The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH—Future Horizons in NASH Management
Abstract
:1. Introduction
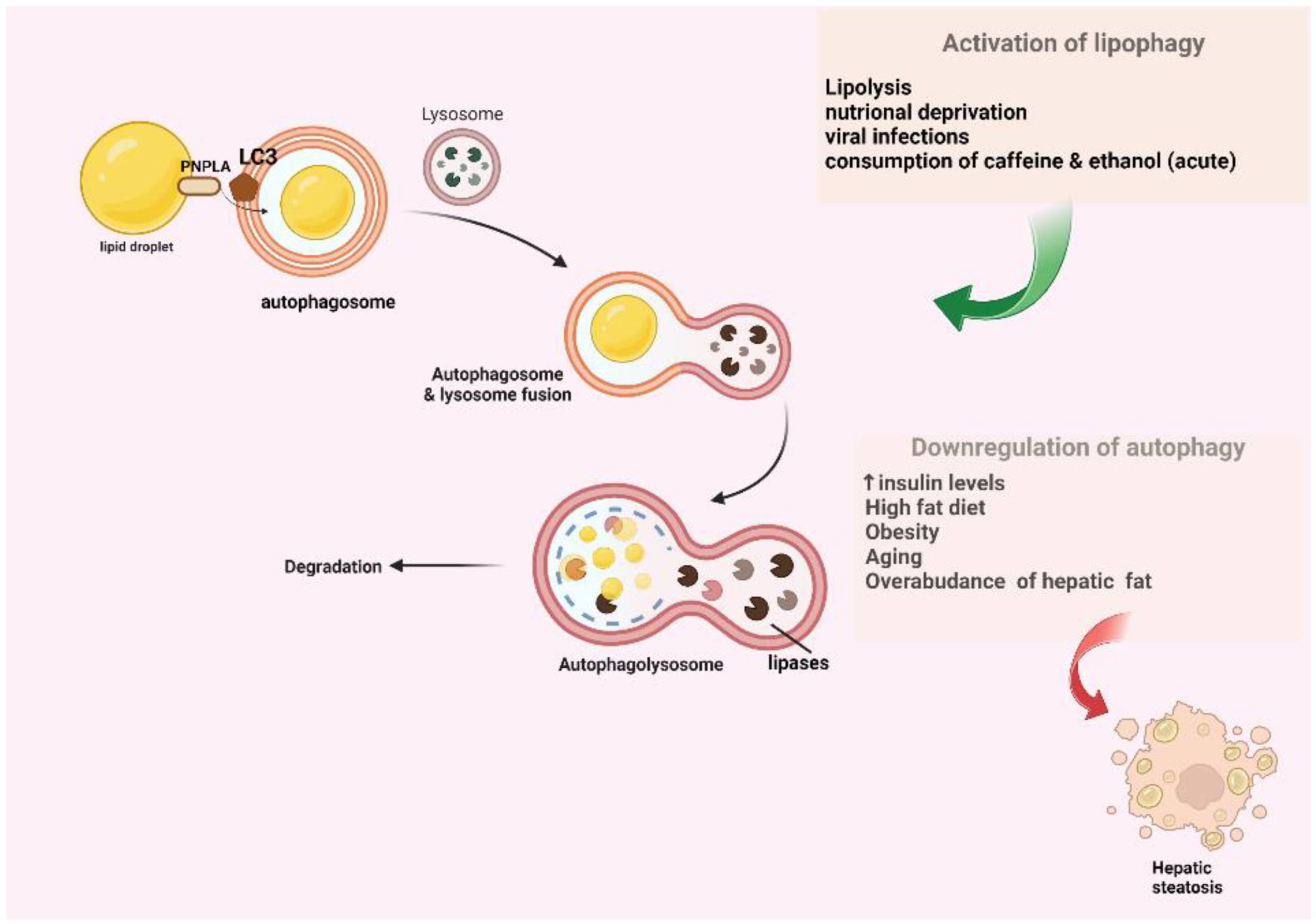
2. An Overview of the Conventional Macroautophagy and Lipophagy Pathway
3. The Association between Autophagy and NASH
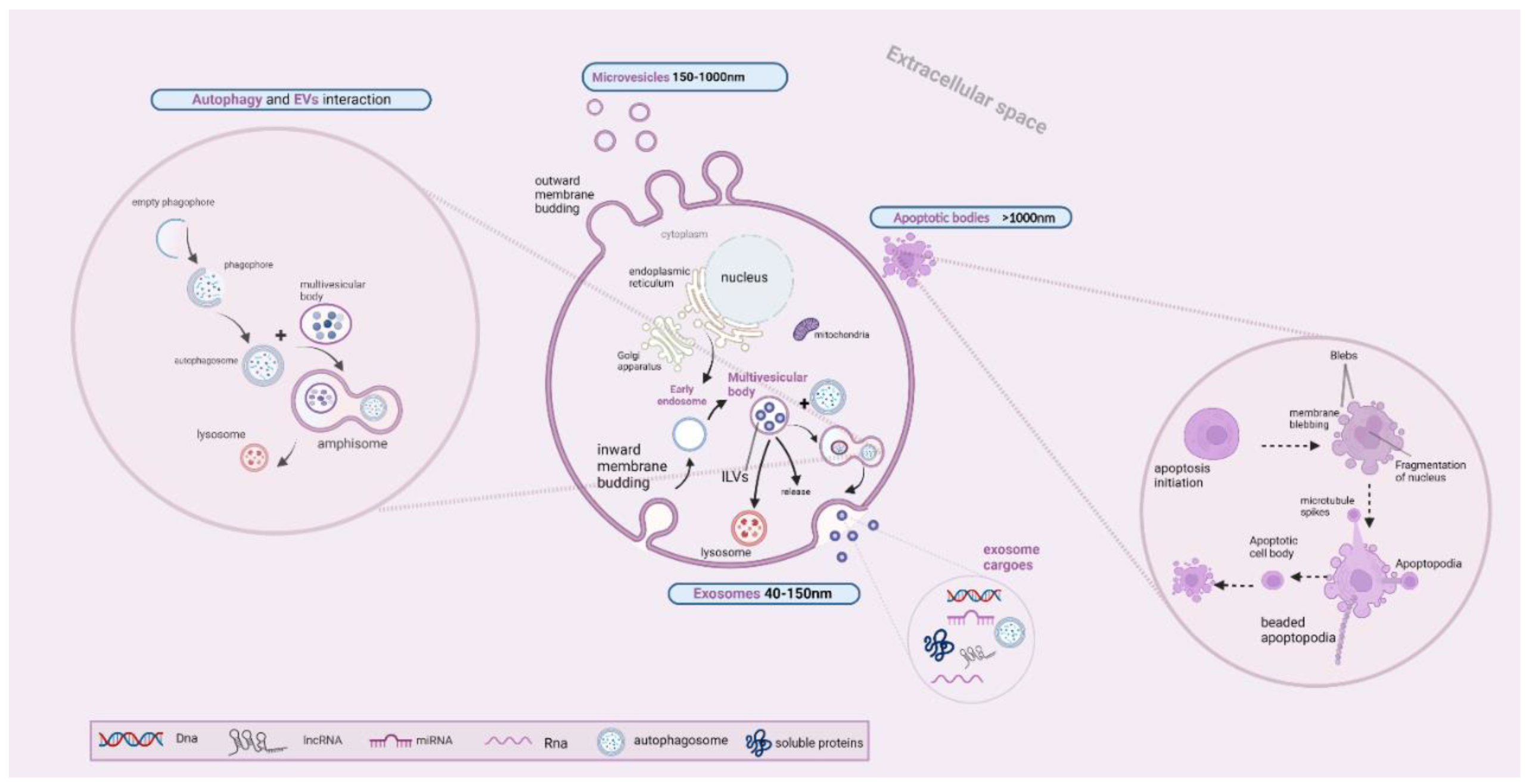
4. An Overview of Extracellular Vesicle Biogenetic Mechanisms
5. The Interplay between EV Secretion and the Autophagy Pathway
6. The Association between EVs and NASH
7. Future Horizons in NASH Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Amnion-derived MSCs | AmMSC |
| Apoptotic bodies | ApoBDs |
| Apoptotic vesicles | ApoEVs |
| Arrestin domain-containing protein-1 | ARRDC1 |
| C-X-C motif chemokine ligand 10 | CXCL10 |
| Endosomal sorting complex | ESCRT |
| Enhanced Liver Fibrosis | ELF |
| Extracellular vesicles | EVs |
| Hepatocellular carcinoma | HCC |
| Human liver stem cells | HLSCs |
| Intraluminal vesicles | ILVs |
| LC3-dependent EV loading and secretion | LDELS |
| Long non-coding RNA | lncRNA |
| Major histocompatibility complex | MHC |
| Mammalian target of rapamycin | mTOR |
| Microtubule-associated protein 1 light chain 3 | LC3 |
| Mitochondrial DNA | mtDNA |
| Mixed lineage kinase 3 | MLK3 |
| MSC-derived extracellular vesicles | MSC-EVs |
| Multivesicular bodies | MVBs |
| Multivesicular endosome | MVE |
| Non-alcoholic fatty liver | NAFL |
| Non-alcoholic fatty liver disease | NAFLD |
| Non-alcoholic steatohepatitis | NASH |
| Peroxisome proliferator-activated receptor | PRAR |
| Phagophore assembly site | PAS |
| Pluripotent stem cells | iPSC |
| Soluble NSF attachment protein receptor | SNARE |
| Sphingosine kinase 1 | SphK1 |
| Sphingosine-1 phosphate | S1P |
| Transcription factor EB | TFEB |
| Trans-Golgi network | TGN |
| Tumor susceptibility gene 101 protein | TSG101 |
| Vanin-1 | VNN1 |
References
- Dufour, J.-F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.-S.; Zelber-Sagi, S. Current Therapies and New Developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current Concepts and Future Challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Carotti, S.; Aquilano, K.; Zalfa, F.; Ruggiero, S.; Valentini, F.; Zingariello, M.; Francesconi, M.; Perrone, G.; Alletto, F.; Antonelli-Incalzi, R.; et al. Lipophagy Impairment Is Associated with Disease Progression in NAFLD. Front. Physiol. 2020, 11, 850. [Google Scholar] [CrossRef]
- Leidal, A.M.; Debnath, J. Emerging Roles for the Autophagy Machinery in Extracellular Vesicle Biogenesis and Secretion. FASEB Bioadv. 2021, 3, 377–386. [Google Scholar] [CrossRef]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular Vesicles: Mediators of Intercellular Communication in Tissue Injury and Disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. Role of Autophagy in Cholangiocarcinoma: An Autophagy-Based Treatment Strategy. World J. Gastrointest. Oncol. 2021, 13, 1229–1243. [Google Scholar] [CrossRef]
- Peker, N.; Gozuacik, D. Autophagy as a Cellular Stress Response Mechanism in the Nervous System. J. Mol. Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef]
- Mizushima, N. The ATG Conjugation Systems in Autophagy. Curr. Opin. Cell Biol. 2020, 63, 1–10. [Google Scholar] [CrossRef]
- Kawabata, T.; Yoshimori, T. Autophagosome Biogenesis and Human Health. Cell Discov. 2020, 6, 33. [Google Scholar] [CrossRef]
- Li, H.-Y.; Peng, Z.-G. Targeting Lipophagy as a Potential Therapeutic Strategy for Nonalcoholic Fatty Liver Disease. Biochem. Pharmacol. 2022, 197, 114933. [Google Scholar] [CrossRef]
- Samovski, D.; Abumrad, N.A. Regulation of Lipophagy in NAFLD by Cellular Metabolism and CD36. J. Lipid Res. 2019, 60, 755–757. [Google Scholar] [CrossRef] [Green Version]
- Fisher, E.A. The Degradation of Apolipoprotein B100: Multiple Opportunities to Regulate VLDL Triglyceride Production by Different Proteolytic Pathways. Biochim. Biophys. Acta 2012, 1821, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Udoh, U.-A.S.; Rajan, P.K.; Nakafuku, Y.; Finley, R.; Sanabria, J.R. Cell Autophagy in NASH and NASH-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 7734. [Google Scholar] [CrossRef]
- Czaja, M.J. Function of Autophagy in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Frendo-Cumbo, S.; Tokarz, V.L.; Bilan, P.J.; Brumell, J.H.; Klip, A. Communication between Autophagy and Insulin Action: At the Crux of Insulin Action-Insulin Resistance? Front. Cell Dev. Biol. 2021, 9, 708431. [Google Scholar] [CrossRef]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting Autophagy in Obesity: From Pathophysiology to Management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.-Y.; Matsui, I.; Matsusaka, T.; et al. High-Fat Diet–Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Tautenhahn, H.-M.; Dirsch, O.; Dahmen, U. Modulation of Autophagy: A Novel “Rejuvenation” Strategy for the Aging Liver. Oxid. Med. Cell. Longev. 2021, 2021, 6611126. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.d.M.; Kowaltowski, A.J.; Kakimoto, P.A. Autophagy in Hepatic Steatosis: A Structured Review. Front. Cell Dev. Biol. 2021, 9, 657389. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-W.; Yang, R.-X.; Fan, J.-G. Chronic Hepatitis B Infection with Concomitant Hepatic Steatosis: Current Evidence and Opinion. World J. Gastroenterol. 2021, 27, 3971–3983. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.B.; Cvjetan, N.; Ayache, J.; Walde, P. Multivesicular Vesicles: Preparation and Applications. ChemSystemsChem 2021, 3, e2000049. [Google Scholar] [CrossRef]
- Frankel, E.B.; Audhya, A. ESCRT-Dependent Cargo Sorting at Multivesicular Endosomes. Semin. Cell Dev. Biol. 2018, 74, 4–10. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Nakano, A. The Golgi Apparatus and Its Next-Door Neighbors. Front. Cell Dev. Biol. 2022, 10, 884360. [Google Scholar] [CrossRef]
- Piper, R.C.; Katzmann, D.J. Biogenesis and Function of Multivesicular Bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef] [Green Version]
- Woodman, P.G.; Futter, C.E. Multivesicular Bodies: Co-Ordinated Progression to Maturity. Curr. Opin. Cell Biol. 2008, 20, 408–414. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoon, J.-Y.; Lee, J.H.; Lee, H.-H.; Knowles, J.C.; Kim, H.-W. Emerging Biogenesis Technologies of Extracellular Vesicles for Tissue Regenerative Therapeutics. J. Tissue Eng. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Hyenne, V.; Labouesse, M.; Goetz, J.G. The Small GTPase Ral Orchestrates MVB Biogenesis and Exosome Secretion. Small GTPases 2016, 9, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extracellular Communication during Cancer Progression. J. Cell Sci. 2010, 123 Pt 10, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Atkin-Smith, G.K.; Poon, I.K.H. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017, 27, 151–162. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. In New Frontiers: Extracellular Vesicles; Part of the Subcellular Biochemistry Book Series; Mathivanan, S., Fonseka, P., Nedeva, C., Atukorala, I., Eds.; Springer: Cham, Switzerland, 2021; Volume 97, pp. 61–88. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef]
- Wei, W.; Pan, Y.; Yang, X.; Chen, Z.; Heng, Y.; Yang, B.; Pu, M.; Zuo, J.; Lai, Z.; Tang, Y.; et al. The Emerging Role of the Interaction of Extracellular Vesicle and Autophagy-Novel Insights into Neurological Disorders. J. Inflamm. Res. 2022, 15, 3395–3407. [Google Scholar] [CrossRef]
- Repnik, U.; Česen, M.H.; Turk, B. The Endolysosomal System in Cell Death and Survival. Cold Spring Harb. Perspect. Biol. 2013, 5, a008755. [Google Scholar] [CrossRef]
- Du, J.; Ji, Y.; Qiao, L.; Liu, Y.; Lin, J. Cellular Endo-Lysosomal Dysfunction in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Liver Int. 2020, 40, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Hikita, H.; Sakane, S.; Takehara, T. Mechanisms of the Autophagosome-Lysosome Fusion Step and Its Relation to Non-Alcoholic Fatty Liver Disease. Liver Res. 2018, 2, 120–124. [Google Scholar] [CrossRef]
- Solvik, T.A.; Nguyen, T.A.; Lin, Y.-H.T.; Marsh, T.; Huang, E.J.; Wiita, A.P.; Debnath, J.; Leidal, A.M. Autophagy Cargo Receptors Are Secreted via Extracellular Vesicles and Particles in Response to Endolysosomal Inhibition or Impaired Autophagosome Maturation. bioRxiv 2021. Erratum in J. Cell Biol. 2022, 221, e202110151. [Google Scholar] [CrossRef]
- Gonzalez, C.D.; Resnik, R.; Vaccaro, M.I. Secretory Autophagy and Its Relevance in Metabolic and Degenerative Disease. Front. Endocrinol. 2020, 11, 266. [Google Scholar] [CrossRef]
- Leidal, A.M.; Debnath, J. LC3-Dependent Extracellular Vesicle Loading and Secretion (LDELS). Autophagy 2020, 16, 1162–1163. [Google Scholar] [CrossRef]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA Packaging into Extracellular Vesicles: An Orchestra of RNA-Binding Proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef]
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.; Goldsmith, J.; Liu, J.Y.; Huang, Y.-H.; et al. The LC3-Conjugation Machinery Specifies the Loading of RNA-Binding Proteins into Extracellular Vesicles. Nat. Cell Biol. 2020, 22, 187–199. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Klionsky, D.J. The LC3-Conjugation Machinery Specifies Cargo Loading and Secretion of Extracellular Vesicles. Autophagy 2020, 16, 1169–1171. [Google Scholar] [CrossRef]
- Ganesan, D.; Cai, Q. Understanding Amphisomes. Biochem. J. 2021, 478, 1959–1976. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Nd, A.M. Non-Alcoholic Fatty Liver Disease, an Overview. Integr. Med. 2019, 18, 42–49. [Google Scholar]
- Liao, C.-Y.; Song, M.J.; Gao, Y.; Mauer, A.S.; Revzin, A.; Malhi, H. Hepatocyte-Derived Lipotoxic Extracellular Vesicle Sphingosine 1-Phosphate Induces Macrophage Chemotaxis. Front. Immunol. 2018, 9, 2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorairaj, V.; Sulaiman, S.A.; Abu, N.; Abdul Murad, N.A. Extracellular Vesicles in the Development of the Non-Alcoholic Fatty Liver Disease: An Update. Biomolecules 2020, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharthi, J.; Latchoumanin, O.; George, J.; Eslam, M. Macrophages in Metabolic Associated Fatty Liver Disease. World J. Gastroenterol. 2020, 26, 1861–1878. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, R.; Kemper, S.; Brigstock, D.R. Extracellular Vesicles from Hepatocytes Are Therapeutic for Toxin-Mediated Fibrosis and Gene Expression in the Liver. Front. Cell Dev. Biol. 2019, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Zisser, A.; Ipsen, D.H.; Tveden-Nyborg, P. Hepatic Stellate Cell Activation and Inactivation in NASH-Fibrosis-Roles as Putative Treatment Targets? Biomedicines 2021, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.K.; Friedman, S.L. Hepatic Stellate Cell-Immune Interactions in NASH. Front. Endocrinol. 2022, 13, 867940. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Dou, G.; Wang, L. MicroRNAs in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2021, 17, 1851–1863. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Tveden-Nyborg, P. Extracellular Vesicles as Drivers of Non-Alcoholic Fatty Liver Disease: Small Particles with Big Impact. Biomedicines 2021, 9, 93. [Google Scholar] [CrossRef]
- Babuta, M.; Szabo, G. Extracellular Vesicles in Inflammation: Focus on the MicroRNA Cargo of EVs in Modulation of Liver Diseases. J. Leukoc. Biol. 2022, 111, 75–92. [Google Scholar] [CrossRef]
- Kostallari, E.; Valainathan, S.; Biquard, L.; Shah, V.H.; Rautou, P.-E. Role of Extracellular Vesicles in Liver Diseases and Their Therapeutic Potential. Adv. Drug Deliv. Rev. 2021, 175, 113816. [Google Scholar] [CrossRef]
- Nakamura, A.; Terauchi, Y. Lessons from Mouse Models of High-Fat Diet-Induced NAFLD. Int. J. Mol. Sci. 2013, 14, 21240–21257. [Google Scholar] [CrossRef] [Green Version]
- Sowa, J.-P.; Fingas, C.D.; Canbay, A. Mixed Lineage Kinase 3 Connects Hepatocellular Lipotoxicity with Macrophage Chemotaxis. Hepatology 2016, 63, 685–687. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinez, I.; Alen, R.; Rada, P.; Valverde, A.M. Insights into Extracellular Vesicles as Biomarker of NAFLD Pathogenesis. Front. Med. 2020, 7, 395. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Bai, F.; Xu, Y.; Liang, X.; Ma, C.; Gao, L. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front. Immunol. 2021, 12, 708978. [Google Scholar] [CrossRef]
- Tang, D.; Cao, F.; Yan, C.; Fang, K.; Ma, J.; Gao, L.; Sun, B.; Wang, G. Extracellular Vesicle/Macrophage Axis: Potential Targets for Inflammatory Disease Intervention. Front. Immunol. 2022, 13, 705472. [Google Scholar] [CrossRef]
- Oates, J.R.; McKell, M.C.; Moreno-Fernandez, M.E.; Damen, M.S.M.A.; Deepe, G.S., Jr.; Qualls, J.E.; Divanovic, S. Macrophage Function in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: The Mac Attack. Front. Immunol. 2019, 10, 2893. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-L.; Pan, Q.; Cao, H.-X.; Xin, F.-Z.; Zhao, Z.-H.; Yang, R.-X.; Zeng, J.; Zhou, H.; Fan, J.-G. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Malhi, H.; Gores, G.J. Nonalcoholic Steatohepatitis Promoting Kinases. Semin. Liver Dis. 2020, 40, 346–357. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Wang, H.; Zhang, M.; Qiu, P.; Zhang, M.; Zhang, R.; Zhao, Q.; Liu, J. Crosstalk between Liver Macrophages and Surrounding Cells in Nonalcoholic Steatohepatitis. Front. Immunol. 2020, 11, 1169. [Google Scholar] [CrossRef]
- Azparren-Angulo, M.; Royo, F.; Gonzalez, E.; Liebana, M.; Brotons, B.; Berganza, J.; Goñi-de-Cerio, F.; Manicardi, N.; Abad-Jordà, L.; Gracia-Sancho, J.; et al. Extracellular Vesicles in Hepatology: Physiological Role, Involvement in Pathogenesis, and Therapeutic Opportunities. Pharmacol. Ther. 2021, 218, 107683. [Google Scholar] [CrossRef]
- Are, V.S.; Vuppalanchi, R.; Vilar-Gomez, E.; Chalasani, N. Enhanced Liver Fibrosis Score Can Be Used to Predict Liver-Related Events in Patients with Nonalcoholic Steatohepatitis and Compensated Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 1292–1293.e3. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Muller, K.; Rowland, A. Circulating Cell-Specific Extracellular Vesicles as Biomarkers for the Diagnosis and Monitoring of Chronic Liver Diseases. Cell. Mol. Life Sci. 2022, 79, 232. [Google Scholar] [CrossRef]
- Welsh, J.A.; Scorletti, E.; Clough, G.F.; Englyst, N.A.; Byrne, C.D. Leukocyte Extracellular Vesicle Concentration Is Inversely Associated with Liver Fibrosis Severity in NAFLD. J. Leukoc. Biol. 2018, 104, 631–639. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.K.; Jeong, S.-Y.; Cho, H.J.; Park, J.; Kim, T.M.; Kim, S. Cargo Proteins in Extracellular Vesicles: Potential for Novel Therapeutics in Non-Alcoholic Steatohepatitis. J. Nanobiotechnol. 2021, 19, 372. [Google Scholar] [CrossRef]
- Povero, D.; Yamashita, H.; Ren, W.; Subramanian, M.G.; Myers, R.P.; Eguchi, A.; Simonetto, D.A.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; et al. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol. Commun. 2020, 4, 1263–1278. [Google Scholar] [CrossRef]
- Liaskou, E.; Zimmermann, H.W.; Li, K.-K.; Oo, Y.H.; Suresh, S.; Stamataki, Z.; Qureshi, O.; Lalor, P.F.; Shaw, J.; Syn, W.-K.; et al. Monocyte Subsets in Human Liver Disease Show Distinct Phenotypic and Functional Characteristics. Hepatology 2013, 57, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.A.; Useckaite, Z.; Johnson, J.; Sorich, M.J.; Hopkins, A.M.; Rowland, A. Selective Isolation of Liver-Derived Extracellular Vesicles Redefines Performance of MiRNA Biomarkers for Non-Alcoholic Fatty Liver Disease. Biomedicines 2022, 10, 195. [Google Scholar] [CrossRef]
- Jiang, H.; Qian, Y.; Shen, Z.; Liu, Y.; He, Y.; Gao, R.; Shen, M.; Chen, S.; Fu, Q.; Yang, T. Circulating MicroRNA-135a-3p in Serum Extracellular Vesicles as a Potential Biological Marker of Non-alcoholic Fatty Liver Disease. Mol. Med. Rep. 2021, 24, 498. [Google Scholar] [CrossRef]
- Povero, D.; Tameda, M.; Eguchi, A.; Ren, W.; Kim, J.; Myers, R.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; Bosch, J.; et al. Protein and MiRNA Profile of Circulating Extracellular Vesicles in Patients with Primary Sclerosing Cholangitis. Sci. Rep. 2022, 12, 3027. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, M.; Jiang, X.; Yu, H.; Qiu, Y.; Yu, J.; Yang, F.; Bao, Z. Identifying SLC27A5 as a Potential Prognostic Marker of Hepatocellular Carcinoma by Weighted Gene Co-Expression Network Analysis and in Vitro Assays. Cancer Cell Int. 2021, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.-F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.-W.; Shen, D.; Zhang, J.-Z.; Zhou, N.; He, J.; et al. Liver Governs Adipose Remodelling via Extracellular Vesicles in Response to Lipid Overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.P.K.; Stevens, M.M. Strategic Design of Extracellular Vesicle Drug Delivery Systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, H.; Wang, H. Extracellular Vesicles in Non-Alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Front. Physiol. 2021, 12, 707429. [Google Scholar] [CrossRef]
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular Vesicles Derived from Mesenchymal Stem/Stromal Cells: The Regenerative Impact in Liver Diseases. Hepatology 2022, 75, 1590–1603. [Google Scholar] [CrossRef]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Therapy against Fibrotic Diseases. Stem Cell Res. Ther. 2021, 12, 435. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed Lineage Kinase 3 Mediates Release of C-X-C Motif Ligand 10-Bearing Chemotactic Extracellular Vesicles from Lipotoxic Hepatocytes. Hepatology 2016, 63, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Povero, D.; Panera, N.; Eguchi, A.; Johnson, C.D.; Papouchado, B.G.; de Araujo Horcel, L.; Pinatel, E.M.; Alisi, A.; Nobili, V.; Feldstein, A.E. Lipid-Induced Hepatocyte-Derived Extracellular Vesicles Regulate Hepatic Stellate Cell via MicroRNAs Targeting PPAR-γ. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 646–663.e4. [Google Scholar] [CrossRef] [Green Version]
- Liss, K.H.H.; Finck, B.N. PPARs and Nonalcoholic Fatty Liver Disease. Biochimie 2017, 136, 65–74. [Google Scholar] [CrossRef]
- Bruno, S.; Herrera Sanchez, M.B.; Pasquino, C.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G. Human Liver-Derived Stem Cells Improve Fibrosis and Inflammation Associated with Nonalcoholic Steatohepatitis. Stem Cells Int. 2019, 2019, 6351091. [Google Scholar] [CrossRef]
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Yuyama, K.; Nakamura, H.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018, 2018, 3212643. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin Sensitizer MSDC-0602K in Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Phase IIb Study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and Inflammation in Non-Alcoholic Fatty Liver Disease: A Randomized, Placebo Controlled Clinical Trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Kofam. Safety and Tolerability of Yaq-001 in Patients with Non-Alcoholic Steatohepatitis. Available online: https://www.kofam.ch/en/snctp-portal/searching-for-a-clinical-trial/study/46475 (accessed on 30 September 2022).
- Zubiete-Franco, I.; García-Rodríguez, J.L.; Martínez-Uña, M.; Martínez-Lopez, N.; Woodhoo, A.; Juan, V.G.-D.; Beraza, N.; Lage-Medina, S.; Andrade, F.; Fernandez, M.L.; et al. Methionine and S-Adenosylmethionine Levels Are Critical Regulators of PP2A Activity Modulating Lipophagy during Steatosis. J. Hepatol. 2016, 64, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Huda, N.; Khambu, B.; Yin, X.-M. Relevance of Autophagy to Fatty Liver Diseases and Potential Therapeutic Applications. Amino Acids 2017, 49, 1965–1979. [Google Scholar] [CrossRef] [Green Version]
- Hösel, M.; Huber, A.; Bohlen, S.; Lucifora, J.; Ronzitti, G.; Puzzo, F.; Boisgerault, F.; Hacker, U.T.; Kwanten, W.J.; Klöting, N.; et al. Autophagy Determines Efficiency of Liver-Directed Gene Therapy with Adeno-Associated Viral Vectors. Hepatology 2017, 66, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Strzyz, P. AMPK against NASH. Nat. Rev. Mol. Cell Biol. 2020, 21, 181. [Google Scholar] [CrossRef]

| Parental Cell | EV-Contained Cargo | Recipient Cell | Effect |
|---|---|---|---|
| Lipotoxic hepatocyte | miR-192 miR-128-3p VNN-1 | HSCs | -Activation of HSCs -Progression of NASH and fibrotic injury [56,57,58,59,60] |
| miR-1 VNN-1 | LSECs | -Activation of LSECs -Promotion of angiogenesis and fibrinogenesis [61,62] | |
| CXCL10 Ceramides [48] Integrin beta-1 TRAIL Oxidized mtDNA mtDNA miR-192-5p | Macrophages Monocytes | -Chemotaxis -Disease Progression [64,65,66,67,68,69] | |
| miRNA let-7e-5p | Preadipocytes | ↑Lipogenesis [83] | |
| LSECs | S1P [48] SphK1 | HSCs | ↑Adipocyte Lipogenesis [61,62] |
| Portal fibroblasts | VEGF | LSECs | -Activation of HSCs -Progression of fibrotic injury and angiogenesis [61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifylli, E.-M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Deutsch, M.; Aloizos, G.; Fortis, S.P.; Papageorgiou, E.G.; Tsagarakis, A.; Manolakopoulos, S. The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH—Future Horizons in NASH Management. Int. J. Mol. Sci. 2022, 23, 12185. https://doi.org/10.3390/ijms232012185
Trifylli E-M, Kriebardis AG, Koustas E, Papadopoulos N, Deutsch M, Aloizos G, Fortis SP, Papageorgiou EG, Tsagarakis A, Manolakopoulos S. The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH—Future Horizons in NASH Management. International Journal of Molecular Sciences. 2022; 23(20):12185. https://doi.org/10.3390/ijms232012185
Chicago/Turabian StyleTrifylli, Eleni-Myrto, Anastasios G. Kriebardis, Evangelos Koustas, Nikolaos Papadopoulos, Melanie Deutsch, Georgios Aloizos, Sotirios P. Fortis, Effie G. Papageorgiou, Ariadne Tsagarakis, and Spilios Manolakopoulos. 2022. "The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH—Future Horizons in NASH Management" International Journal of Molecular Sciences 23, no. 20: 12185. https://doi.org/10.3390/ijms232012185
APA StyleTrifylli, E. -M., Kriebardis, A. G., Koustas, E., Papadopoulos, N., Deutsch, M., Aloizos, G., Fortis, S. P., Papageorgiou, E. G., Tsagarakis, A., & Manolakopoulos, S. (2022). The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH—Future Horizons in NASH Management. International Journal of Molecular Sciences, 23(20), 12185. https://doi.org/10.3390/ijms232012185








