PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Influence of PCSK9 on Astrocyte Cholesterol Metabolism
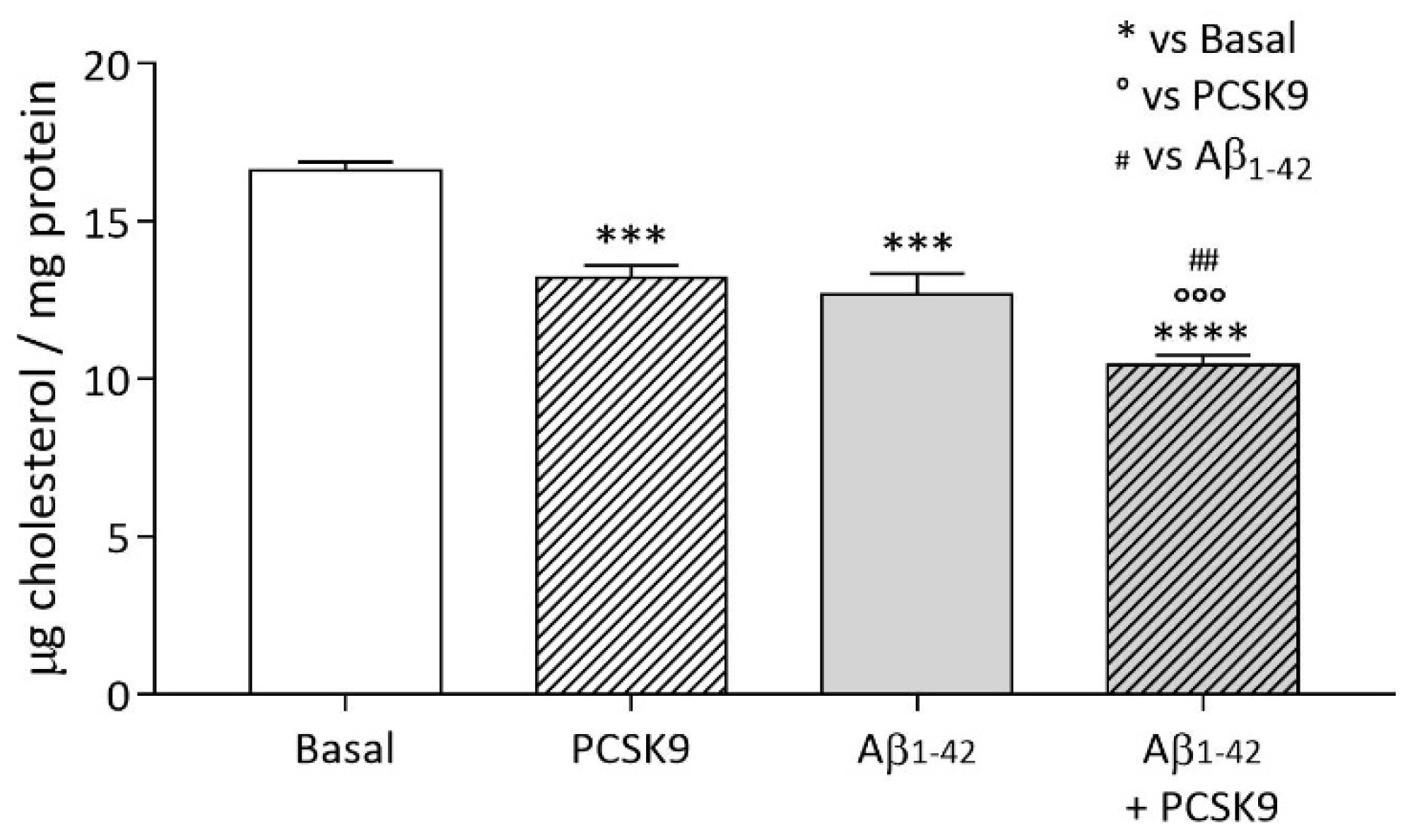
2.1.1. Intracellular Cholesterol Content
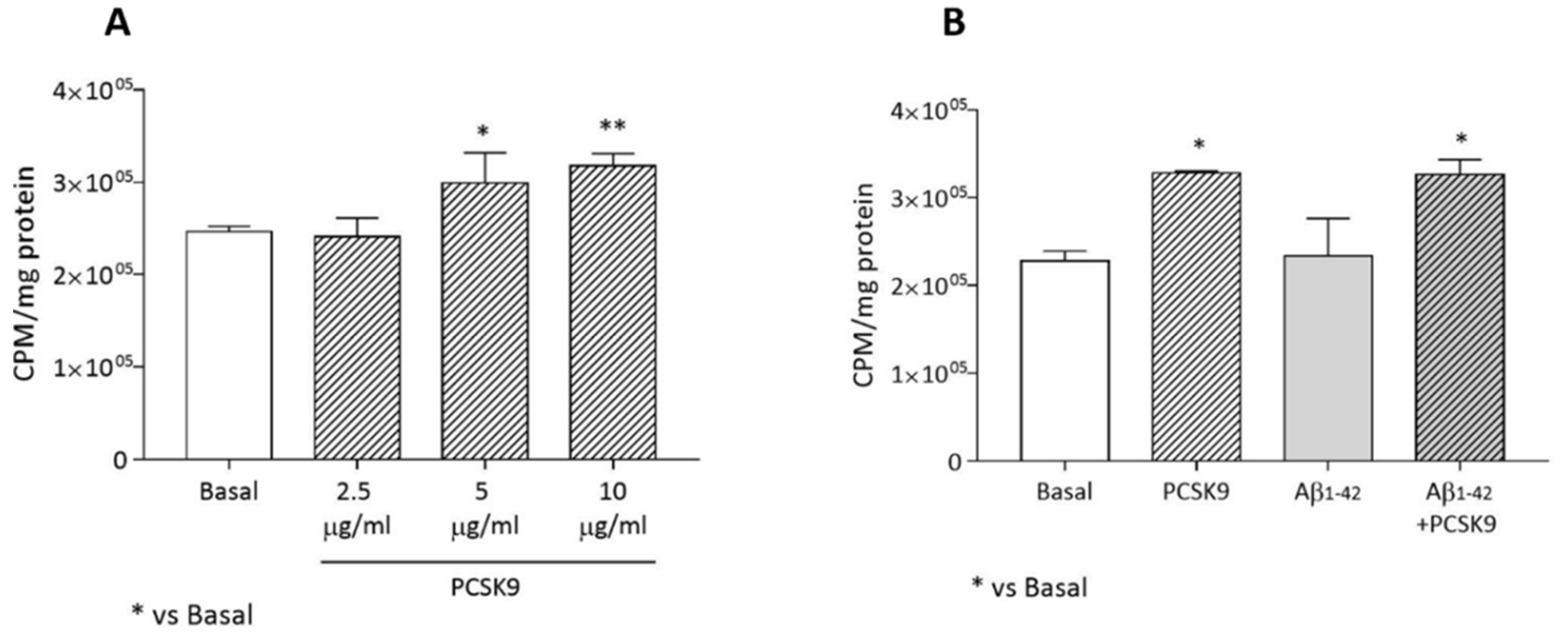
2.1.2. Endogenous Cholesterol Biosynthesis
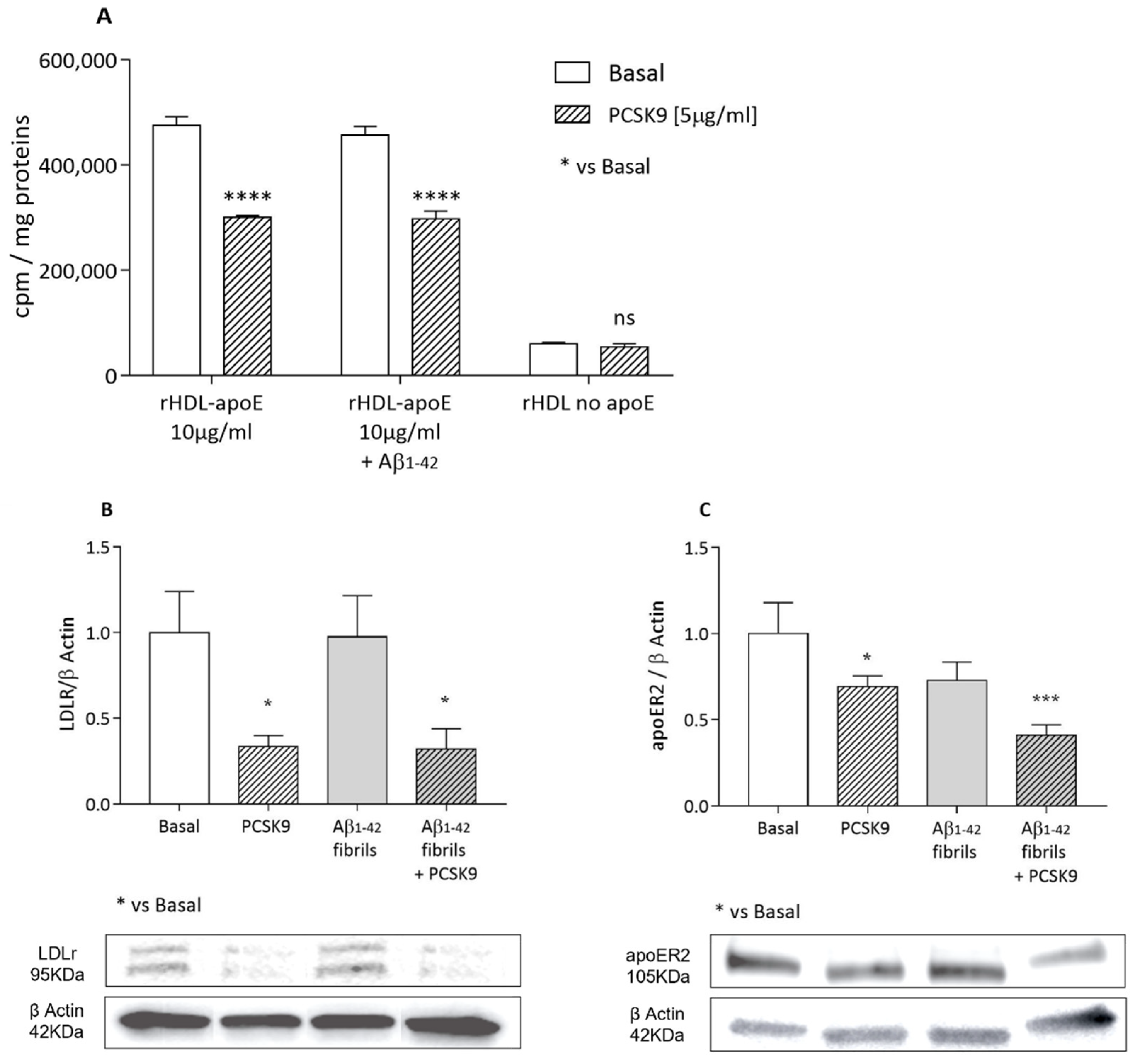
2.1.3. Cholesterol Uptake from apoE-rHDL and Lipoprotein Receptors Expression
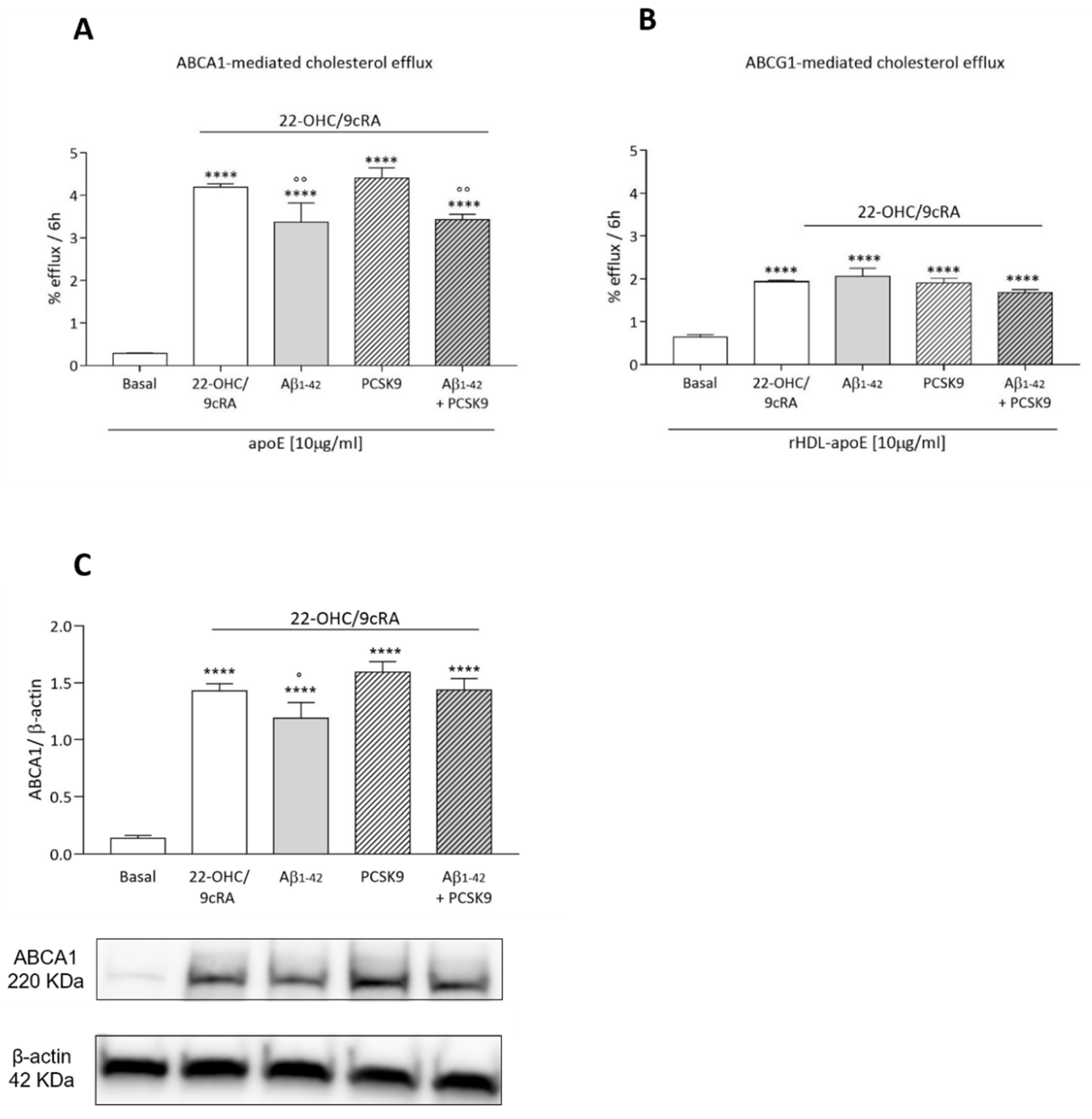
2.1.4. Cholesterol Efflux
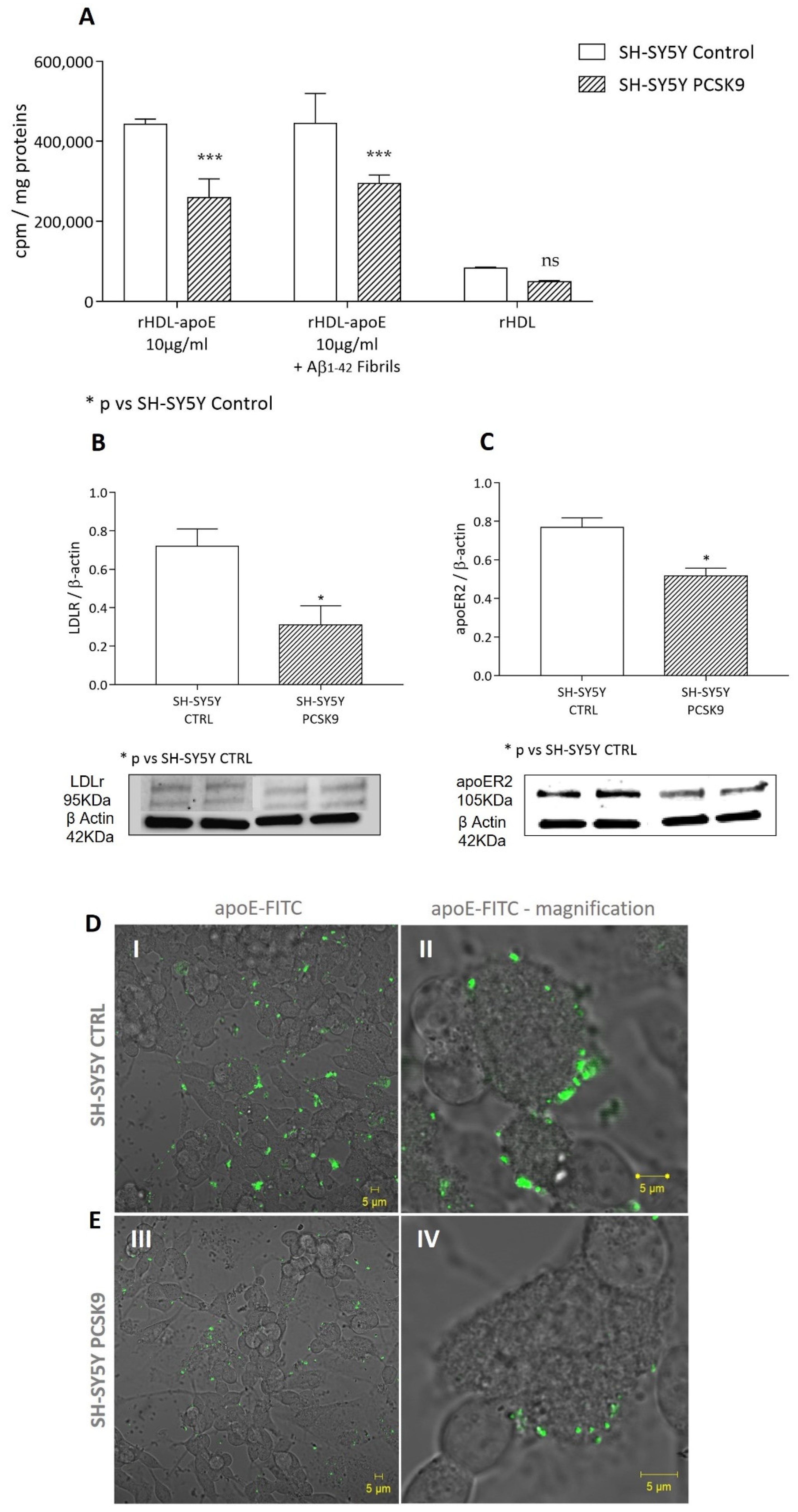
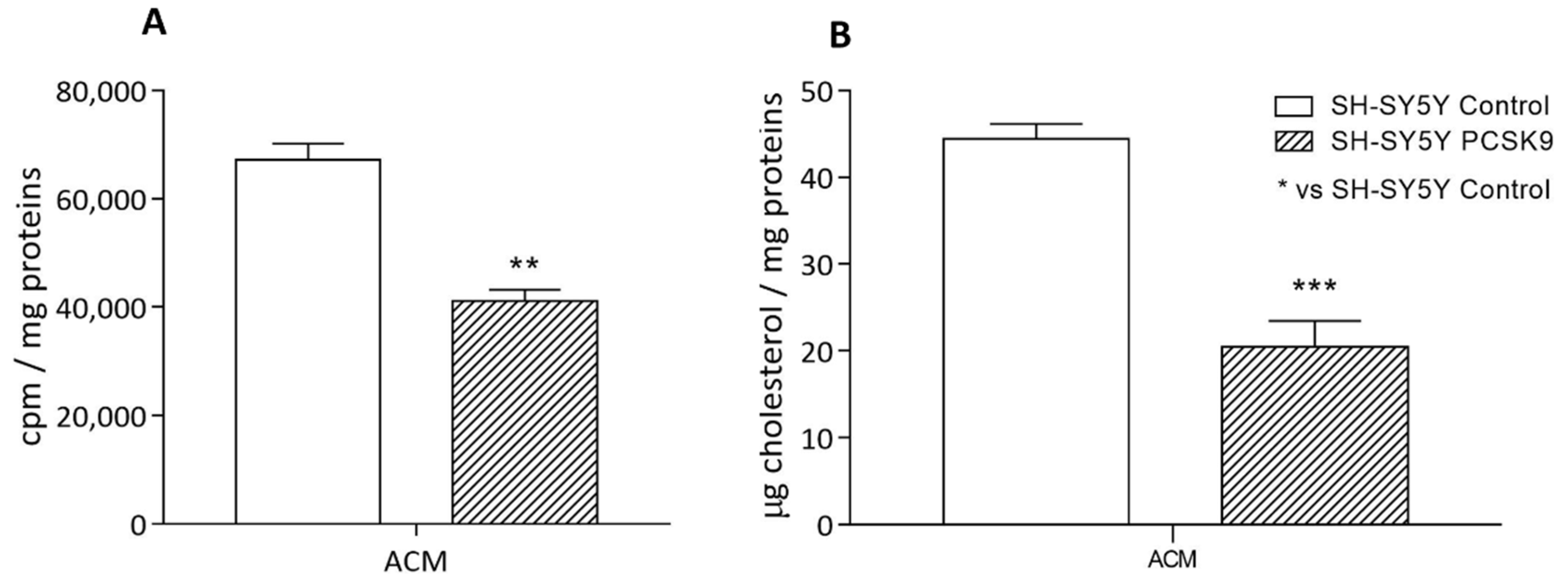
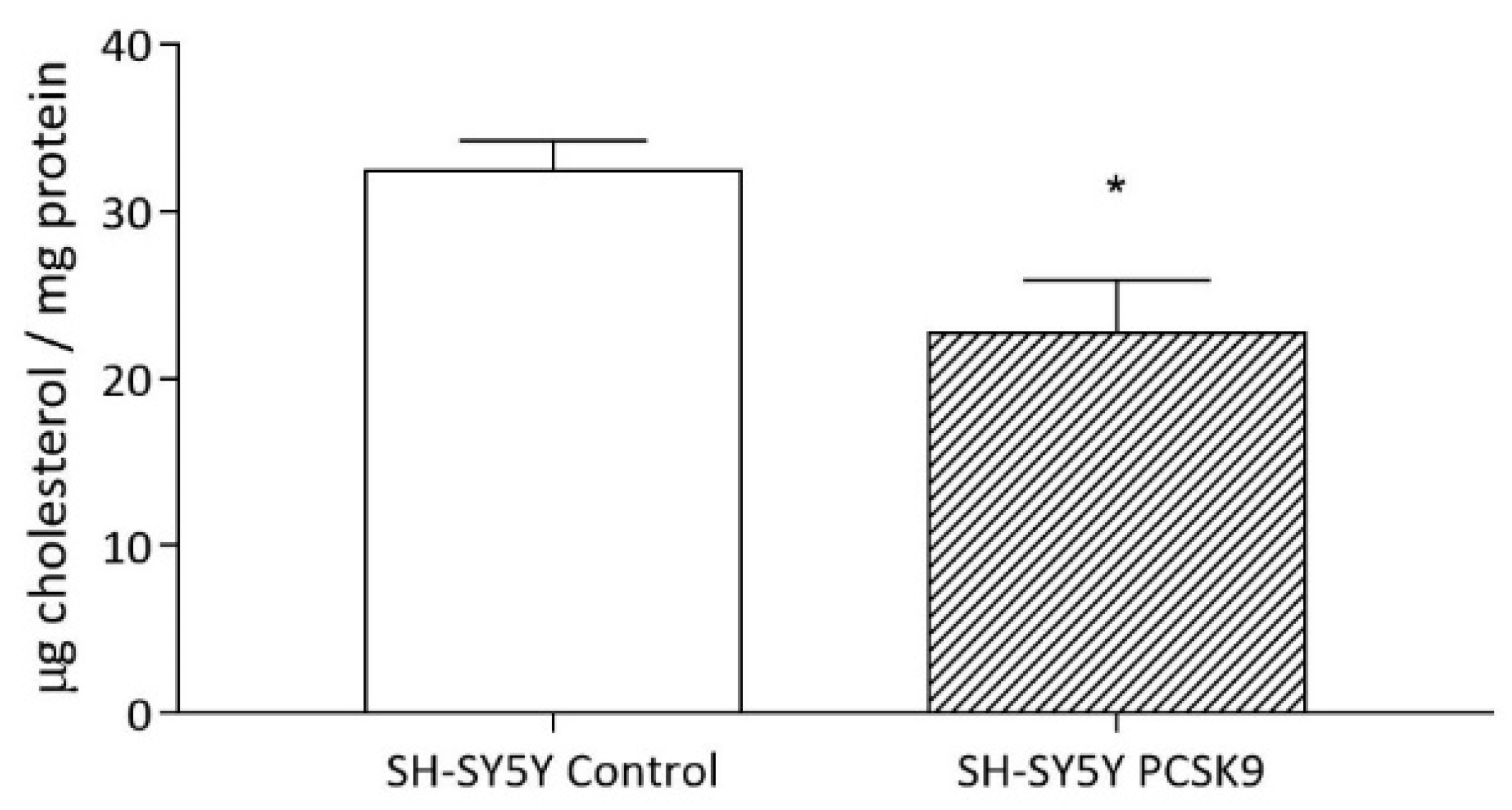
2.2. Influence of PCSK9 on Neuron Cholesterol Supply
2.2.1. Cholesterol Uptake by apoE-rHDL
2.2.2. Cholesterol Uptake by Astrocyte-Conditioned Medium
2.2.3. Intracellular Cholesterol Content
2.3. Influence of PCSK9 on Neurotoxicity
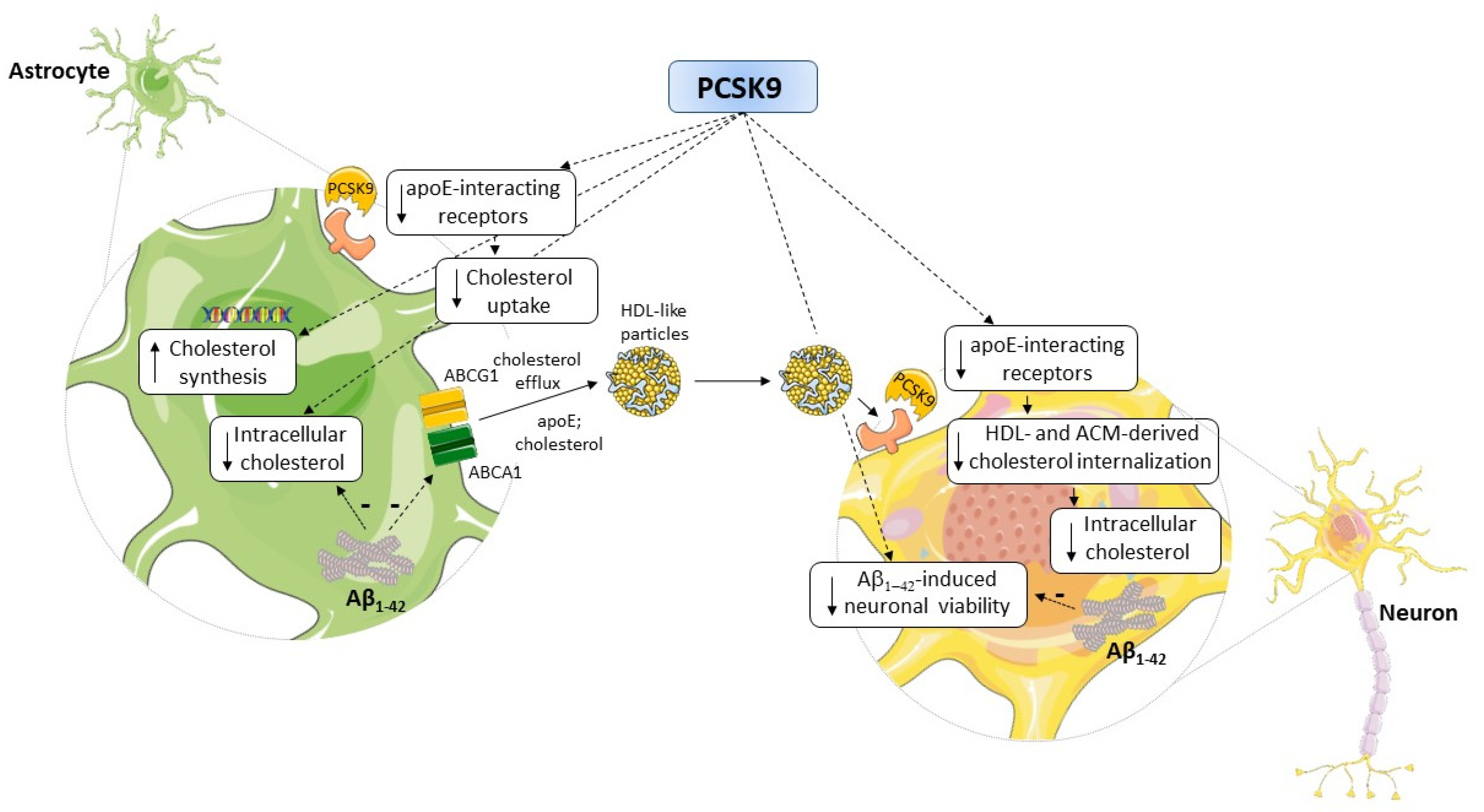
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. ApoE-Containing Reconstituted HDL
4.3. Astrocytes-Conditioned Medium (ACM)
4.4. Aβ1-42 Oligomers and Fibrils Preparation
4.5. Cellular Viability Assay
4.6. Cholesterol Metabolism Parameters
4.6.1. Endogenous Cholesterol Biosynthesis
4.6.2. Intracellular Cholesterol Content
4.6.3. Cholesterol Efflux
4.6.4. Cholesterol Uptake
4.7. Western Blot Analyses
4.8. Confocal Microscopy
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferri, N. Proprotein convertase subtilisin/kexin type 9: From the discovery to the development of new therapies for cardiovascular diseases. Scientifica 2012, 2012, 927352. [Google Scholar] [CrossRef] [Green Version]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Bélanger Jasmin, S.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Prat, A.; Marcinkiewicz, E.; Paquin, J.; Chitramuthu, B.P.; Baranowski, D.; Cadieux, B.; Bennett, H.P.J.; Seidah, N.G. Implication of the proprotein convertase NARC-1/PCSK9 in the development of the nervous system. J. Neurochem. 2006, 98, 838–850. [Google Scholar] [CrossRef]
- Oldham, C.E.; Powell, R.S.; Williams, A.B.; Dixon, S.; Wooten, C.J.; Melendez, Q.M.; Lopez, D. Potential Link between Proprotein Convertase Subtilisin/Kexin Type 9 and Alzheimer’s Disease. Int. J. Biomed. Investig. 2018, 1, 106. [Google Scholar] [CrossRef]
- Courtemanche, H.; Bigot, E.; Pichelin, M.; Guyomarch, B.; Boutoleau-Bretonnière, C.; Le May, C.; Derkinderen, P.; Cariou, B. PCSK9 Concentrations in Cerebrospinal Fluid Are Not Specifically Increased in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1519–1525. [Google Scholar] [CrossRef]
- Zimetti, F.; Caffarra, P.; Ronda, N.; Favari, E.; Adorni, M.P.; Zanotti, I.; Bernini, F.; Barocco, F.; Spallazzi, M.; Galimberti, D.; et al. Increased PCSK9 cerebrospinal fluid concentrations in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Picard, C.; Poirier, A.; Bélanger, S.; Labonté, A.; Auld, D.; Poirier, J. Proprotein convertase subtilisin/kexin type 9 (PCSK9) in Alzheimer’s disease: A genetic and proteomic multi-cohort study. PLoS ONE 2019, 14, e0220254. [Google Scholar] [CrossRef] [Green Version]
- Paquette, M.; Saavedra, Y.G.L.; Poirier, J.; Théroux, L.; Dea, D.; Baass, A.; Dufour, R. Loss-of-Function PCSK9 Mutations Are Not Associated with Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2018, 31, 90–96. [Google Scholar] [CrossRef]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef] [Green Version]
- Rosoff, D.B.; Bell, A.S.; Jung, J.; Wagner, J.; Mavromatis, L.A.; Lohoff, F.W. Mendelian Randomization Study of PCSK9 and HMG-CoA Reductase Inhibition and Cognitive Function. J. Am. Coll. Cardiol. 2022, 80, 653–662. [Google Scholar] [CrossRef]
- Turri, M.; Marchi, C.; Adorni, M.P.; Calabresi, L.; Zimetti, F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159123. [Google Scholar] [CrossRef]
- Zuin, M.; Cervellati, C.; Trentini, A.; Passaro, A.; Rosta, V.; Zimetti, F.; Zuliani, G. Association between Serum Concentrations of Apolipoprotein A-I (ApoA-I) and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 984. [Google Scholar] [CrossRef]
- Marchi, C.; Adorni, M.P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1- And ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. J. Lipid Res. 2019, 60, 1449–1456. [Google Scholar] [CrossRef]
- Feringa, F.M.; van der Kant, R. Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front. Aging Neurosci. 2021, 13, 690372. [Google Scholar] [CrossRef]
- Marais, A.D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 2019, 51, 165–176. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ruscica, M.; Ferri, N.; Bernini, F.; Zimetti, F. Proprotein Convertase Subtilisin/Kexin Type 9, Brain Cholesterol Homeostasis and Potential Implication for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Kysenius, K.; Muggalla, P.; Mätlik, K.; Arumäe, U.; Huttunen, H.J. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol. Life Sci. 2012, 69, 1903–1916. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, E.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363–2372. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Ferris, H.A.; Perry, R.J.; Moreira, G.V.; Shulman, G.I.; Horton, J.D.; Kahn, C.R. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, 1189–1194. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Saher, G.; Xiao, L.; Möbius, W.; Nave, K.-A. Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci. 2007, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Pfrieger, F.W. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? Bioessays 2003, 25, 72–78. [Google Scholar] [CrossRef]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Borràs, C.; Mercer, A.; Sirisi, S.; Alcolea, D.; Escolà-Gil, J.C.; Blanco-Vaca, F.; Tondo, M. HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2022, 23, 9356. [Google Scholar] [CrossRef]
- Zhao, X.-S.; Wu, Q.; Peng, J.; Pan, L.-H.; Ren, Z.; Liu, H.-T.; Jiang, Z.-S.; Wang, G.-X.; Tang, Z.-H.; Liu, L.-S. Hyperlipidemia-induced apoptosis of hippocampal neurons in apoE(-/-) mice may be associated with increased PCSK9 expression. Mol. Med. Rep. 2017, 15, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-S.; Bai, X.-Q.; Gao, Y.; Wu, Q.; Ren, Z.; Li, Q.; Pan, L.-H.; He, N.-Y.; Peng, J.; Tang, Z.-H. PCSK9 Promotes oxLDL-Induced PC12 Cell Apoptosis Through the Bcl-2/Bax-Caspase 9/3 Signaling Pathway. J. Alzheimer’s Dis. 2017, 57, 723–734. [Google Scholar] [CrossRef]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef]
- Kollmer, M.; Close, W.; Funk, L.; Rasmussen, J.; Bsoul, A.; Schierhorn, A.; Schmidt, M.; Sigurdson, C.J.; Jucker, M.; Fändrich, M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 2019, 10, 4760. [Google Scholar] [CrossRef] [Green Version]
- Azizidoost, S.; Babaahmadi-Rezaei, H.; Nazeri, Z.; Cheraghzadeh, M.; Kheirollah, A. Amyloid beta increases ABCA1 and HMGCR protein expression, and cholesterol synthesis and accumulation in mice neurons and astrocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159069. [Google Scholar] [CrossRef]
- Sierri, G.; Dal Magro, R.; Vergani, B.; Leone, B.E.; Formicola, B.; Taiarol, L.; Fagioli, S.; Kravicz, M.; Tremolizzo, L.; Calabresi, L.; et al. Reduced Levels of ABCA1 Transporter Are Responsible for the Cholesterol Efflux Impairment in β-Amyloid-Induced Reactive Astrocytes: Potential Rescue from Biomimetic HDLs. Int. J. Mol. Sci. 2021, 23, 102. [Google Scholar] [CrossRef]
- Fan, Q.W.; Iosbe, I.; Asou, H.; Yanagisawa, K.; Michikawa, M. Expression and regulation of apolipoprotein E receptors in the cells of the central nervous system in culture: A review. J. Am. Aging Assoc. 2001, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Foss, D.; Mahley, R.W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 1987, 917, 148–161. [Google Scholar] [CrossRef]
- Krimbou, L.; Denis, M.; Haidar, B.; Carrier, M.; Marcil, M.; Genest, J.J. Molecular interactions between apoE and ABCA1: Impact on apoE lipidation. J. Lipid Res. 2004, 45, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Schwendeman, A.; Sviridov, D.O.; Yuan, W.; Guo, Y.; Morin, E.E.; Yuan, Y.; Stonik, J.; Freeman, L.; Ossoli, A.; Thacker, S.; et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015, 56, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Papotti, B.; Adorni, M.P.; Marchi, C.; Zimetti, F.; Ronda, N.; Panighel, G.; Lupo, M.G.; Vilella, A.; Giuliani, D.; Ferri, N.; et al. (Department of Food and Drug, University of Parma, 43124 Parma, Italy). 2022; Unpublished work. [Google Scholar]
- Favari, E.; Zanotti, I.; Zimetti, F.; Ronda, N.; Bernini, F.; Rothblat, G.H. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2345–2350. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhao, Z.-W.; Zeng, P.-H.; Zhou, Y.-J.; Yin, W.-J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 2022, 21, 1121–1139. [Google Scholar] [CrossRef]
- Cazzaniga, E.; Bulbarelli, A.; Lonati, E.; Orlando, A.; Re, F.; Gregori, M.; Masserini, M. Abeta peptide toxicity is reduced after treatments decreasing phosphatidylethanolamine content in differentiated neuroblastoma cells. Neurochem. Res. 2011, 36, 863–869. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [Green Version]
- Staurenghi, E.; Giannelli, S.; Testa, G.; Sottero, B.; Leonarduzzi, G.; Gamba, P. Cholesterol Dysmetabolism in Alzheimer’s Disease: A Starring Role for Astrocytes? Antioxidants 2021, 10, 1890. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.G.; Marchianò, S.; Adorni, M.P.; Zimetti, F.; Ruscica, M.; Greco, M.F.; Corsini, A.; Ferri, N. PCSK9 Induces Rat Smooth Muscle Cell Proliferation and Counteracts the Pleiotropic Effects of Simvastatin. Int. J. Mol. Sci. 2021, 22, 4114. [Google Scholar] [CrossRef]
- Sobati, S.; Shakouri, A.; Edalati, M.; Mohammadnejad, D.; Parvan, R.; Masoumi, J.; Abdolalizadeh, J. PCSK9: A Key Target for the Treatment of Cardiovascular Disease (CVD). Adv. Pharm. Bull. 2020, 10, 502–511. [Google Scholar] [CrossRef] [PubMed]
- van Deijk, A.-L.F.; Camargo, N.; Timmerman, J.; Heistek, T.; Brouwers, J.F.; Mogavero, F.; Mansvelder, H.D.; Smit, A.B.; Verheijen, M.H.G. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia 2017, 65, 670–682. [Google Scholar] [CrossRef]
- Canepa, E.; Borghi, R.; Viña, J.; Traverso, N.; Gambini, J.; Domenicotti, C.; Marinari, U.M.; Poli, G.; Pronzato, M.A.; Ricciarelli, R. Cholesterol and amyloid-β: Evidence for a cross-talk between astrocytes and neuronal cells. J. Alzheimer’s Dis. 2011, 25, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, D.; Cheng, R.; Zhu, X.; Wan, T.; Liu, J.; Zhang, R. Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-β proteins. PLoS ONE 2014, 9, e99939. [Google Scholar] [CrossRef] [Green Version]
- Rousselet, E.; Marcinkiewicz, J.; Kriz, J.; Zhou, A.; Hatten, M.E.; Prat, A.; Seidah, N.G. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 2011, 52, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazura, A.D.; Ohler, A.; Storck, S.E.; Kurtyka, M.; Scharfenberg, F.; Weggen, S.; Becker-Pauly, C.; Pietrzik, C.U. PCSK9 acts as a key regulator of Aβ clearance across the blood-brain barrier. Cell Mol. Life Sci. 2022, 79, 212. [Google Scholar] [CrossRef]
- Liu, M.; Wu, G.; Baysarowich, J.; Kavana, M.; Addona, G.H.; Bierilo, K.K.; Mudgett, J.S.; Pavlovic, G.; Sitlani, A.; Renger, J.J.; et al. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J. Lipid Res. 2010, 51, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, K.N.; Manelli, A.M.; Stine, W.B.J.; Baker, L.K.; Krafft, G.A.; LaDu, M.J. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002, 277, 32046–32053. [Google Scholar] [CrossRef] [Green Version]
- Bingham, B.; Shen, R.; Kotnis, S.; Lo, C.F.; Ozenberger, B.A.; Ghosh, N.; Kennedy, J.D.; Jacobsen, J.S.; Grenier, J.M.; DiStefano, P.S.; et al. Proapoptotic effects of NARC 1 (= PCSK9), the gene encoding a novel serine proteinase. Cytom. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 1123–1131. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Shi, J.; Jiang, Q.; Wang, H.; Li, X.; Hao, D. Inhibition of proprotein convertase subtilisin/kexin type 9 attenuates neuronal apoptosis following focal cerebral ischemia via apolipoprotein E receptor 2 downregulation in hyperlipidemic mice. Int. J. Mol. Med. 2018, 42, 2098–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Trotter, J.; Zhang, J.; Peters, M.M.; Cheng, H.; Bao, J.; Han, X.; Weeber, E.J.; Bu, G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 2010, 30, 17068–17078. [Google Scholar] [CrossRef] [Green Version]
- El Hajj, A.; Herzine, A.; Calcagno, G.; Désor, F.; Djelti, F.; Bombail, V.; Denis, I.; Oster, T.; Malaplate, C.; Vigier, M.; et al. Targeted Suppression of Lipoprotein Receptor LSR in Astrocytes Leads to Olfactory and Memory Deficits in Mice. Int. J. Mol. Sci. 2022, 23, 2049. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, L.-L.; Yang, H.; Li, L.; Liu, J.; Chen, L.; Hong, W.-J.; Wang, C.-G.; Ma, J.-J.; Huang, J.; et al. Effect of High Cholesterol Regulation of LRP1 and RAGE on Aβ Transport Across the Blood-Brain Barrier in Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 18, 428–442. [Google Scholar] [CrossRef]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Melo, H.M.; Lopes, S.C.; Ribeiro, C.T.; Delanogare, E.; Moreira, J.C.F.; Gelain, D.P.; Prediger, R.D.; et al. LDL Receptor Deficiency Does not Alter Brain Amyloid-β Levels but Causes an Exacerbation of Apoptosis. J. Alzheimer’s Dis. 2020, 73, 585–596. [Google Scholar] [CrossRef]
- Mulder, M.; Koopmans, G.; Wassink, G.; Al Mansouri, G.; Simard, M.-L.; Havekes, L.M.; Prickaerts, J.; Blokland, A. LDL receptor deficiency results in decreased cell proliferation and presynaptic bouton density in the murine hippocampus. Neurosci. Res. 2007, 59, 251–256. [Google Scholar] [CrossRef]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Donizetti, A.; Iannotta, L.; Aliperti, V.; Cupidi, C.; Bruni, A.C.; Cigliano, L. Brain-derived neurotrophic factor modulates cholesterol homeostasis and Apolipoprotein E synthesis in human cell models of astrocytes and neurons. J. Cell Physiol. 2018, 233, 6925–6943. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Maresca, B.; Mollica, M.P.; Cavaliere, G.; Cefaliello, C.; Trinchese, G.; Esposito, M.G.; Scudiero, R.; Crispino, M.; Abrescia, P.; et al. Haptoglobin increases with age in rat hippocampus and modulates Apolipoprotein E mediated cholesterol trafficking in neuroblastoma cell lines. Front. Cell Neurosci. 2014, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Favari, E.; Zimetti, F.; Bortnick, A.E.; Adorni, M.P.; Zanotti, I.; Canavesi, M.; Bernini, F. Impaired ATP-binding cassette transporter A1-mediated sterol efflux from oxidized LDL-loaded macrophages. FEBS Lett. 2005, 579, 6537–6542. [Google Scholar] [CrossRef] [Green Version]
- Greco, D.; Kocyigit, D.; Adorni, M.P.; Marchi, C.; Ronda, N.; Bernini, F.; Gurses, K.M.; Canpinar, H.; Guc, D.; Oguz, S.H.; et al. Vitamin D replacement ameliorates serum lipoprotein functions, adipokine profile and subclinical atherosclerosis in pre-menopausal women. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 822–829. [Google Scholar] [CrossRef]
- Favari, E.; Calabresi, L.; Adorni, M.P.; Jessup, W.; Simonelli, S.; Franceschini, G.; Bernini, F. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry 2009, 48, 11067–11074. [Google Scholar] [CrossRef]
- Kawanobe, T.; Shiranaga, N.; Kioka, N.; Kimura, Y.; Ueda, K. Apolipoprotein A-I directly interacts with extracellular domain 1 of human ABCA1. Biosci. Biotechnol. Biochem. 2019, 83, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Petraroia, M.; Lerza, L.; Pensabene, D.; Martella, N.; Pallottini, V.; Segatto, M. NGF Modulates Cholesterol Metabolism and Stimulates ApoE Secretion in Glial Cells Conferring Neuroprotection against Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 4842. [Google Scholar] [CrossRef]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-beta1-42 peptide. Biomaterials 2010, 31, 6519–6529. [Google Scholar] [CrossRef] [PubMed]
- Balducci, C.; Beeg, M.; Stravalaci, M.; Bastone, A.; Sclip, A.; Biasini, E.; Tapella, L.; Colombo, L.; Manzoni, C.; Borsello, T.; et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc. Natl. Acad. Sci. USA 2010, 107, 2295–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigna, G.B.; Satta, E.; Bernini, F.; Boarini, S.; Bosi, C.; Giusto, L.; Pinotti, E.; Tarugi, P.; Vanini, A.; Volpato, S.; et al. Flow-mediated dilation, carotid wall thickness and HDL function in subjects with hyperalphalipoproteinemia. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Rothblat, G.H.; Arbogast, L.Y.; Ray, E.K. Stimulation of esterified cholesterol accumulation in tissue culture cells exposed to high density lipoproteins enriched in free cholesterol. J. Lipid Res. 1978, 19, 350–358. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Apaijai, N.; Moisescu, D.M.; Palee, S.; McSweeney, C.M.; Saiyasit, N.; Maneechote, C.; Boonnag, C.; Chattipakorn, N.; Chattipakorn, S.C. Pretreatment With PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation. J. Am. Heart Assoc. 2019, 8, e010838. [Google Scholar] [CrossRef] [Green Version]
- Arunsak, B.; Pratchayasakul, W.; Amput, P.; Chattipakorn, K.; Tosukhowong, T.; Kerdphoo, S.; Jaiwongkum, T.; Thonusin, C.; Palee, S.; Chattipakorn, N.; et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor exerts greater efficacy than atorvastatin on improvement of brain function and cognition in obese rats. Arch. Biochem. Biophys. 2020, 689, 108470. [Google Scholar] [CrossRef]
- Abuelezz, S.A.; Hendawy, N. HMGB1/RAGE/TLR4 axis and glutamate as novel targets for PCSK9 inhibitor in high fat cholesterol diet induced cognitive impairment and amyloidosis. Life Sci. 2021, 273, 119310. [Google Scholar] [CrossRef] [PubMed]
- Schlunk, F.; Fischer, P.; Princen, H.M.G.; Rex, A.; Prinz, V.; Foddis, M.; Lütjohann, D.; Laufs, U.; Endres, M. No effects of PCSK9-inhibitor treatment on spatial learning, locomotor activity, and novel object recognition in mice. Behav. Brain Res. 2021, 396, 112875. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papotti, B.; Adorni, M.P.; Marchi, C.; Zimetti, F.; Ronda, N.; Panighel, G.; Lupo, M.G.; Vilella, A.; Giuliani, D.; Ferri, N.; et al. PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12192. https://doi.org/10.3390/ijms232012192
Papotti B, Adorni MP, Marchi C, Zimetti F, Ronda N, Panighel G, Lupo MG, Vilella A, Giuliani D, Ferri N, et al. PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(20):12192. https://doi.org/10.3390/ijms232012192
Chicago/Turabian StylePapotti, Bianca, Maria Pia Adorni, Cinzia Marchi, Francesca Zimetti, Nicoletta Ronda, Giovanni Panighel, Maria Giovanna Lupo, Antonietta Vilella, Daniela Giuliani, Nicola Ferri, and et al. 2022. "PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 20: 12192. https://doi.org/10.3390/ijms232012192
APA StylePapotti, B., Adorni, M. P., Marchi, C., Zimetti, F., Ronda, N., Panighel, G., Lupo, M. G., Vilella, A., Giuliani, D., Ferri, N., & Bernini, F. (2022). PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. International Journal of Molecular Sciences, 23(20), 12192. https://doi.org/10.3390/ijms232012192







