Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Generation and Metabolic Pathways of H2S
2.1. Metabolism and Production of H2S In Vivo
2.1.1. Metabolism of H2S In Vivo
2.1.2. H2S Production In Vivo
2.2. Development of H2S-Based Therapeutics
2.2.1. Natural Sulfur-Containing Organic Compounds
2.2.2. Inorganic Sulfide Salts
2.2.3. Lawesson’s Reagent
2.2.4. Dithiolthiones
| H2S Donors | Chemical Compound | Bioactivity | Drawbacks | Ref. |
|---|---|---|---|---|
| Inorganic salts | NaHS/CaS/NaS2 | Anti-inflammation, cardioprotective effects, diabetes amelioration | Action time short, uncontrollable | [68] |
| Lawesson’s reagent | GYY4137 | Anti-inflammation, vasodilation | Slow hydrolysis rate, metabolized to CO | [62,69] |
| DTTs | ADT-OH | Reducing cell viability | Poor selectivity | [70] |
| DTT-NSAID | Anti-inflammation | Increasing arterial pressure | [71] | |
| Derivatives of Allium sativum extracts | DATS/DADS | Regulating blood vessels | Poor water solubility, generating byproducts | [72] |
| SPRC | Anti-inflammation, anti-oxidation | Unstable and short half-life. | [55,73] | |
| Derivatives of thioamino acids | Thioglycine/Thiovaline | Vasodilation | Poor selectivity, slow release rate | [74] |
| Derivatives of anti-inflammatory drugs | S-aspirin | Anti-inflammation, cardiovascular protection | Complications in the upper gastrointestinal tract | [71] |
| Derivatives of anti-inflammatory drugs | S-diclofenac | Anti-inflammation, gastrointestinal protection | High cardiovascular risk | [75] |
| Derivatives of anti-inflammatory drugs | ATB-429 | Anti-inflammation | Increasing arterial pressure | [76] |
| Derivatives of anti-inflammatory drugs | ATB-346 | Anti-inflammation, antipyretic, analgesic | Increasing arterial pressure | [77] |
| Thiol-triggered donors | N-Benzoylthiobenzamides | Cardioprotection | Poor selectivity | [78] |
| Thiol-triggered donors | Acyl perthiols | Cardioprotection | Poor selectivity | [79] |
| Thiol-triggered donors | Dithioperoxyanhydrides | Vasodilation | Poor selectivity | [80] |
| Thiol-triggered donors | Arylthioamides | Vasodilation | Poor selectivity | [81] |
| Thiol-triggered donors | S-Aroylthiooximes | Anti-cancer proliferation | Poor selectivity | [82] |
| Photosensitive H2S Donor | Geminal-dithiols | Restores anti-microbial resistance | Poor selectivity | [83] |
| Photosensitive H2S Donor | Ketoprofenate photocages | Unknown | [84] | |
| Photosensitive H2S Donor | α-Thioetherketones | Anti-inflammation | Poor selectivity | [85] |
| Enzyme-triggered H2S donor | BW-HP-101 | Esterase triggered, anti-inflammation | Unknown | [86] |
| pH-triggered H2S donor | JK-1/JK-2 | MI/R protection | Unknown | [87] |
| Dual COS/H2S donor | N-Thiocarboxyanhydrides | Angiogenesis | Unknown | [88] |
| Dual COS/H2S donor | Arylboronate thiocarbamates | Cardioprotection | Unknown | [89] |
| Dual COS/H2S donor | o-Nitrobenzyl thiocarbamates | Unknown | Unknown | [90] |
3. Association between H2S Level and NAFLD In Vivo
4. Physiological Mechanism of H2S in Alleviating NAFLD
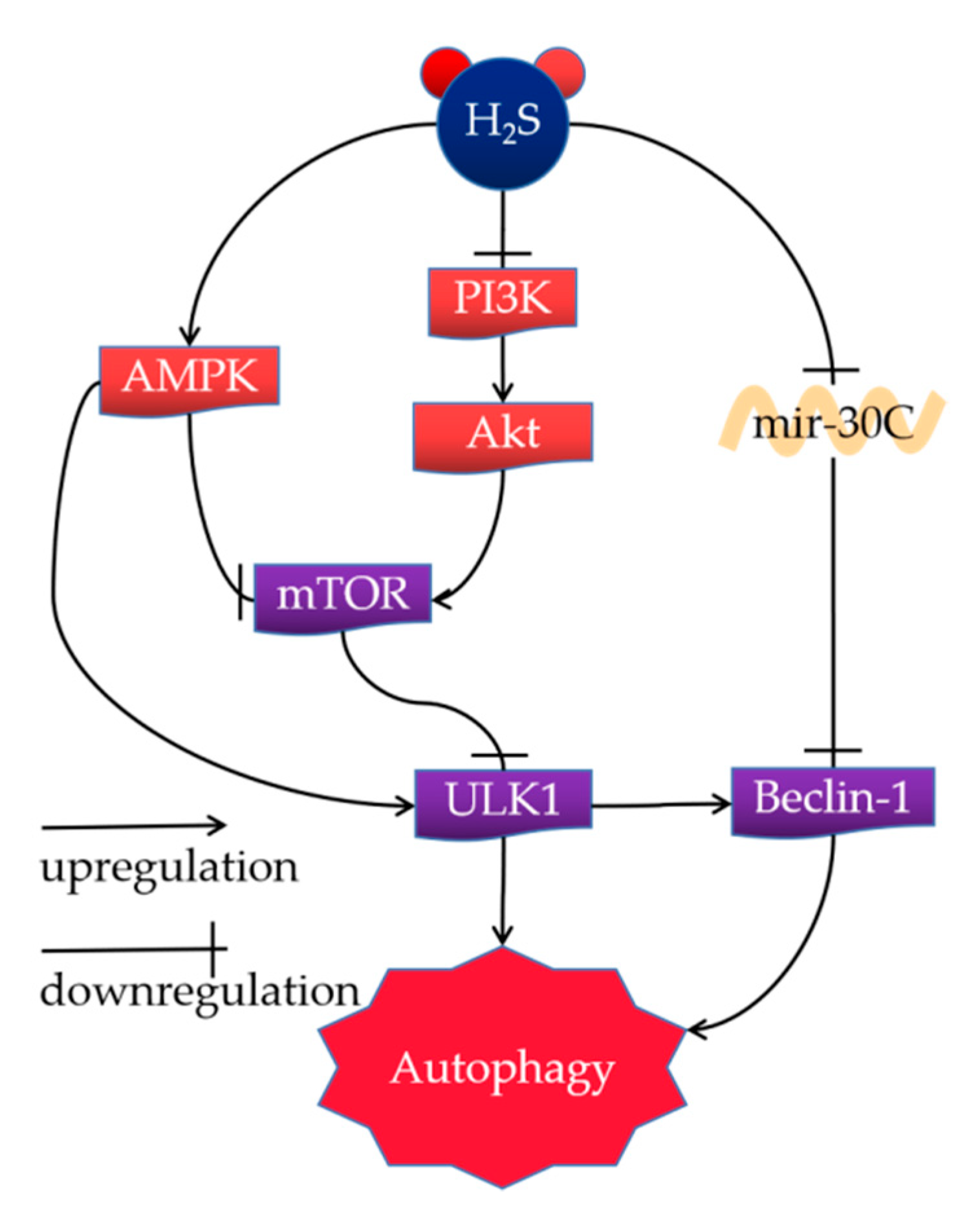
4.1. H2S Alleviates NAFLD by Activating Autophagy
4.1.1. AMPK-mTOR Pathway
4.1.2. PI3K/Akt/mTOR Signaling Pathway
4.1.3. Mir-30c Signaling Pathway
4.2. H2S Alleviates NAFLD by Regulating Inflammation
4.3. H2S Alleviates NAFLD by Improving Oxidative Stress
4.4. H2S Regulates Lipid and Glucose Metabolism
4.5. H2S Alleviates NAFLD by Improving ER Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malone Rubright, S.L.; Pearce, L.L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daldal, H.; Beder, B.; Serin, S.; Sungurtekin, H. Hydrogen sulfide toxicity in a thermal spring: A fatal outcome. Clin. Toxicol. 2010, 48, 755–756. [Google Scholar] [CrossRef]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Raiola, I.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; et al. Hydrogen sulfide, the third gaseous signaling molecule with cardiovascular properties, is decreased in hemodialysis patients. J. Ren. Nutr. 2010, 20, S11–S14. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Vlasov, B.Y.; Kolesnikova, L.I. Hydrogen Sulfide as a Third Essential Gas Molecule in Living Tissues. Vestn. Ross. Akad. Meditsinskikh Nauk. 2015, 70, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ding, Y.L.; Zhang, J.L.; Zhang, P.; Wang, J.Q.; Li, Z.H. Alpinetin improved high fat diet-induced non-alcoholic fatty liver disease (NAFLD) through improving oxidative stress, inflammatory response and lipid metabolism. Biomed Pharm. 2018, 97, 1397–1408. [Google Scholar] [CrossRef]
- Hirsova, P.; Bohm, F.; Dohnalkova, E.; Nozickova, B.; Heikenwalder, M.; Gores, G.J.; Weber, A. Hepatocyte apoptosis is tumor promoting in murine nonalcoholic steatohepatitis. Cell Death Dis. 2020, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Guo, Y.Y.; Li, B.Y.; Peng, W.Q.; Chang, X.X.; Gao, X.; Tang, Q.Q. Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J. Biol. Chem. 2019, 294, 11805–11816. [Google Scholar] [CrossRef]
- Kashyap, M.L.; Ganji, S.; Nakra, N.K.; Kamanna, V.S. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): Novel use for an old drug? J. Clin. Lipidol. 2019, 13, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Seko, Y.; Yamaguchi, K.; Itoh, Y. The genetic backgrounds in nonalcoholic fatty liver disease. Clin. J. Gastroenterol. 2018, 11, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Han, C.Y.; Rho, H.S.; Kim, A.; Kim, T.H.; Jang, K.; Jun, D.W.; Kim, J.W.; Kim, B.; Kim, S.G. FXR Inhibits Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome in Hepatocytes and Ameliorates Liver Injury. Cell Rep. 2018, 24, 2985–2999. [Google Scholar] [CrossRef]
- Margini, C.; Dufour, J.F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.K.K.; Zhang, L.; Chan, M.T.V. Autophagy, NAFLD and NAFLD-Related HCC. Adv. Exp. Med. Biol. 2018, 1061, 127–138. [Google Scholar] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J. Clin. Transl. Hepatol. 2020, 8, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.L.; Fan, J.G. Prevalence and harm of nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 10. [Google Scholar]
- Xu, S.; Liu, Z.; Liu, P. Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis. Int. J. Cardiol. 2014, 172, 313–317. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Teng, T.; Duan, S.; Ji, A.; Li, Y. Hydrogen sulfide and autophagy: A double edged sword. Pharmacol. Res. 2018, 131, 120–127. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.; Fago, A. Reactions of ferric hemoglobin and myoglobin with hydrogen sulfide under physiological conditions. J. Inorg. Biochem. 2018, 182, 133–140. [Google Scholar] [CrossRef]
- Zuhra, K.; Tomé, C.S.; Masi, L.; Giardina, G.; Paulini, G.; Malagrinò, F.; Forte, E.; Vicente, J.B.; Giuffrè, A. N-Acetylcysteine Serves as Substrate of 3-Mercaptopyruvate Sulfurtransferase and Stimulates Sulfide Metabolism in Colon Cancer Cells. Cells 2019, 8, 828. [Google Scholar] [CrossRef]
- Kashfi, K. The dichotomous role of H2S in cancer cell biology? Deja vu all over again. Biochem. Pharm. 2018, 149, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Shen, X.; Carlström, M.; Borniquel, S.; Jädert, C.; Kevil, C.G.; Lundberg, J.O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free. Radic. Biol. Med. 2013, 60, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Awano, N.; Wada, M.; Mori, H.; Nakamori, S.; Takagi, H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005, 71, 4149–4152. [Google Scholar] [CrossRef] [Green Version]
- Khalil, N.A.; Walton, G.E.; Gibson, G.R.; Tuohy, K.M.; Andrews, S.C. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int. J. Food Sci. Nutr. 2014, 65, 79–88. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, M.; Andriamihaja, M.; Lan, A.; Khodorova, N.; Audebert, M.; Blouin, J.M.; Grauso, M.; Lancha, L.; Benetti, P.H.; Benamouzig, R.; et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free. Radic. Biol. Med. 2016, 93, 155–164. [Google Scholar] [CrossRef]
- Koj, A.; Frendo, J.; Janik, Z. [35S]thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem. J. 1967, 103, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B(6). Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [Green Version]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N. Production of H(2)S, H(2)S(n), and persulfide species (CysSSH and GSSH) by 3-mercaptopyruvate sulfurtransferase. Nihon Yakurigaku Zasshi Folia Pharmacol. Jpn. 2018, 152, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Renga, B. Hydrogen sulfide generation in mammals: The molecular biology of cystathionine-β- synthase (CBS) and cystathionine-γ-lyase (CSE). Inflamm. Allergy Drug Targets 2011, 10, 85–91. [Google Scholar] [CrossRef]
- Yamamoto, J.; Sato, W.; Kosugi, T.; Yamamoto, T.; Kimura, T.; Taniguchi, S.; Kojima, H.; Maruyama, S.; Imai, E.; Matsuo, S.; et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin. Exp. Nephrol. 2013, 17, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Signaling by hydrogen sulfide (H(2)S) and polysulfides (H(2)S(n)) in the central nervous system. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Emmez, H.; Borcek, A.O.; Gonul, I.I.; Belen, H.B.; Solaroglu, I.; Baykaner, M.K. The Effect of Hydrogen Sulphide on Experimental Cerebral Vasospasm. Turk. Neurosurg. 2017, 27, 374–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yan, Q.; Liu, X.; Li, P.; Li, X.; Chen, Y.; Simoncini, T.; Liu, J.; Zhu, D.; Fu, X. 17β-Estradiol nongenomically induces vascular endothelial H(2)S release by promoting phosphorylation of cystathionine γ-lyase. J. Biol. Chem. 2019, 294, 15577–15592. [Google Scholar] [CrossRef]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef]
- Majtan, T.; Krijt, J.; Sokolova, J.; Krizkova, M.; Ralat, M.A.; Kent, J.; Gregory, J.F., 3rd; Kozich, V.; Kraus, J.P. Biogenesis of Hydrogen Sulfide and Thioethers by Cystathionine Beta-Synthase. Antioxid Redox Signal 2018, 28, 311–323. [Google Scholar] [CrossRef]
- Braunstein, A.E.; Goryachenkova, E.V.; Tolosa, E.A.; Willhardt, I.H.; Yefremova, L.L. Specificity and some other properties of liver serine sulphhydrase: Evidence for its identity with cystathionine -synthase. Biochim. Biophys. Acta 1971, 242, 247–260. [Google Scholar] [CrossRef]
- Miles, E.W.; Kraus, J.P. Cystathionine beta-synthase: Structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.H.; Cui, L.B.; Wu, K.; Zheng, X.L.; Cayabyab, F.S.; Chen, Z.W.; Tang, C.K. Hydrogen sulfide as a potent cardiovascular protective agent. Clin. Chim. Acta 2014, 437, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Nirasawa, T.; Yoshii, T.; Niimura, Y. Is novel signal transducer sulfur oxide involved in the redox cycle of persulfide at the catalytic site cysteine in a stable reaction intermediate of mercaptopyruvate sulfurtransferase? Antioxid. Redox Signal. 2012, 16, 747–753. [Google Scholar] [CrossRef]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H(2)S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Kiss, D.J.; Ferenczy, G.G. A detailed mechanism of the oxidative half-reaction of d-amino acid oxidase: Another route for flavin oxidation. Org. Biomol. Chem. 2019, 17, 7973–7984. [Google Scholar] [CrossRef]
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Nin, D.S.; Binte Idres, S.; Song, Z.; Moore, P.K.; Deng, L.-W. Biological effects of GYY4137 and other phosphorothioate-based hydrogen sulfide donors. Antioxid. Redox Signal. 2020, 32, 145–158. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, X.; Ji, K.; Wang, B. Hydrogen sulfide prodrugs-a review. Acta Pharm. Sin. B 2015, 5, 367–377. [Google Scholar] [CrossRef]
- Kang, J.; Neill, D.L.; Xian, M. Phosphonothioate-Based Hydrogen Sulfide Releasing Reagents: Chemistry and Biological Applications. Front. Pharmacol. 2017, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Luo, S.; Liu, J.; Xie, S.; Liu, Y.; Xu, J.; Zhu, Z.; Xu, S. Controllable thioester-based hydrogen sulfide slow-releasing donors as cardioprotective agents. Chem. Commun. 2019, 55, 6193–6196. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective Smell of Hydrogen Sulfide and Polysulfide in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Kan, J.; Liu, X.; Ma, F.; Tran, B.H.; Zou, Y.; Wang, S.; Zhu, Y.Z. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. PLoS ONE 2013, 8, e69205. [Google Scholar] [CrossRef] [Green Version]
- Martelli, A.; Citi, V.; Testai, L.; Brogi, S.; Calderone, V. Organic Isothiocyanates as Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 110–144. [Google Scholar] [CrossRef]
- Zhen, Y.; Wu, Q.; Ding, Y.; Zhang, W.; Zhai, Y.; Lin, X.; Weng, Y.; Guo, R.; Zhang, Y.; Feng, J.; et al. Exogenous hydrogen sulfide promotes hepatocellular carcinoma cell growth by activating the STAT3-COX-2 signaling pathway. Oncol. Lett. 2018, 15, 6562–6570. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.B.M.; Souza, L.K.M.; Sousa, N.A.; Araujo, T.S.L.; de Araujo, S.; Pacifico, D.M.; Silva, I.S.; Silva, R.O.; Nicolau, L.A.D.; Souza, F.M.; et al. H2S is a key antisecretory molecule against cholera toxin-induced diarrhoea in mice: Evidence for non-involvement of the AC/cAMP/PKA pathway and AMPK. Nitric Oxide 2018, 76, 152–163. [Google Scholar] [CrossRef]
- Malagrino, F.; Zuhra, K.; Mascolo, L.; Mastronicola, D.; Vicente, J.B.; Forte, E.; Giuffre, A. Hydrogen Sulfide Oxidation: Adaptive Changes in Mitochondria of SW480 Colorectal Cancer Cells upon Exposure to Hypoxia. Oxid. Med. Cell. Longev. 2019, 2019, 8102936. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tang, W.; Zhu, Y.Z. An Update on AMPK in Hydrogen Sulfide Pharmacology. Front. Pharmacol. 2017, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.; Li, X.; Shirhan, M.; Peh, M.T.; Xie, L.; Zhou, S.; et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(-/-) mice. Br. J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Yuan, Y.; Sheng, Y.; Yuan, B.; Wang, Y.; Zheng, J.; Liu, C.F.; Zhang, X.; Hu, L.F. GYY4137, an H2S Slow-Releasing Donor, Prevents Nitrative Stress and alpha-Synuclein Nitration in an MPTP Mouse Model of Parkinson’s Disease. Front. Pharmacol. 2017, 8, 741. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Xu, Q.; Jia, J.; Ao, G.; Sun, Y.; Hu, L.; Alkayed, N.J.; Wang, C.; Cheng, J. Hydrogen sulfide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux. Biochem. Biophys. Res. Commun. 2015, 458, 632–638. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Kashfi, K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid. Redox Signal. 2014, 20, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; De La Cruz, L.K.; Roy Choudhury, M.; Anifowose, A.; Wang, B. Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med. Res. Rev. 2018, 38, 57–100. [Google Scholar] [CrossRef]
- Lee, Z.W.; Zhou, J.; Chen, C.S.; Zhao, Y.; Tan, C.H.; Li, L.; Moore, P.K.; Deng, L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef] [Green Version]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide Biol. Chem. 2014, 41, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, M.; Kodela, R.; Nath, N.; Dastagirzada, Y.M.; Velázquez-Martínez, C.A.; Boring, D.; Kashfi, K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: A general property and evidence of a tissue type-independent effect. Biochem. Pharmacol. 2012, 83, 715–722. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Zhu, Y.Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids 2011, 41, 205–215. [Google Scholar] [CrossRef]
- Zhou, Z.; von Wantoch Rekowski, M.; Coletta, C.; Szabo, C.; Bucci, M.; Cirino, G.; Topouzis, S.; Papapetropoulos, A.; Giannis, A. Thioglycine and L-thiovaline: Biologically active H₂S-donors. Bioorganic Med. Chem. 2012, 20, 2675–2678. [Google Scholar] [CrossRef]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free. Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Elsheikh, W.; Blackler, R.W.; Flannigan, K.L.; Wallace, J.L. Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer. Nitric Oxide Biol. Chem. 2014, 41, 131–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xian, M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Aguilar, H.C.; Lefer, D.J.; et al. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Roger, T.; Raynaud, F.; Bouillaud, F.; Ransy, C.; Simonet, S.; Crespo, C.; Bourguignon, M.P.; Villeneuve, N.; Vilaine, J.P.; Artaud, I.; et al. New biologically active hydrogen sulfide donors. Chembiochem A Eur. J. Chem. Biol. 2013, 14, 2268–2271. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef]
- Foster, J.C.; Powell, C.R.; Radzinski, S.C.; Matson, J.B. S-aroylthiooximes: A facile route to hydrogen sulfide releasing compounds with structure-dependent release kinetics. Org. Lett. 2014, 16, 1558–1561. [Google Scholar] [CrossRef]
- Devarie-Baez, N.O.; Bagdon, P.E.; Peng, B.; Zhao, Y.; Park, C.M.; Xian, M. Light-induced hydrogen sulfide release from “caged” gem-dithiols. Org. Lett. 2013, 15, 2786–2789. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, N.; Ieda, N.; Sasakura, K.; Nagano, T.; Hanaoka, K.; Suzuki, T.; Miyata, N.; Nakagawa, H. Synthesis of a photocontrollable hydrogen sulfide donor using ketoprofenate photocages. Chem. Commun. 2014, 50, 587–589. [Google Scholar] [CrossRef]
- Xiao, Z.; Bonnard, T.; Shakouri-Motlagh, A.; Wylie, R.A.L.; Collins, J.; White, J.; Heath, D.E.; Hagemeyer, C.E.; Connal, L.A. Triggered and Tunable Hydrogen Sulfide Release from Photogenerated Thiobenzaldehydes. Chemistry 2017, 23, 11294–11300. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 4514–4518. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.M.; Yang, C.T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef]
- Steiger, A.K.; Zhao, Y.; Pluth, M.D. Emerging Roles of Carbonyl Sulfide in Chemical Biology: Sulfide Transporter or Gasotransmitter? Antioxid. Redox Signal. 2018, 28, 1516–1532. [Google Scholar] [CrossRef] [Green Version]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Steiger, A.K.; Yang, Y.; Royzen, M.; Pluth, M.D. Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H(2)S). Chem. Commun. 2017, 53, 1378–1380. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Cui, C.; Cui, C.; Chen, Z.; Zhang, H.; Cui, Q.; Xu, G.; Fan, J.; Han, Y.; Tang, L.; et al. Hepatocellular cystathionine gamma lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology 2022. [Google Scholar] [CrossRef]
- Mateus, I.; Prip-Buus, C. Hydrogen sulphide in liver glucose/lipid metabolism and non-alcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13680. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Zhao, Y.; Yang, G.; Wang, C.; Zhao, Z.; Li, S. Chondroitin sulfate stimulates the secretion of H2S by Desulfovibrio to improve insulin sensitivity in NAFLD mice. Int. J. Biol. Macromol. 2022, 213, 631–638. [Google Scholar] [CrossRef]
- Werge, M.P.; McCann, A.; Galsgaard, E.D.; Holst, D.; Bugge, A.; Albrechtsen, N.J.W.; Gluud, L.L. The Role of the Transsulfuration Pathway in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 1081. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Fan, M.; Pan, S.; Li, S.; Chen, M.; Wang, H. Metabolomic-proteomic combination analysis reveals the targets and molecular pathways associated with hydrogen sulfide alleviating NAFLD. Life Sci. 2021, 264, 118629. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Wang, X.Y.; Bian, J.S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Luo, Z.L.; Tang, L.J.; Wang, T.; Dai, R.W.; Ren, J.D.; Cheng, L.; Xiang, K.; Tian, F.Z. Effects of treatment with hydrogen sulfide on methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. J. Gastroenterol. Hepatol. 2014, 29, 215–222. [Google Scholar] [CrossRef]
- Sarna, L.K.; Sid, V.; Wang, P.; Siow, Y.L.; House, J.D.; Karmin, O. Tyrosol Attenuates High Fat Diet-Induced Hepatic Oxidative Stress: Potential Involvement of Cystathionine beta-Synthase and Cystathionine gamma-Lyase. Lipids 2016, 51, 583–590. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Wang, H.; Gao, C.; Chang, P.; Chen, X.; Shan, H.; Zhang, M.; Tao, L. Hydrogen sulfide protects against cell damage through modulation of PI3K/Akt/Nrf2 signaling. Int. J. Biochem. Cell Biol. 2019, 117, 105636. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Yu, C.; Pan, Z.; Liu, Y.; Zhao, J.; Wang, X.; Yun, F.; Zhao, H.; Yan, S.; et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E925–E935. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, L.; Gao, Z.; Sun, X.; Yu, M.; Dong, S.; Wu, J.; Zhao, Y.; Xu, C.; Zhang, W.; et al. Exogenous H2S Protects Against Diabetic Cardiomyopathy by Activating Autophagy via the AMPK/mTOR Pathway. Cell. Physiol. Biochem. 2017, 43, 1168–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.; Li, L.; Qu, F.; Zhang, G.; Wang, Y.; Bai, X.; Pan, S.; Xue, D.; Wang, G.; Sun, B. Hydrogen sulphide exacerbates acute pancreatitis by over-activating autophagy via AMPK/mTOR pathway. J. Cell. Mol. Med. 2016, 20, 2349–2361. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.C.; Wang, X.J.; Yu, L.; Chan, F.K.; Cheng, A.S.; Yu, J.; Sung, J.J.; Wu, W.K.; Cho, C.H. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS ONE 2012, 7, e37572. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Liu, H.; Yang, Y.; Lan, T.; Wang, H.; Wu, D. Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. Int. J. Mol. Sci. 2022, 23, 7170. [Google Scholar] [CrossRef]
- Iqbal, I.K.; Bajeli, S.; Sahu, S.; Bhat, S.A.; Kumar, A. Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2-SIRT1 interaction to initiate autophagy. Autophagy 2021, 17, 3511–3529. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, Y.H.; Chen, N.; Wang, L.J.; Chen, D.X.; Weng, H.L.; Dooley, S.; Ding, H.G. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017, 8, e2688. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, X.; Cai, H.; Sun, H.; Hu, Y.; Huang, X.; Kong, W.; Kong, W. The role of sodium hydrosulfide in attenuating the aging process via PI3K/AKT and CaMKKbeta/AMPK pathways. Redox Biol. 2017, 12, 987–1003. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.; Wang, H. Hydrogen sulfide plays an important protective role by influencing autophagy in diseases. Physiol. Res. 2019, 68, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, H.K.; Li, Y.P.; Guo, Y.P. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J. Biomed. Sci. 2015, 22, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, C.; Wu, D.; Zhang, A.; Gu, T.; Wang, L.; Wang, C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS ONE 2012, 7, e41147. [Google Scholar] [CrossRef]
- Sutti, S.; Locatelli, I.; Bruzzi, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Albano, E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin. Sci. 2015, 129, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Wang, D.; Hu, X.; Li, S.; Li, S. Hydrogen Sulfide Gas Exposure Induces Necroptosis and Promotes Inflammation through the MAPK/NF-kappaB Pathway in Broiler Spleen. Oxidative Med. Cell. Longev. 2019, 2019, 8061823. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Z.; Liang, M.; Deng, X.; Lai, M.; Shang, L.; Su, X. Exogenous hydrogen sulfide protects fatty liver against ischemia-reperfusion injury by regulating endoplasmic reticulum stress-induced autophagy in macrophage through mediating the class A scavenger receptor pathway in rats. Cell Biol. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Wang, D.Y.; Li, H.M.; Guo, J.C.; Duan, S.F.; Ji, X.Y. Hydrogen Sulfide as a Novel Regulatory Factor in Liver Health and Disease. Oxid. Med. Cell. Longev. 2019, 2019, 3831713. [Google Scholar] [CrossRef] [Green Version]
- Koike, S.; Ogasawara, Y.; Shibuya, N.; Kimura, H.; Ishii, K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013, 587, 3548–3555. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine gamma lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor gamma activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim. Et Biophys. Acta 2016, 1861, 419–429. [Google Scholar] [CrossRef]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Et Biophys. Acta 2012, 1821, 809–818. [Google Scholar] [CrossRef]
- Suppli, M.P.; Rigbolt, K.T.G.; Veidal, S.S.; Heebøll, S.; Eriksen, P.L. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. American journal of physiology Gastrointest. Liver Physiol. 2019, 316, G462–G472. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, J.S. Hydrogen sulfide: A neuromodulator and neuroprotectant in the central nervous system. ACS Chem. Neurosci. 2014, 5, 876–883. [Google Scholar] [CrossRef]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.; Gao, X.H.; Willard, B.; Hatzoglou, M.; Banerjee, R.; Kabil, O. Hydrogen sulfide modulates eukaryotic translation initiation factor 2α (eIF2α) phosphorylation status in the integrated stress-response pathway. J. Biol. Chem. 2017, 292, 13143–13153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H(2)S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zheng, N.; Qi, K.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas. Res. 2015, 5, 1. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jiang, K.; Ruan, Y.; Zhao, S.; Zhao, Y.; He, Y.; Wang, Z.; Wei, J.; Li, Q.; Yang, C.; et al. Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 12202. https://doi.org/10.3390/ijms232012202
Li X, Jiang K, Ruan Y, Zhao S, Zhao Y, He Y, Wang Z, Wei J, Li Q, Yang C, et al. Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2022; 23(20):12202. https://doi.org/10.3390/ijms232012202
Chicago/Turabian StyleLi, Xianghui, Kaixin Jiang, Yantian Ruan, Siyuan Zhao, Yiming Zhao, Yuhua He, Zhili Wang, Jiacun Wei, Qiming Li, Changyong Yang, and et al. 2022. "Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease" International Journal of Molecular Sciences 23, no. 20: 12202. https://doi.org/10.3390/ijms232012202
APA StyleLi, X., Jiang, K., Ruan, Y., Zhao, S., Zhao, Y., He, Y., Wang, Z., Wei, J., Li, Q., Yang, C., Li, Y., & Teng, T. (2022). Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 23(20), 12202. https://doi.org/10.3390/ijms232012202









