Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin
Abstract
:1. Introduction
2. Results
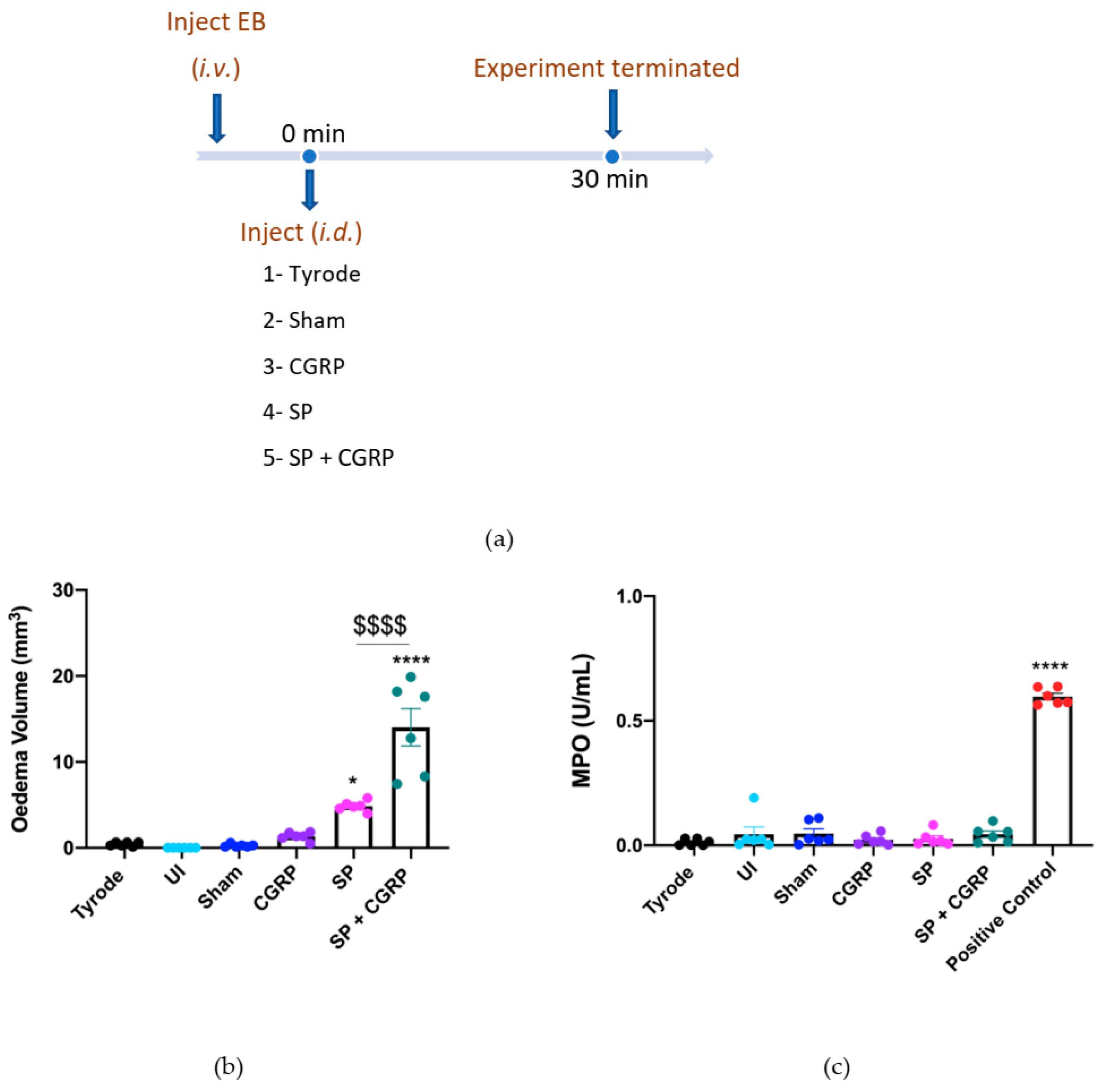
2.1. CGRP Potentiation of SP-Induced Oedema Formation over 30 min
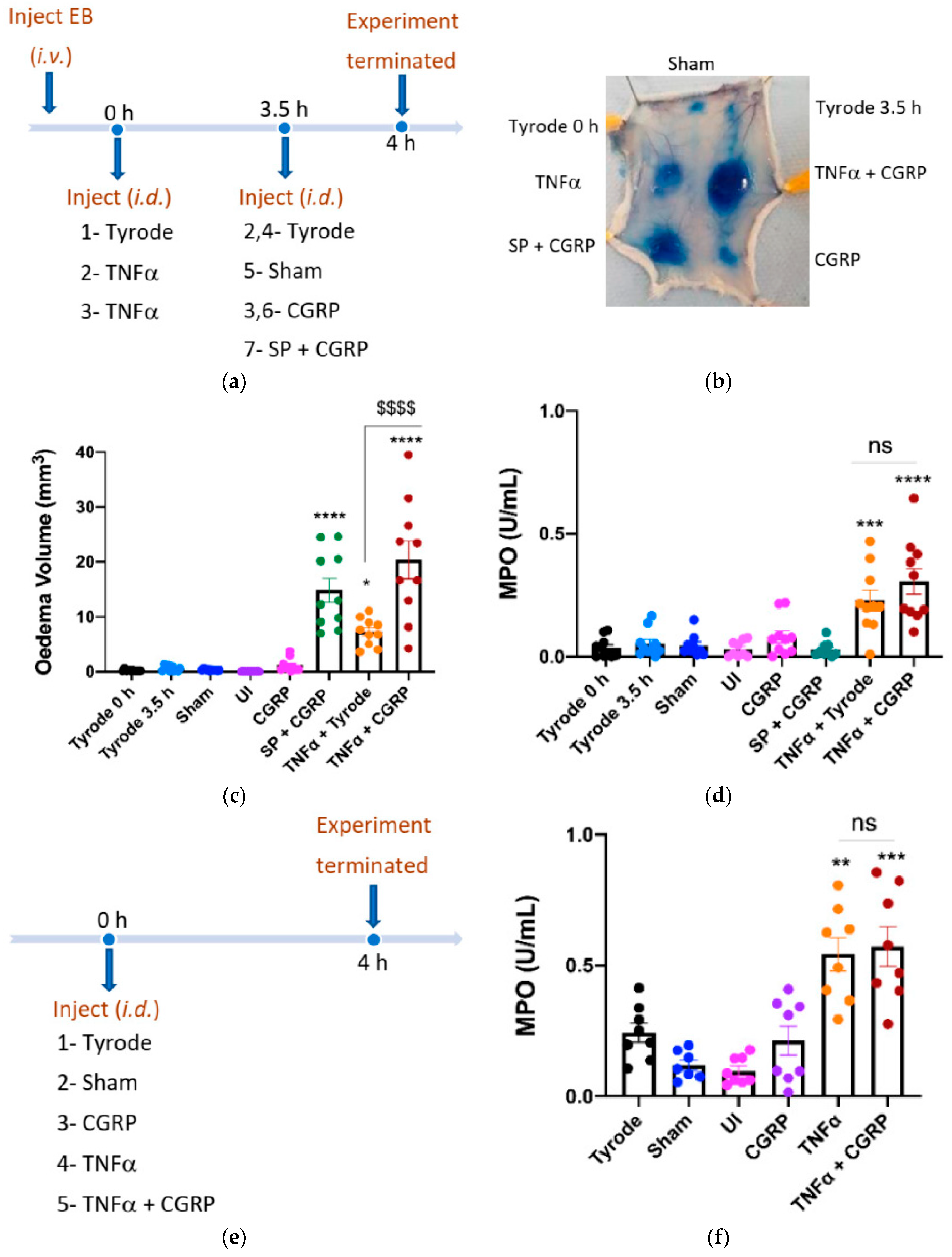
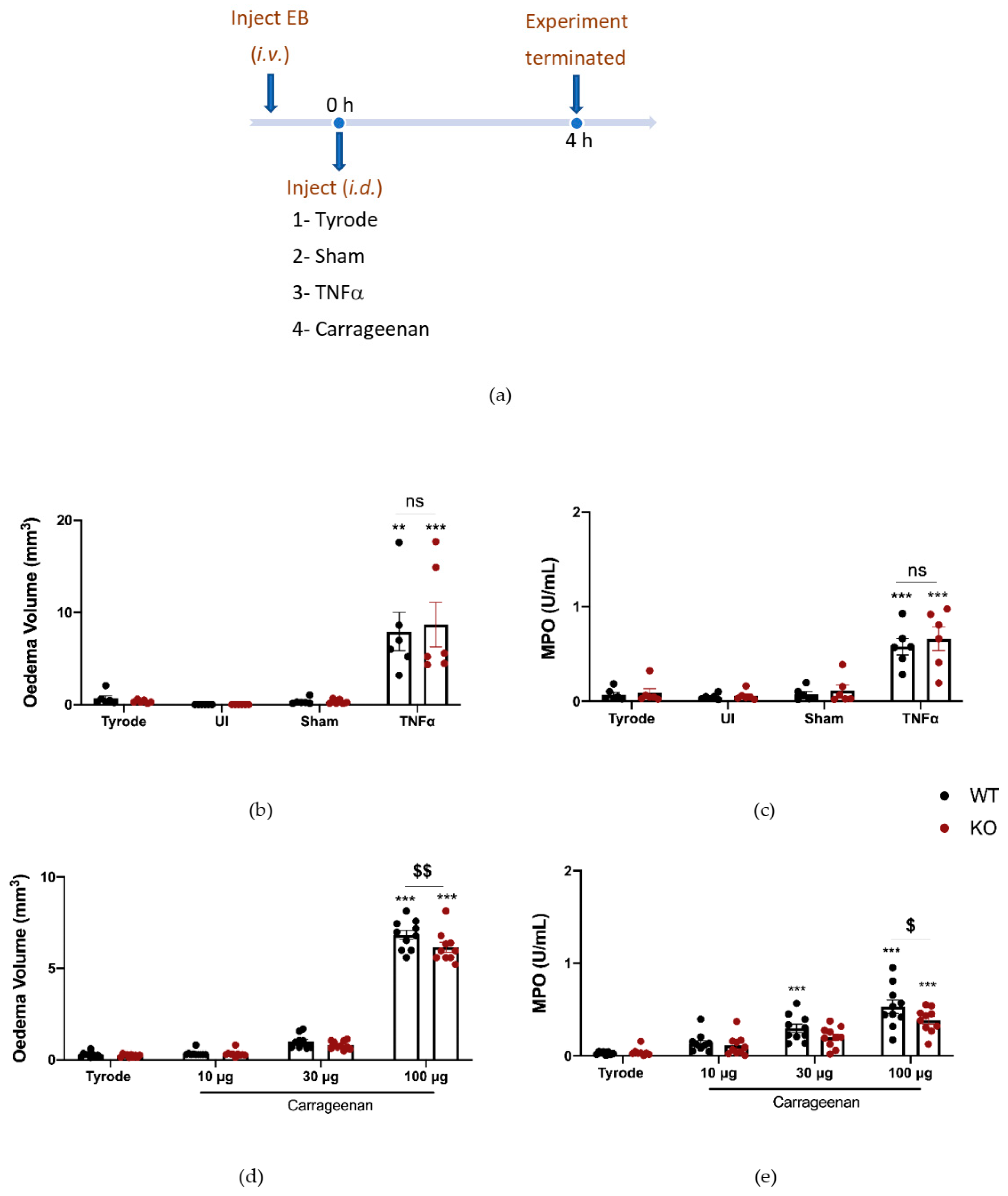
2.2. CGRP Potentiation of TNFα-Induced Oedema Formation over 4 h
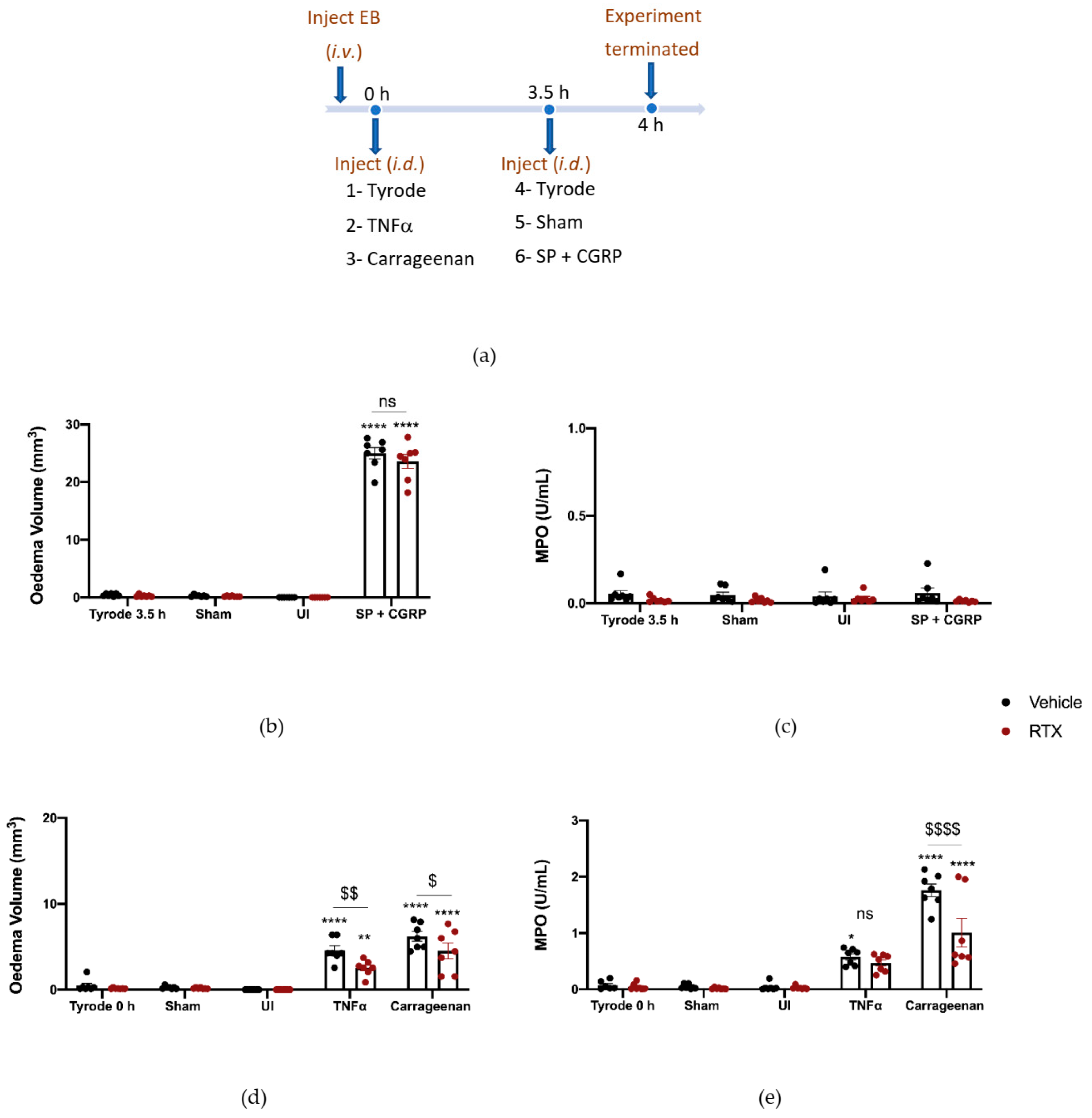
2.3. The Effect of Depleting Sensory Nerves on TNFα and Carrageenan-Induced Oedema Formation and Neutrophil Accumulation
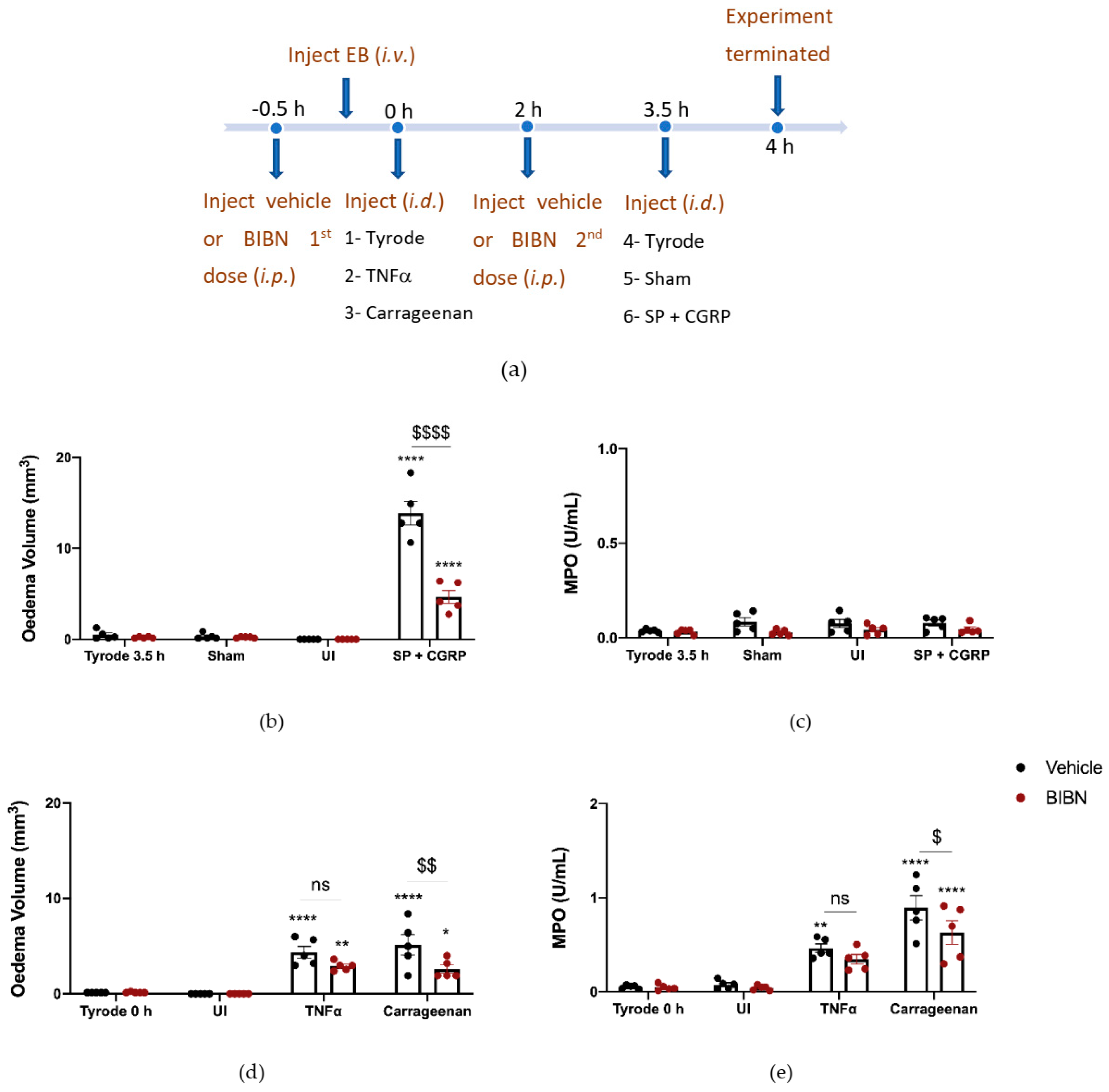
2.4. The Effect of CGRP Antagonist BIBN4096BS on TNFα and Carrageenan-Induced Oedema Formation and Neutrophil Accumulation
2.5. The Effect of Deleting αCGRP Peptide on TNFα and Carrageenan-Induced Oedema Formation and Neutrophil Accumulation
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. In Vivo Dorsal Skin Oedema Inflammation Model
4.3. Obtaining an Internal Standard via Zymosan-Induced Peritoneal Inflammation
4.4. Sensory Nerve Depletion—Resiniferatoxin (RTX) Pre-Treatment
4.5. Evaluation of Sensory Nerve Depletion—Hot Plate Test
4.6. Systemic Treatments for Skin Assay
4.7. Ex Vivo Assay of Myeloperoxidase (MPO) Accumulation
4.8. Quantitative Polymerase Chain Reaction
4.9. Measurement of Blood Pressure via Carotid Artery Cannulation
5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGRP | Calcitonin gene related peptide |
| COX | Cyclo-oxygenase |
| CLR | Calcitonin receptor-like receptor |
| DMSO | Dimethyl Sulfoxide |
| EB | Evans Blue |
| HTAB | Hexadecyltrimethylammonium bromide |
| 5-HT | 5-Hydroxytryptamine |
| ICAM-1 | Intracellular Adhesion Molecule-1 |
| KO | Knockout |
| MPO | Myeloperoxidase |
| NSAID | Non-steroidal anti-inflammatory drug |
| OD | Optical density |
| RAMP1 | Receptor activity modifying protein 1 |
| RTX | Resiniferatoxin |
| SEM | Standard error of the mean |
| SP | Substance P |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| TNFα | Tumour necrosis factor alpha |
| TRPV1 | transient receptor potential vanilloid 1 |
| VEGF | Vascular endothelial growth factor |
| VCAM-1 | Vascular Cell Adhesion molecule 1 |
| WT | Wildtype |
References
- Brain, S.D.; Grant, A.D. Vascular Actions of Calcitonin Gene-Related Peptide and Adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin Gene-Related Peptide Is a Potent Vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef]
- Van der Schueren, B.J.; Rogiers, A.; Vanmolkot, F.H.; van Hecken, A.; Depré, M.; Kane, S.A.; de Lepeleire, I.; Sinclair, S.R.; de Hoon, J.N. Calcitonin Gene-Related Peptide8-37 Antagonizes Capsaicin-Induced Vasodilation in The Skin: Evaluation of A Human In Vivo Pharmacodynamic Model. J. Pharmacol. Exp. Ther. 2008, 325, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Argunhan, F.; Thapa, D.; Aubdool, A.A.; Carlini, E.; Arkless, L.; Hendrikse, E.R.; de Valente, J.S.; Kodji, X.; Barrett, B.; Ricciardi, C.A.; et al. Calcitonin Gene-Related Peptide Protects Against Cardiovascular Dysfunction Independently of Nitric Oxide In Vivo. Hypertension 2021, 77, 1178–1190. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [Green Version]
- Aubdool, A.A.; Thakore, P.; Argunhan, F.; Smillie, S.-J.; Schnelle, M.; Srivastava, S.; Alawi, K.M.; Wilde, E.; Mitchell, J.; Farrell-Dillon, K.; et al. A Novel A-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, And Heart Failure. Circulation 2017, 136, 367–383. [Google Scholar] [CrossRef]
- Kee, Z.; Kodji, X.; Brain, S.D. The Role of Calcitonin Gene Related Peptide (CGRP) In Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 2018, 9, 1249. [Google Scholar] [CrossRef] [Green Version]
- Brain, S.D.; Williams, T.J. Inflammatory Oedema Induced by Synergism Between Calcitonin Gene-Related Peptide (CGRP) And Mediators of Increased Vascular Permeability. Br. J. Pharmacol. 1985, 86, 855–860. [Google Scholar] [CrossRef]
- Grant, A.D.; Tam, C.W.; Lazar, Z.; Shih, M.K.; Brain, S.D. The Calcitonin Gene-Related Peptide (CGRP) Receptor Antagonist BIBN4096BS Blocks CGRP And Adrenomedullin Vasoactive Responses in The Microvasculature. Br. J. Pharmacol. 2004, 142, 1091–1098. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Brain, S.D. A Historical Perspective on The Role of Sensory Nerves in Neurogenic Inflammation. Semin. Immunopathol. 2018, 40, 229–236. [Google Scholar] [CrossRef]
- Cao, T.; Gerard, N.P.; Brain, S.D. Use of Nk1knockout Mice to Analyze Substance P-Induced Edema Formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R476–R481. [Google Scholar] [CrossRef] [Green Version]
- Brain, S.D.; Williams, T.J. Substance P Regulates the Vasodilator Activity of Calcitonin Gene-Related Peptide. Nature 1988, 335, 73–75. [Google Scholar] [CrossRef]
- McDonald, D.; Thurston, G.; Baluk, P. Endothelial Gaps as Sites for Plasma Leakage in Inflammation. Microcirculation 1999, 6, 7–22. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Panayi, G.S. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-A) In Autoimmune Disease and Current TNF-A Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Hong, J.J.; Hadeler, E.K.; Mosca, M.L.; Brownstone, N.D.; Bhutani, T.; Liao, W.J. TNF-Alpha Inhibitors and Ustekinumab for The Treatment of Psoriasis: Therapeutic Utility in The Era Of IL-17 And IL-23 Inhibitors. J. Psoriasis Psoriatic Arthritis 2022, 7, 79–92. [Google Scholar] [CrossRef]
- Petrofsky, M.; Bermudez, L.E. Neutrophils Frommycobacterium Avium-Infected Mice Produce TNF-A, IL-12, And IL-1Β And Have a Putative Role in Early Host Response. Clin. Immunol. 1999, 91, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Finsterbusch, M.; Voisin, M.-B.; Beyrau, M.; Williams, T.J.; Nourshargh, S. Neutrophils Recruited by Chemoattractants In Vivo Induce Microvascular Plasma Protein Leakage Through Secretion Of TNF. J. Exp. Med. 2014, 211, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G. Neutrophil–Endothelial Cell Interactions: Mechanisms of Neutrophil Adherence to Vascular Endothelium. J. Investig. Dermatol. 1989, 93, S53–S58. [Google Scholar] [CrossRef]
- Wedmore, C.V.; Williams, T.J. Control of Vascular Permeability by Polymorphonuclear Leukocytes in Inflammation. Nature 1981, 289, 646–650. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of The Rat as An Assay for Antiinflammatory Drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Cao, T.; Pintér, E.; Al-Rashed, S.; Gerard, N.; Hoult, J.R.; Brain, S.D. Neurokinin-1 Receptor Agonists Are Involved in Mediating Neutrophil Accumulation in The Inflamed, But Not Normal, Cutaneous Microvasculature: An In Vivo Study Using Neurokinin-1 Receptor Knockout Mice. J. Immunol. 2000, 164, 5424–5429. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.C.C.; Fernandes, E.S.; Quintão, N.L.M.; Campos, M.M.; Calixto, J.B. Relevance of Tumour Necrosis Factor-A For the Inflammatory and Nociceptive Responses Evoked by Carrageenan In The Mouse Paw. Br. J. Pharmacol. 2006, 148, 688–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, I.; Smillie, S.-J.; Bodkin, J.V.; Fernandes, E.S.; O’Byrne, K.T.; Brain, S.D. The Vasoactive Potential of Kisspeptin-10 in the Peripheral Vasculature. PLoS ONE 2011, 6, e14671. [Google Scholar] [CrossRef] [PubMed]
- Schierwagen, C.; Bylund-Fellenius, A.-C.; Lundberg, C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J. Pharmacol. Methods 1990, 23, 179–186. [Google Scholar] [CrossRef]
- Kodji, X.; Arkless, K.L.; Kee, Z.; Cleary, S.J.; Aubdool, A.A.; Evans, E.; Caton, P.; Pitchford, S.C.; Brain, S.D. Sensory nerves mediate spontaneous behaviors in addition to inflammation in a murine model of psoriasis. FASEB J. 2018, 33, 1578–1594. [Google Scholar] [CrossRef] [Green Version]
- Aubdool, A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newbold, P.; Brain, S. The modulation of inflammatory oedema by calcitonin gene-related peptide. J. Cereb. Blood Flow Metab. 1993, 108, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Gautam, N.; Olofsson, M.; Herwald, H.; Iversen, L.F.; Lundgren-Åkerlund, E.; Hedqvist, P.; Arfors, K.-E.; Flodgaard, H.; Lindbom, L. Heparin-binding protein (HBP/CAP37): A missing link in neutrophil-evoked alteration of vascular permeability. Nat. Med. 2001, 7, 1123–1127. [Google Scholar] [CrossRef]
- Cain, R.J.; Vanhaesebroeck, B.; Ridley, A.J. The PI3K p110α isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J. Cell Biol. 2010, 188, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Guo, X.; Zhang, X.; Wang, Z.; Zhou, J.; Xia, L.; Zhang, Y.; Wen, J.; Jin, D. The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J. Recept. Signal Transduct. 2013, 33, 114–123. [Google Scholar] [CrossRef]
- Crossman, D.; Dashwood, M.; Brain, S.; McEwan, J.; Pearson, J. Action of calcitonin gene-related peptide upon bovine vascular endothelial and smooth muscle cells grown in isolation and co-culture. J. Cereb. Blood Flow Metab. 1990, 99, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.J.; Mullooly, N.; Safitri, D.; Harris, M.; de Vries, T.; MaassenVanDenBrink, A.; Poyner, D.R.; Gianni, D.; Wigglesworth, M.; Ladds, G. CGRP, adrenomedullin and adrenomedullin 2 display endogenous GPCR agonist bias in primary human cardiovascular cells. Commun. Biol. 2021, 4, 776. [Google Scholar] [CrossRef]
- Cohen, J.A.; Edwards, T.N.; Liu, A.W.; Hirai, T.; Jones, M.R.; Wu, J.; Li, Y.; Zhang, S.; Ho, J.; Davis, B.M.; et al. Cutaneous TRPV1+ Neurons Trigger Protective Innate Type 17 Anticipatory Immunity. Cell 2019, 178, 919–932.e14. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Szállási, A.; Szabó, T.; Bíró, T.; Modarres, S.; Blumberg, P.M.; Krause, J.E.; Cortright, D.N.; Appendino, G.B. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. J. Cereb. Blood Flow Metab. 1999, 128, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Bencze, N.; Schvarcz, C.; Kriszta, G.; Danics, L.; Szőke, É.; Balogh, P.; Szállási, Á.; Hamar, P.; Helyes, Z.; Botz, B. Desensitization of Capsaicin-Sensitive Afferents Accelerates Early Tumor Growth via Increased Vascular Leakage in a Murine Model of Triple Negative Breast Cancer. Front. Oncol. 2021, 11, 685297. [Google Scholar] [CrossRef]
- Pan, H.-L.; Khan, G.M.; Alloway, K.D.; Chen, S.-R. Resiniferatoxin Induces Paradoxical Changes in Thermal and Mechanical Sensitivities in Rats: Mechanism of Action. J. Neurosci. 2003, 23, 2911–2919. [Google Scholar] [CrossRef] [Green Version]
- Schattenkirchner, M. Meloxicam:a selective COX-2 inhibitor non-steroidal anti-inflammatory drug. Expert Opin. Investig. Drugs 1997, 6, 321–334. [Google Scholar] [CrossRef]
- Johnston, B.M.; Owen, D.A. Histamine, histamine antagonists and regional blood flow. Eur. J. Pharmacol. 1977, 44, 355–363. [Google Scholar] [CrossRef]
- Sufka, K.J.; Schomburg, F.M.; Giordano, J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol. Biochem. Behav. 1992, 41, 53–56. [Google Scholar] [CrossRef]
- Koehler, P.J. Use of corticosteroids in neuro-oncology. Anti-Cancer Drugs 1995, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, H.; Hunt, M.; Nagasaka, H.; Yaksh, T. Systematic Review of Systemic and Neuraxial Effects of Acetaminophen in Preclinical Models of Nociceptive Processing. J. Pain Res. 2021, 14, 3521–3552. [Google Scholar] [CrossRef]
- Louis, S.; Johnstone, D.; Russell, N.; Jamieson, A.; Dockray, G. Antibodies to calcitonin-gene related peptide reduce inflammation induced by topical mustard oil but not that due to carrageenin in the rat. Neurosci. Lett. 1989, 102, 257–260. [Google Scholar] [CrossRef]
- Salmon, A.-M.; Damaj, I.M.; Marubio, L.M.; Epping-Jordan, M.P.; Pich, E.M.; Changeux, J.-P. Altered Neuroadaptation in Opiate Dependence and Neurogenic Inflammatory Nociception in αCGRP Deficient Mice. Sci. World J. 2001, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- De Queiroz, B.F.; de Almeida, M.P.; Bakhle, Y.; Francischi, J.N. Calcitonin-gene related peptide is a potent inducer of oedema in rat orofacial tissue. Neuropeptides 2018, 68, 43–48. [Google Scholar] [CrossRef]
- Sung, C.-P.; Arleth, A.J.; Aiyar, N.; Bhatnagar, P.K.; Lysko, P.G.; Feuerstein, G. CGRP stimulates the adhesion of leukocytes to vascular endothelial cells. Peptides 1992, 13, 429–434. [Google Scholar] [CrossRef]
- Ansel, J.C.; Armstrong, C.A.; Song, I.; Quinlan, K.L.; Olerud, J.E.; Caughman, S.W.; Bunnett, N.W. Interactions of the skin and nervous system. J. Investig. Dermatol. Symp. Proc. 1997, 2, 23–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, T.; Brain, S.; Rampart, M.; Williams, T. Time-dependent synergistic interactions between the vasodilator neuropeptide, calcitonin gene-related peptide (CGRP) and mediators of inflammation. J. Cereb. Blood Flow Metab. 1991, 103, 1515–1519. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, A.; Perretti, M. Calcitonin gene-related peptides modulate the acute inflammatory response induced by interleukin-1 in the mouse. Eur. J. Pharmacol. 1994, 264, 407–415. [Google Scholar] [CrossRef]
- Sampson, A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy 2000, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Quellier, D.; Lamb, J.; Voisin, T.; Baral, P.; Bock, F.; Schönberg, A.; Mirchev, R.; Pier, G.; Chiu, I.; et al. Pseudomonas aeruginosa–induced nociceptor activation increases susceptibility to infection. PLOS Pathog. 2021, 17, e1009557. [Google Scholar] [CrossRef]
- Jochheim, L.S.; Odysseos, G.; Hidalgo-Sastre, A.; Zhong, S.; Staufer, L.M.; Kroiss, M.; Kabacaoglu, D.; Lange, S.; Engleitner, T.; Hartmann, D.; et al. The neuropeptide receptor subunit RAMP1 constrains the innate immune response during acute pancreatitis in mice. Pancreatology 2019, 19, 541–547. [Google Scholar] [CrossRef]
- Matsui, S.; Tanaka, M.; Kamiyoshi, A.; Sakurai, T.; Ichikawa-Shindo, Y.; Kawate, H.; Dai, K.; Cui, N.; Wei, Y.; Tanaka, M.; et al. Endogenous Calcitonin Gene–Related Peptide Deficiency Exacerbates Postoperative Lymphedema by Suppressing Lymphatic Capillary Formation and M2 Macrophage Accumulation. Am. J. Pathol. 2019, 189, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Czirják, L.; Sándor, Z.; Helyes, Z.; László, T.; Elekes, K.; Czömpöly, T.; Starr, A.; Brain, S.; Szolcsányi, J.; et al. Investigation of sensory neurogenic components in a bleomycin-induced scleroderma model using transient receptor potential vanilloid 1 receptor– and calcitonin gene-related peptide–knockout mice. Arthritis Care Res. 2007, 58, 292–301. [Google Scholar] [CrossRef]
- Ding, W.; Stohl, L.L.; Saab, J.; Azizi, S.; Zhou, X.K.; Mehta, D.; Granstein, R.D. Regulation of Cutaneous Immunity In Vivo by Calcitonin Gene–Related Peptide Signaling through Endothelial Cells. J. Immunol. 2022, 208, 633–641. [Google Scholar] [CrossRef]
- Reich, A.; Orda, A.; Wiśnicka, B.; Szepietowski, J.C. Plasma concentration of selected neuropeptides in patients suffering from psoriasis. Exp. Dermatol. 2007, 16, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Sun, L.; Cai, X.; Lou, F.; Sun, Y.; Wang, B.; Jiang, B.; Bao, L.; Li, X.; Song, N.; et al. Lidocaine Ameliorates Psoriasis by Obstructing Pathogenic CGRP Signaling—Mediated Sensory Neuron—Dendritic Cell Communication. J. Investig. Dermatol. 2022, 142, 2173–2183.e6. [Google Scholar] [CrossRef]
- Salmon, A.-M.; Damaj, I.; Sekine, S.; Picciotto, M.R.; Marubio, L.; Changeux, J.-P. Modulation of morphine analgesia in αCGRP mutant mice. NeuroReport 1999, 10, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.A.; Miles, E.M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J. Physiol. 1952, 118, 228–257. [Google Scholar] [CrossRef]
- Zarban, A.A.; Chaudhry, H.; Maselli, D.; Kodji, X.; Valente, J.D.S.; Joachim, J.; Trevelin, S.C.; van Baardewijk, J.; Argunhan, F.; Ivetic, A.; et al. Enhancing techniques for determining inflammatory edema formation and neutrophil accumulation in murine skin. JID Innov. 2022; in press. [Google Scholar] [CrossRef]
- Macdonald, A.D.; Woolfe, G.; Bergel, F.; Morrison, A.L.; Rinderknecht, H. ANALGESIC ACTION OF PETHIDINE DERIVATIVES AND RELATED COMPOUNDS. Br. J. Pharmacol. Chemother. 1946, 1, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minett, M.S.; Quick, K.; Wood, J.N. Behavioral Measures of Pain Thresholds. Curr. Protoc. Mouse Biol. 2011, 1, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.D.S.; Alawi, K.M.; Keringer, P.; Bharde, S.; Ayaz, F.; Saleque, N.; Kodji, X.; Thapa, D.; Argunhan, F.; Brain, S.D. Examining the role of transient receptor potential canonical 5 (TRPC5) in osteoarthritis. Osteoarthr. Cartil. Open 2020, 2, 100119. [Google Scholar] [CrossRef] [PubMed]

| Expt Aim | Deplete Neuropeptides | Deplete Neuropeptides | Antagonise CGRP | Antagonise CGRP | CGRP Lacking | CGRP Lacking |
|---|---|---|---|---|---|---|
| Treatments/ Assays | RTX Oedema | RTX MPO | BIBN4096 Oedema | BIBN4096 MPO | CGRPKO Oedema | CGRPKO MPO |
| Tyrode | ns | ns | ns | ns | ns | ns |
| Carrageenan | p < 0.05 | p < 0.001 | p < 0.01 | p < 0.05 | p < 0.01 | p < 0.05 |
| TNFα | p < 0.01 | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarban, A.A.; Chaudhry, H.; de Sousa Valente, J.; Argunhan, F.; Ghanim, H.; Brain, S.D. Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin. Int. J. Mol. Sci. 2022, 23, 12246. https://doi.org/10.3390/ijms232012246
Zarban AA, Chaudhry H, de Sousa Valente J, Argunhan F, Ghanim H, Brain SD. Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin. International Journal of Molecular Sciences. 2022; 23(20):12246. https://doi.org/10.3390/ijms232012246
Chicago/Turabian StyleZarban, Ali A., Hiba Chaudhry, João de Sousa Valente, Fulye Argunhan, Hala Ghanim, and Susan D. Brain. 2022. "Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin" International Journal of Molecular Sciences 23, no. 20: 12246. https://doi.org/10.3390/ijms232012246
APA StyleZarban, A. A., Chaudhry, H., de Sousa Valente, J., Argunhan, F., Ghanim, H., & Brain, S. D. (2022). Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin. International Journal of Molecular Sciences, 23(20), 12246. https://doi.org/10.3390/ijms232012246







