Biological Activity of Different Forms of Oxidized Parathyroid Hormone
Abstract
1. Introduction
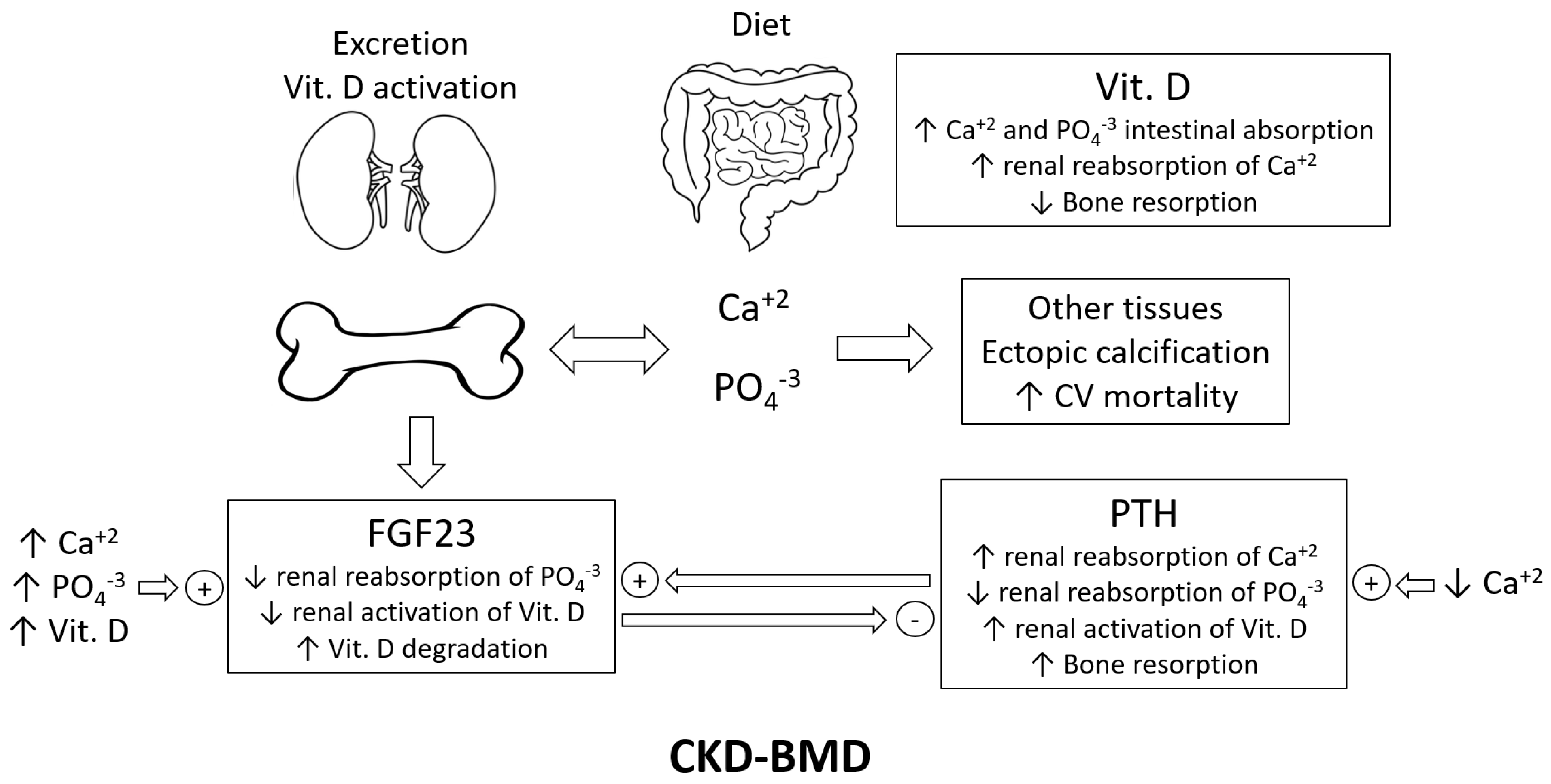
2. Chronic Kidney Disease–Mineral Bone Disorder (CKD-MBD) and Uremic Calcification
3. Oxidative Stress in CKD
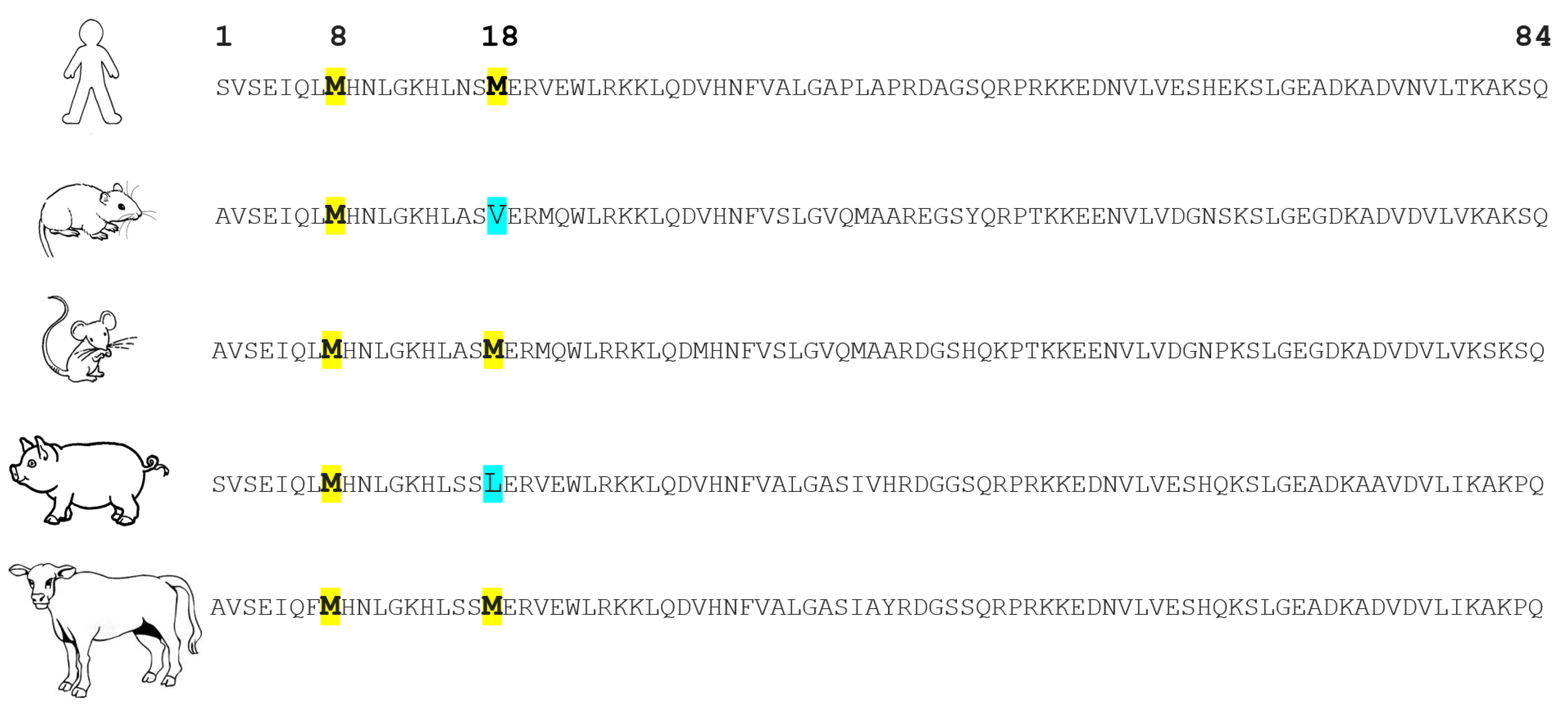
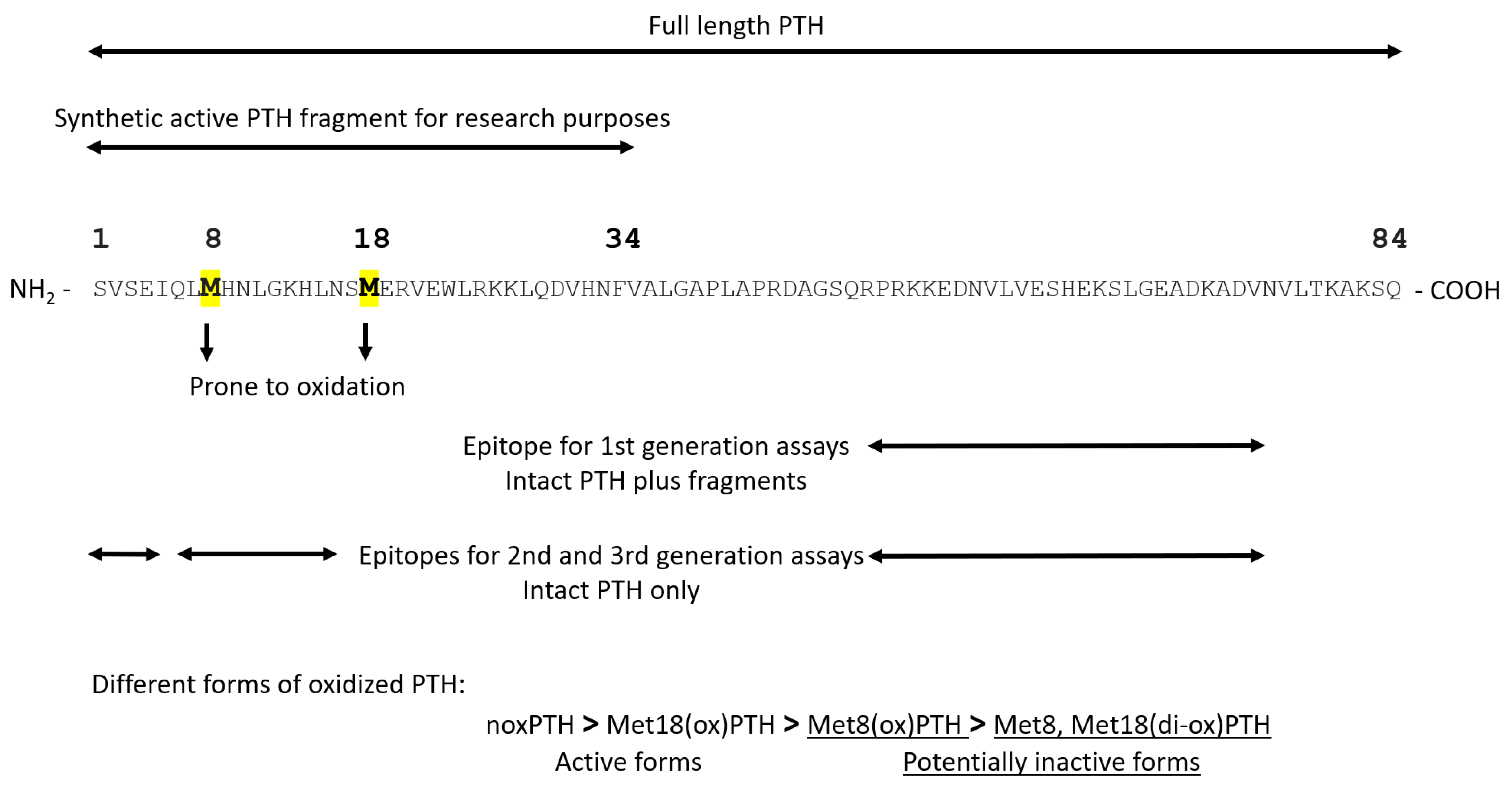
4. Oxidation of PTH and Its Available Assays
5. Preclinical Studies Investigating the Oxidation of PTH
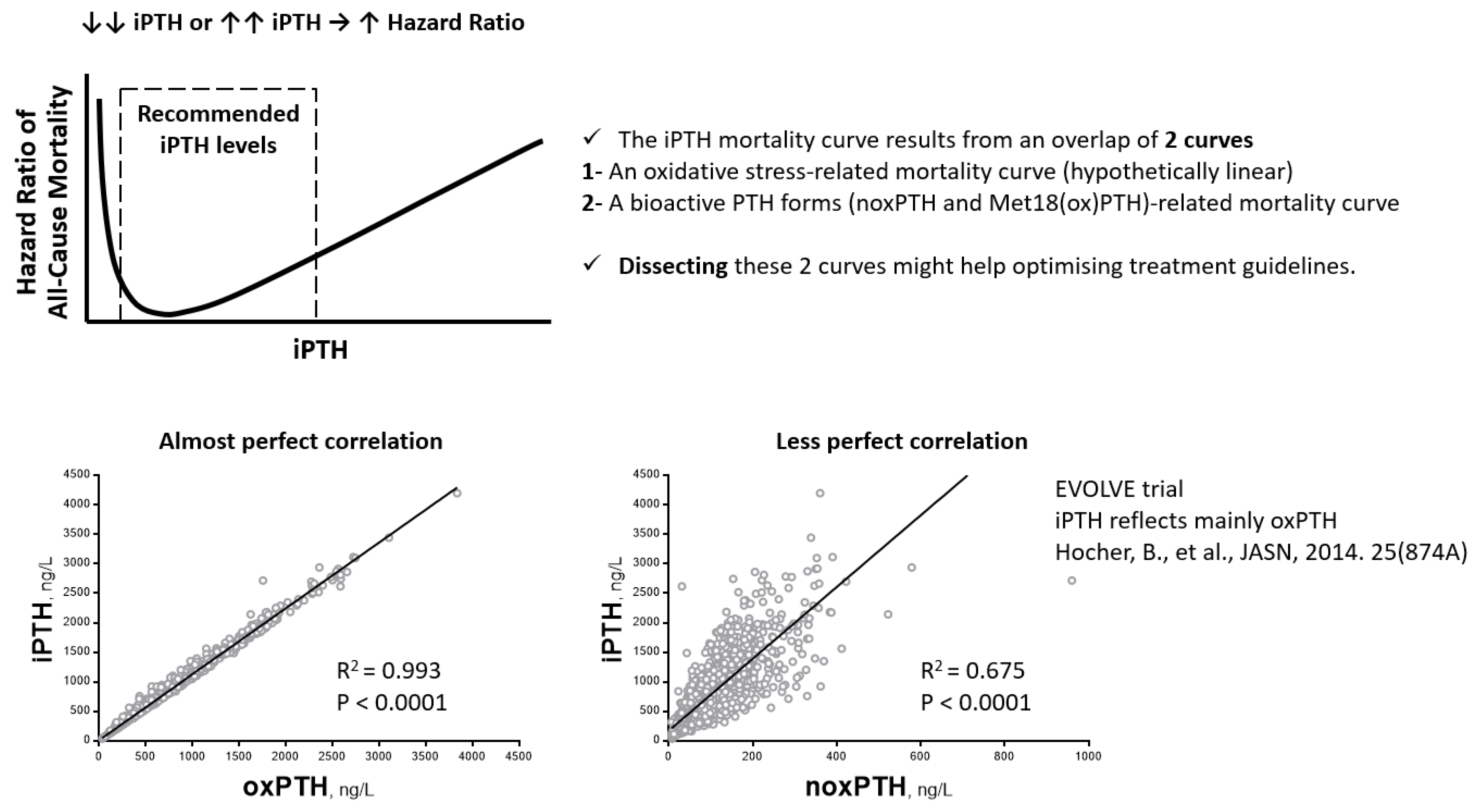
6. Clinical Studies Investigating the Oxidation of PTH
7. Conclusions and Open Questions
Funding
Conflicts of Interest
References
- Goltzman, D. Physiology of Parathyroid Hormone. Endocrinol. Metab. Clin. N. Am. 2018, 47, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Ursem, S.R.; Vervloet, M.G.; de Jongh, R.T.; Heijboer, A.C. Oxidation of parathyroid hormone. Clin. Chim. Acta 2020, 506, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.D.; Ahmad, A.M.; Vora, J.P. The physiology of the circadian rhythm of parathyroid hormone and its potential as a treatment for osteoporosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 437–444. [Google Scholar] [CrossRef]
- Arnold, A.; Dennison, E.; Kovacs, C.S.; Mannstadt, M.; Rizzoli, R.; Brandi, M.L.; Clarke, B.; Thakker, R.V. Hormonal regulation of biomineralization. Nat. Rev. Endocrinol. 2021, 17, 261–275. [Google Scholar] [CrossRef]
- Kakani, E.; Elyamny, M.; Ayach, T.; El-Husseini, A. Pathogenesis and management of vascular calcification in CKD and dialysis patients. Semin. Dial. 2019, 32, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R.; De Leon, J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Mace, M.L.; Egstrand, S.; Morevati, M.; Olgaard, K.; Lewin, E. New Insights to the Crosstalk between Vascular and Bone Tissue in Chronic Kidney Disease–Mineral and Bone Disorder. Metabolites 2021, 11, 849. [Google Scholar] [CrossRef]
- Sprague, S.M.; Martin, K.J.; Coyne, D.W. Phosphate Balance and CKD–Mineral Bone Disease. Kidney Int. Rep. 2021, 6, 2049–2058. [Google Scholar] [CrossRef]
- Leifheit-Nestler, M.; Haffner, D. How FGF23 shapes multiple organs in chronic kidney disease. Mol. Cell. Pediatr. 2021, 8, 12. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Carrio, J.; Bande-Fernández, J.J.; Alonso-Montes, C.; Carrillo-López, N. Chronic Kidney Disease—Mineral and Bone Disorders: Pathogenesis and Management. Calcif. Tissue Res. 2021, 108, 410–422. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, M.E.; Rodríguez, M. Recent advances in understanding and managing secondary hyperparathyroidism in chronic kidney disease. F1000Research 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Covic, A.; Vervloet, M.; Massy, Z.A.; Torres, P.U.; Goldsmith, D.; Brandenburg, V.; Mazzaferro, S.; Evenepoel, P.; Bover, J.; Apetrii, M.; et al. Bone and mineral disorders in chronic kidney disease: Implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol. 2018, 6, 319–331. [Google Scholar] [CrossRef]
- Hamano, T. Mineral and bone disorders in conventional hemodialysis: Challenges and solutions. Semin. Dial. 2018, 31, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Ciceri, P.; Galassi, A.; Mangano, M.; Carugo, S.; Capelli, I.; Cianciolo, G. The Key Role of Phosphate on Vascular Calcification. Toxins 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Jovanovich, A.; Kendrick, J. Personalized Management of Bone and Mineral Disorders and Precision Medicine in End-Stage Kidney Disease. Semin. Nephrol. 2018, 38, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Ravarotto, V.; Simioni, F.; Pagnin, E.; Davis, P.A.; Calò, L.A. Oxidative stress—Chronic kidney disease—Cardiovascular disease: A vicious circle. Life Sci. 2018, 210, 125–131. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2018, 34, 975–991. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef] [PubMed]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, S.B.; Stoyanova, E.; Coll, E.; Pastor, S.; Reyes, J.; Andrés, E.; Ballarin, J.; Xamena, N.; Marcos, R. Genetic damage in chronic renal failure patients is associated with the glomerular filtration rate index. Mutagenesis 2010, 25, 603–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buemi, M.; Costa, C.; Floccari, F.; Coppolino, G.; Campo, S.; Bolignano, D.; Sturiale, A.; Lacquaniti, A.; Buemi, A.; Loddo, S.; et al. Genomic damage in endothelial progenitor cells from uremic patients in hemodialysis. J. Nephrol. 2010, 23, 328–334. [Google Scholar] [PubMed]
- Habener, J.F.; Amherdt, M.; Ravazzola, M.; Orci, L. Parathyroid hormone biosynthesis. Correlation of conversion of biosynthetic precursors with intracellular protein migration as determined by electron microscope autoradiography. J. Cell Biol. 1979, 80, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Zeng, S. Clear the Fog around Parathyroid Hormone Assays: What Do iPTH Assays Really Measure? Clin. J. Am. Soc. Nephrol. 2018, 13, 524–526. [Google Scholar] [CrossRef]
- Hocher, B.; Armbruster, F.P.; Stoeva, S.; Reichetzeder, C.; Grön, H.J.; Lieker, I.; Khadzhynov, D.; Slowinski, T.; Roth, H.J. Measuring parathyroid hormone (PTH) in patients with oxidative stress--do we need a fourth generation parathyroid hormone assay? PLoS ONE 2012, 7, e40242. [Google Scholar] [CrossRef]
- Hocher, B.; Oberthür, D.; Slowinski, T.; Querfeld, U.; Schaefer, F.; Doyon, A.; Tepel, M.; Roth, H.J.; Grön, H.J.; Reichetzeder, C.; et al. Modeling of Oxidized PTH (oxPTH) and Non-oxidized PTH (n-oxPTH) Receptor Binding and Relationship of Oxidized to Non-Oxidized PTH in Children with Chronic Renal Failure, Adult Patients on Hemodialysis and Kidney Transplant Recipients. Kidney Blood Press. Res. 2013, 37, 240–251. [Google Scholar] [CrossRef]
- Zull, J.E.; Smith, S.K.; Wiltshire, R. Effect of methionine oxidation and deletion of amino-terminal residues on the conformation of parathyroid hormone. Circular dichroism studies. J. Biol. Chem. 1990, 265, 5671–5676. [Google Scholar] [CrossRef]
- Frelinger, A.L., 3rd; Zull, J.E. The role of the methionine residues in the structure and function of parathyroid hormone. Arch Biochem. Biophys. 1986, 244, 641–649. [Google Scholar] [CrossRef]
- Sutcliffe, H.S.; Martin, T.J.; Eisman, J.A.; Pilczyk, R. Binding of parathyroid hormone to bovine kidney-cortex plasma membranes. Biochem. J. 1973, 134, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Frelinger, A.L., 3rd; Zull, J.E. Oxidized forms of parathyroid hormone with biological activity. Separation and characterization of hormone forms oxidized at methionine 8 and methionine 18. J. Biol. Chem. 1984, 259, 5507–5513. [Google Scholar] [CrossRef]
- Pitts, T.O.; Puschett, J.B.; Rose, M.E.; Zull, J.E. Effects of selective oxidation of 1-34 bovine parathyroid hormone on its renal actions in the rabbit. Miner. Electrolyte Metab. 1989, 15, 267–375. [Google Scholar]
- Nabuchi, Y.; Fujiwara, E.; Ueno, K.; Kuboniwa, H.; Asoh, Y.; Ushio, H. Oxidation of Recombinant Human Parathyroid Hormone: Effect of Oxidized Position on the Biological Activity. Pharm. Res. 1995, 12, 2049–2052. [Google Scholar] [CrossRef]
- Yee, J.A. Stimulation of alkaline phosphatase activity in cultured neonatal mouse calvarial bone cells by parathyroid hormone. Calcif. Tissue Res. 1985, 37, 530–538. [Google Scholar] [CrossRef]
- Pang, P.K.T.; Yang, M.C.M.; Kenny, A.D.; Tenner, T.E. Structure and Vascular Activity Relationship of Parathyroid Hormone and Some Hypotensive Peptides. Clin. Exp. Hypertens. Part A Theory Pract. 1982, 4, 189–199. [Google Scholar] [CrossRef]
- Yen, Y.C.; Yang, M.C.M.; Kenny, A.D.; Pang, P.K.T. Parathyroid hormone (PTH) fragments relax the guinea-pig trachea in vitro. Can. J. Physiol. Pharmacol. 1983, 61, 1324–1328. [Google Scholar] [CrossRef]
- Sham, J.; Kenny, A.; Pang, P. Cardiac actions and structural-activity relationship of parathyroid hormone on isolated frog atrium. Gen. Comp. Endocrinol. 1984, 55, 373–377. [Google Scholar] [CrossRef]
- Laethem, R.; Zull, J.E. Characterization of the interaction of parathyroid hormone with the mitochondrial ATPase. Arch. Biochem. Biophys. 1990, 282, 161–169. [Google Scholar] [CrossRef]
- Zeng, S.; Querfeld, U.; Feger, M.; Haffner, D.; Hasan, A.A.; Chu, C.; Slowinski, T.; Bernd Dschietzig, T.; Schäfer, F.; Xiong, Y.; et al. Relationship between GFR, intact PTH, oxidized PTH, non-oxidized PTH as well as FGF23 in patients with CKD. FASEB J. 2020, 34, 15269–15281. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H. Effect of Oxidation and Reduction upon the Biological Activity of Parathyroid Hormone. Science 1958, 128, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N. Effects of oxidation of human parathyroid hormone on its biological activity in continuously infused, thyroparathyroidectomized rats. J. Bone Miner. Res. 1988, 3, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Dacke, C.G.; Kenny, A.D. Avian Bioassay Method for Parathyroid Hormone1. Endocrinology 1973, 92, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Godes, M.; Reichetzeder, C.; Tsuprykov, O.; Chertow, G.M.; Parfrey, P.S.; Floege, J.; Kubo, Y.; Dehmel, B.; Drueke, T.B. Non-oxidized PTH (n-oxPTH) is associated with cardiovascular events and all-cause mortality in patients with secondary hyperparathyroidism undergoing hemodialysis who participated in the EVOLVE trial. J. Am. Soc. Nephrol. 2014, 25, 874A. [Google Scholar]
- Ursem, S.R.; Heijboer, A.C.; D’Haese, P.C.; Behets, G.J.; Cavalier, E.; Vervloet, M.G.; Evenepoel, P. Non-oxidized parathyroid hormone (PTH) measured by current method is not superior to total PTH in assessing bone turnover in chronic kidney disease. Kidney Int. 2021, 99, 1173–1178. [Google Scholar] [CrossRef]
- Seiler-Mussler, S.; Limbach, A.S.; Emrich, I.E.; Pickering, J.W.; Roth, H.J.; Fliser, D.; Heine, G.H. Association of Nonoxidized Parathyroid Hormone with Cardiovascular and Kidney Disease Outcomes in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 569–576. [Google Scholar] [CrossRef]
- Lu, Y.P.; Zeng, S.; Chu, C.; Hasan, A.A.; Slowinski, T.; Yin, L.H.; Krämer, B.K.; Hocher, B. Non-oxidized PTH (n-oxPTH) is associated with graft loss in kidney transplant recipients. Clin. Chim. Acta 2020, 508, 92–97. [Google Scholar] [CrossRef]
- Tepel, M.; Armbruster, F.P.; Grön, H.J.; Scholze, A.; Reichetzeder, C.; Roth, H.J.; Hocher, B. Nonoxidized, Biologically Active Parathyroid Hormone Determines Mortality in Hemodialysis Patients. J. Clin. Endocrinol. Metab. 2013, 98, 4744–4751. [Google Scholar] [CrossRef]
- Floege, J.; Kim, J.; Ireland, E.; Chazot, C.; Drueke, T.; de Francisco, A.; Kronenberg, F.; Marcelli, D.; Passlick-Deetjen, J.; Schernthaner, G.; et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol. Dial. Transplant. 2010, 26, 1948–1955. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kuwae, N.; Regidor, D.; Kovesdy, C.; Kilpatrick, R.; Shinaberger, C.; McAllister, C.; Budoff, M.; Salusky, I.; Kopple, J. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006, 70, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, L.; Zuo, L.; Jin, C.G.; Li, W.G.; Chen, J.-B. Association of CKD-MBD Markers with All-Cause Mortality in Prevalent Hemodialysis Patients: A Cohort Study in Beijing. PLoS ONE 2017, 12, e0168537. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-J.; Chen, L.-P.; Pan, Y.-L.; Lu, Y.; Sun, L.-H.; Zhao, H.-Y.; Wang, W.-Q.; Tao, B.; Liu, J.-M. An inverted U-shaped relationship between parathyroid hormone and body weight, body mass index, body fat. Endocrine 2021, 72, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Y.; Luo, Y. The optimal range of serum intact parathyroid hormone for a lower risk of mortality in the incident hemodialysis patients. Ren. Fail. 2021, 43, 599–605. [Google Scholar] [CrossRef]
| Reference | Subject and/or Model | Readouts | Conclusions | |
|---|---|---|---|---|
| 1 | [29] | In silico modeling | noxPTH and oxPTH interaction with PTH1R | PTH oxidation → 3D structure changes → altered PTH-PTH1R interaction |
| 2 | [30] | Circular dichroism | The secondary structure of PTH | Oxidation at Met18 → small impact Oxidation at Met8 → substantial changes Oxidation at both Met8 and Met18 → greater changes |
| 3 | [31] | In vitro renal membrane adenylyl cyclase assay | cAMP formation as a surrogate measure of renal adenyl cyclase activity | Oxidation at Met18 → full agonist with ↓ potency Oxidation at Met8 → partial agonist with ↓↓ potency Oxidation at both Met8 and Met18 → partial agonist with ↓↓↓ potency |
| 4 | [32] | In vitro plasma membranes purified from bovine renal cortex | Binding affinity and cAMP formation as a surrogate measure of renal adenyl cyclase activity | Oxidation of PTH → loss of the ability to bind plasma membranes and to activate adenylate cyclase |
| 5 | [33] | In vitro renal membrane adenylyl cyclase assay | cAMP formation as a surrogate measure of renal adenyl cyclase activity | The degree of bioactivity: noxPTH > Met18(ox)PTH > Met8(ox)PTH > Met8, Met18(di-ox)PTH |
| 6 | [34] | In vivo rabbits; In vitro isolated perfused rabbit proximal tubules | Renal electrolyte handling and adenylate cyclase stimulation | PTH → phosphaturia, anticalciuria, ↑ renal proximal tubular adenylate cyclase, and ↑ renal cortical cAMP Oxidized forms → weaker or no activity |
| 7 | [35] | In vitro adenylate cyclase assay using rat osteosarcoma cells | cAMP formation as a surrogate measure of adenyl cyclase activity | All oxidized forms possessed reduced biological activity, more so for oxidation at Met8 than at Met18. |
| 8 | [36] | In vitro primary cultures of neonatal mouse calvarial cells | The activity of alkaline phosphatase | Oxidation of PTH → loss of the ability to activate alkaline phosphatase |
| 9 | [37] | In vitro coronary, renal, hepatic, and visceral vascular beds | Direct vasodilatory action | noxPTH but not oxPTH (at Met8 and Met18) has direct vasodilatory action |
| 10 | [38] | In vitro guinea-pig trachea constricted with histamine in vitro | Relaxation of guinea-pig trachea constricted with histamine | noxPTH but not oxPTH (at Met8 and Met18) can relax guinea-pig trachea constricted with histamine |
| 11 | [39] | In vitro isolated frog atrium | Cardiac action (chronotropic and inotropic effects) | noxPTH → +ve chronotropic and inotropic effects oxPTH → abolished cardiac action |
| 12 | [40] | In vitro intact mitochondria, submitochondrial particles, and purified mitochondrial F1 ATPase | The affinity (specific binding) to mitochondrial ATPase and its activation | PTH → ↑ affinity and activation of the mitochondrial ATPase Oxidation of Met18 → 50% ↓ affinity Oxidation of Met8 → 95% ↓ affinity Oxidation of both Met8 and Met18 → further ↓ affinity |
| 13 | [41] | In vitro UMR106 osteoblast-like cells | Fgf23 gene expression | noxPTH → ↑ Fgf23 mRNA synthesis Oxidation of PTH, in particular, at Met8 → ↓ ↑ Fgf23 mRNA synthesis |
| 14 | [42] | In vivo parathyroidectomized rats on low-calcium diet | Blood calcium | Oxidation of PTH → loss of the ability to increase blood calcium |
| 15 | [43] | In vivo parathyroidectomized rats and vit. D-deficient rats | Serum calcium, serum phosphate, urine calcium, urine phosphate, and urine cAMP, as well as renal 1,25 (OH)2D3 and 24,25(OH)2D3 production | noxPTH → ↑ serum calcium, ↓ serum phosphate, ↓ urine calcium, ↑ urine phosphate, ↑ urine cAMP, ↑ renal 1,25 (OH)2D3, and ↓ renal 24,25(OH)2D3 Oxidation at both Met8 and Met18 → loss of all above-mentioned biological activities |
| 16 | [44] | In vivo immature birds (Japanese quail) | Hypercalcemic response | Oxidation of PTH → no hypercalcemic response |
| Reference | Design | Individuals (n) | Population Characteristics | Conclusions | Remarks | |
|---|---|---|---|---|---|---|
| 1 | [28] | Observational study | 18 | Patients on intermittent hemodialysis | The % of oxPTH was 7–34% | No follow-up for the patients; No investigation of the associations between the different forms of PTH and clinical outcomes |
| 2 | [45] | Retrospective study (EVOLVE trial, NCT00345839) | 2867 | Maintenance hemodialysis | Only noxPTH, but not iPTH or oxPTH, was associated with cardiovascular events and mortality | Follow-up for up to 64 months |
| 3 | [46] | Prospective observational bone biopsy study | 31 | Patients with ESKD and low bone turnover, normal bone turnover, or high bone turnover | Measuring noxPTH has no added value compared to total PTH as an indicator of bone turnover in patients with kidney failure | Bone turnover markers showed similar correlation coefficients to noxPTH and total PTH |
| 4 | [47] | Observational study | 535 | Patients with CKD | PTH was associated with all-cause mortality; there was no association of noxPTH with any of the clinical outcomes examined | Follow-up over 5.1 years for the occurrence of acute heart failure, atherosclerotic events, CKD progression, or all-cause death |
| 5 | [48] | Prospective observational study | 600 | Kidney transplant recipients | Only noxPTH, but not oxPTH or iPTH, was associated with graft loss in stable kidney transplant recipients | Follow-up for graft loss for 3 years |
| 6 | [49] | Prospective observational study | 340 | Hemodialysis patients | Measurements of noxPTH may reflect the hormone status more precisely. The iPTH-associated mortality most likely describes oxidative-stress-related mortality. | The follow-up period was 5 years |
| 7 | [41] | Retrospective studies in 2 independent cohorts | 620 (4C study) | Children with CKD | In both clinical cohorts, noxPTH but not oxPTH was significantly associated with FGF23 concentrations, independent of known confounding factors. | With progressive deterioration of kidney function, total PTH and oxPTH substantially increased, whereas noxPTH only moderately increased. The increase in PTH with decreasing GFR is mainly due to an increase in oxPTH in more advanced stages of CKD. |
| 600 | Stable renal transplant recipients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.A.; Hocher, C.-F.; Kleuser, B.; Krämer, B.K.; Hocher, B. Biological Activity of Different Forms of Oxidized Parathyroid Hormone. Int. J. Mol. Sci. 2022, 23, 12228. https://doi.org/10.3390/ijms232012228
Hasan AA, Hocher C-F, Kleuser B, Krämer BK, Hocher B. Biological Activity of Different Forms of Oxidized Parathyroid Hormone. International Journal of Molecular Sciences. 2022; 23(20):12228. https://doi.org/10.3390/ijms232012228
Chicago/Turabian StyleHasan, Ahmed A., Carl-Friedrich Hocher, Burkhard Kleuser, Bernhard K. Krämer, and Berthold Hocher. 2022. "Biological Activity of Different Forms of Oxidized Parathyroid Hormone" International Journal of Molecular Sciences 23, no. 20: 12228. https://doi.org/10.3390/ijms232012228
APA StyleHasan, A. A., Hocher, C.-F., Kleuser, B., Krämer, B. K., & Hocher, B. (2022). Biological Activity of Different Forms of Oxidized Parathyroid Hormone. International Journal of Molecular Sciences, 23(20), 12228. https://doi.org/10.3390/ijms232012228






