acupunct. 2014, 26, 264–270. [Google Scholar] [CrossRef]
- Vanderploeg, K.; Yi, X. Acupuncture in modern society. J. Acupunct. Meridian Stud. 2009, 2, 26–33. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, Y.; Park, H.J.; Hahm, D.H. Coarse needle surface potentiates analgesic effect elicited by acupuncture with twirling manipulation in rats with nociceptive pain. BMC Complement. Altern. Med. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.B. Acupuncture in dentistry: Its possible role and application. Proc. Singap. Healthc. 2012, 21, 48–56. [Google Scholar] [CrossRef]
- Cui, J.; Song, W.; Jin, Y.; Xu, H.; Fan, K.; Lin, D.; Hao, Z.; Lin, J. Research Progress on the Mechanism of the Acupuncture Regulating Neuro-Endocrine-Immune Network System. Vet. Sci. 2021, 8, 149. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.; Lee, J.; Park, C.; Moon, D.E. The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Can. J. Anaesth. 2006, 53, 470–477. [Google Scholar] [CrossRef]
- Baricich, A.; Picelli, A.; Santamato, A.; Carda, S.; de Sire, A.; Smania, N.; Cisari, C.; Invernizzi, M. Safety Profile of High-Dose Botulinum Toxin Type A in Post-Stroke Spasticity Treatment. Clin. Drug Investig. 2018, 38, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.; Clapp, L.; Erickson, M.; Jabbari, B. Botulinum toxin A and chronic low back pain. A randomized; double-blind study. Neurology 2001, 56, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; Folli, A.; D’Abrosca, F.; Grana, E.; Baricich, A.; Carda, S.; Invernizzi, M. Multidimensional Effectiveness of Botulinum Toxin in Neuropathic Pain: A Systematic Review of Randomized Clinical Trials. Toxins 2022, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Bernetti, A.; Agostini, F.; de Sire, A.; Mangone, M.; Tognolo, L.; Di Cesare, A.; Ruiu, P.; Paolucci, T.; Invernizzi, M.; Paoloni, M. Neuropathic Pain and Rehabilitation: A Systematic Review of International Guidelines. Diagnostics 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Lora, V.R.M.; Dugonjić Okroša, A.; Matak, I.; Del Bel Cury, A.A.; Kalinichev, M.; Lacković, Z. Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins 2022, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Meng, J.; Wang, J. New Engineered-Botulinum Toxins Inhibit the Release of Pain-Related Mediators. Int. J. Mol. Sci. 2019, 21, 262. [Google Scholar] [CrossRef]
- Antoniazzi, C.; Belinskaia, M.; Zurawski, T.; Kaza, S.K.; Dolly, J.O.; Lawrence, G.W. Botulinum Neurotoxin Chimeras Suppress Stimulation by Capsaicin of Rat Trigeminal Sensory Neurons In Vivo and In Vitro. Toxins 2022, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–43. [Google Scholar] [CrossRef]
- Teixeira, J.M.; Abdalla, H.B.; Basting, R.T.; Hammock, B.D.; Napimoga, M.H.; Clemente-Napimoga, J.T. Peripheral soluble epoxide hydrolase inhibition reduces hypernociception and inflammation in albumin-induced arthritis in temporomandibular joint of rats. Int. Immunopharmacol. 2020, 87, 106841. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- Paolucci, T.; Agostini, F.; Bernetti, A.; Paoloni, M.; Mangone, M.; Santilli, V.; Pezzi, L.; Bellomo, R.G.; Saggini, R. Integration of focal vibration and intra-articular oxygen-ozone therapy in rehabilitation of painful knee osteoarthritis. J. Int. Med. Res. 2021, 49, 300060520986705. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tallón, F.J.; Torres-Morera, L.M.; Baeza-Noci, J.; Carrillo-Izquierdo, M.D.; Pinto-Bonilla, R. Updated Review on Ozone Therapy in Pain Medicine. Front. Physiol. 2022, 13, 840623. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. The Clinical Application of Ozonetherapy. In OZONE; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103. [Google Scholar] [PubMed]
- Emodi-Perlman, A.; Eli, I.; Smardz, J.; Uziel, N.; Wieckiewicz, G.; Gilon, E.; Grychowska, N.; Wieckiewicz, M. Temporomandibular Disorders and Bruxism Outbreak as a Possible Factor of Orofacial Pain Worsening during the COVID-19 Pandemic-Concomitant Research in Two Countries. J. Clin. Med. 2020, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Emodi-Perlman, A.; Eli, I. One year into the COVID-19 pandemic–temporomandibular disorders and bruxism: What we have learned and what we can do to improve our manner of treatment. Dent. Med. Probl. 2021, 58, 215–218. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2; JohnWiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrillo, M.; Giudice, A.; Marotta, N.; Fortunato, F.; Di Venere, D.; Ammendolia, A.; Fiore, P.; de Sire, A. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 12164. https://doi.org/10.3390/ijms232012164
Ferrillo M, Giudice A, Marotta N, Fortunato F, Di Venere D, Ammendolia A, Fiore P, de Sire A. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. International Journal of Molecular Sciences. 2022; 23(20):12164. https://doi.org/10.3390/ijms232012164
Chicago/Turabian StyleFerrillo, Martina, Amerigo Giudice, Nicola Marotta, Francesco Fortunato, Daniela Di Venere, Antonio Ammendolia, Pietro Fiore, and Alessandro de Sire. 2022. "Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review" International Journal of Molecular Sciences 23, no. 20: 12164. https://doi.org/10.3390/ijms232012164
APA StyleFerrillo, M., Giudice, A., Marotta, N., Fortunato, F., Di Venere, D., Ammendolia, A., Fiore, P., & de Sire, A. (2022). Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. International Journal of Molecular Sciences, 23(20), 12164. https://doi.org/10.3390/ijms232012164










