KLK12 Regulates MMP-1 and MMP-9 via Bradykinin Receptors: Biomarkers for Differentiating Latent and Active Bovine Tuberculosis
Abstract
1. Introduction
2. Results
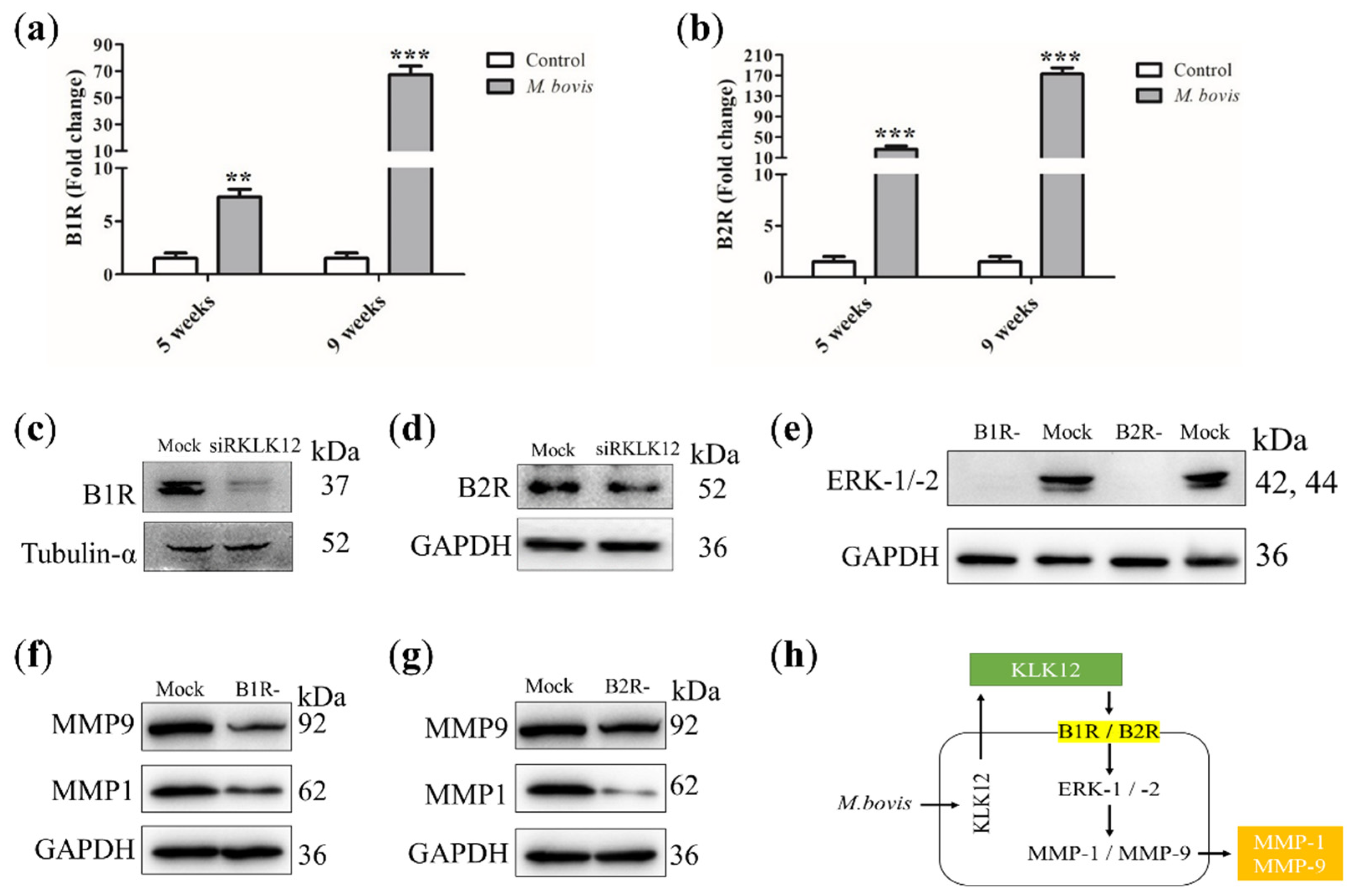
2.1. KLK12 Mediates MMP-1 and MMP-9 Expression
2.2. KLK12 Mediates MMP-1 and MMP-9 Expression via BR/ERK Signaling
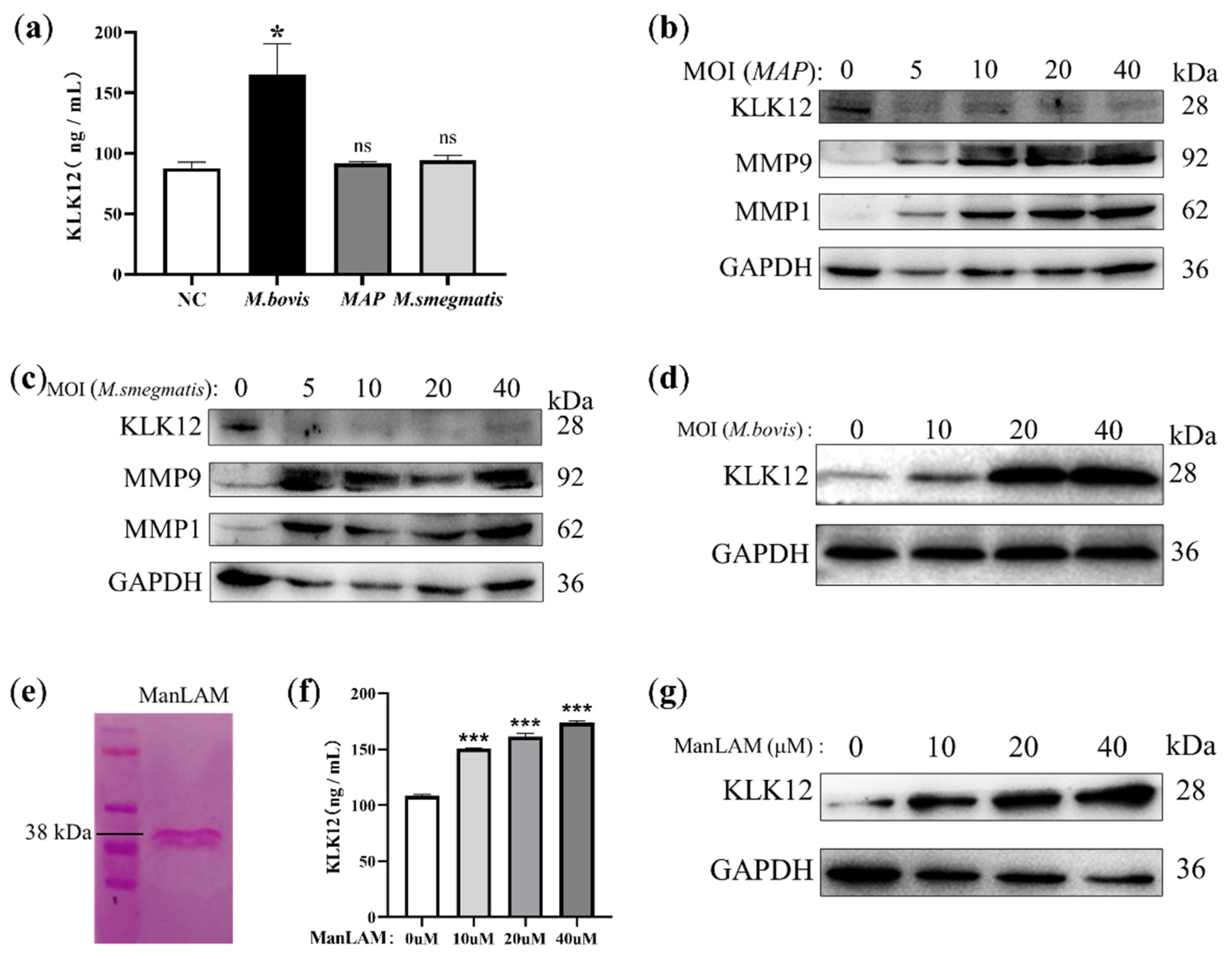
2.3. KLK12 Upregulation Is Mediated by the M. bovis-Specific Antigen ManLAM
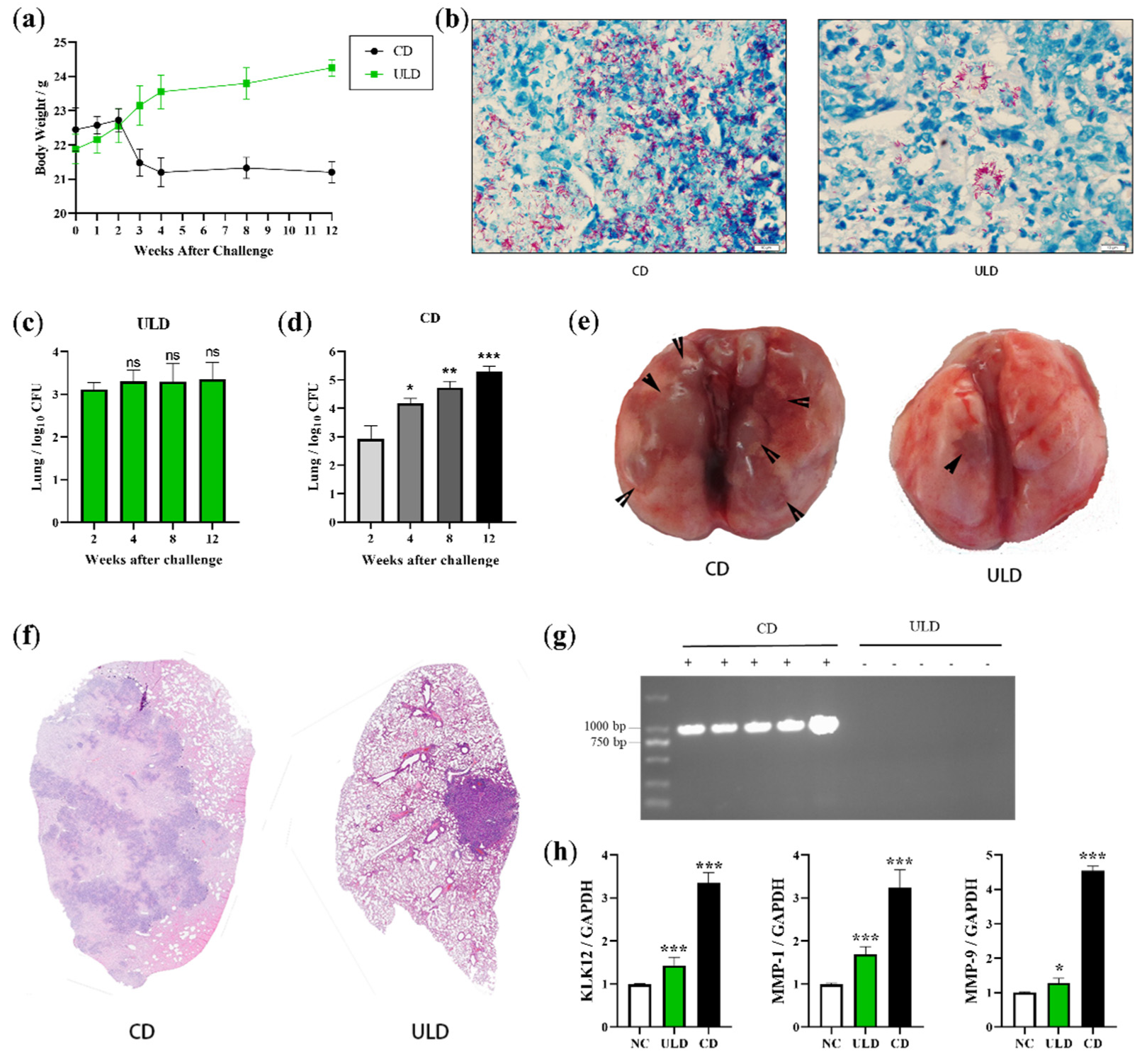
2.4. Establishment of LTBI Model in Mice Infected with Ultra-Low Doses of M. bovis
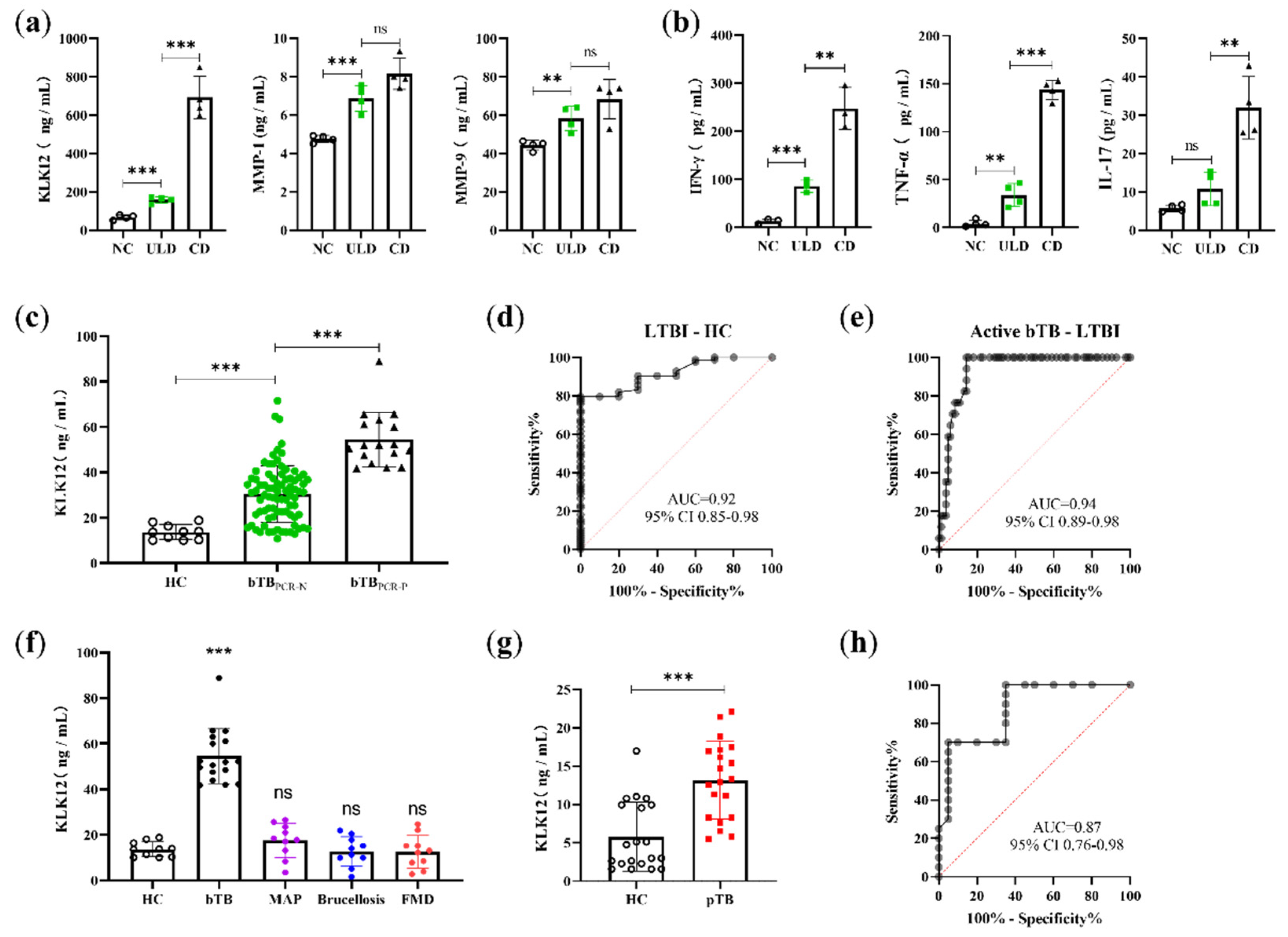
2.5. KLK12 Could Serve as a Serological Biomarker to Detect bTB Disease States
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Bacterial Culture
4.3. Extraction Method of ManLAM
4.4. siRNA Transfection
4.5. Bacterial Infection
4.6. Western Blot
4.7. Quantitative Real-Time PCR
4.8. ELISA
4.9. CFU Assay
4.10. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
| BR | Bradykinin receptors |
| MOI | Multiplicity of infection |
| POC | Point-of-care |
| ULD | Ultra-low dose |
| CD | Conventional dose |
| H&E | Hematoxylin and eosin |
| NC | Negative control |
| HC | Healthy control |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| CI | Confidence interval |
| FMD | Foot and mouth disease |
| TST | Tuberculin skin test |
References
- Pesciaroli, M.; Alvarez, J.; Boniotti, M.B.; Cagiola, M.; Marco, V.D.; Marianelli, C.; Pacciarini, M.; Pasquali, P. Tuberculosis in domestic animal species. Res. Vet. Sci. 2014, 97, S78–S85. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.A.; Whitney, E.; Raghunathan, P.; Cosivi, O. Epidemiology of selected mycobacteria that infect humans and other animals. Rev. Sci. Tech. 2001, 20, 325–337. [Google Scholar] [CrossRef] [PubMed]
- de la Rua-Domenech, R. Human Mycobacterium bovis infection in the United Kingdom: Incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 2006, 86, 77–109. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Gao, G.F.; Liu, C.H. Insights into battles between Mycobacterium tuberculosis and macrophages. Protein Cell 2014, 5, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007, 5, 39–47. [Google Scholar] [CrossRef]
- Russell, D.G.; Barry, C.E., 3rd; Flynn, J.L. Tuberculosis: What we don’t know can, and does, hurt us. Science 2010, 328, 852–856. [Google Scholar] [CrossRef]
- Houghton, A.M. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015, 44, 167–174. [Google Scholar] [CrossRef]
- Azad, A.K.; Sadee, W.; Schlesinger, L.S. Innate immune gene polymorphisms in tuberculosis. Infect. Immun. 2012, 80, 3343–3359. [Google Scholar] [CrossRef]
- Simmer, J.P.; Richardson, A.S.; Smith, C.E.; Hu, Y.; Hu, J.C. Expression of kallikrein-related peptidase 4 in dental and non-dental tissues. Eur. J. Oral Sci. 2011, 119, 226–233. [Google Scholar] [CrossRef]
- Waeckel, L.; Potier, L.; Richer, C.; Roussel, R.; Bouby, N.; Alhenc-Gelas, F. Pathophysiology of genetic deficiency in tissue kallikrein activity in mouse and man. Thromb. Haemost. 2013, 110, 476–483. [Google Scholar] [CrossRef]
- Cerqueira, C.; Ventayol, P.S.; Vogeley, C.; Schelhaas, M. Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space to Facilitate Entry into Host Cells. J. Virol. 2015, 89, 7038–7052. [Google Scholar] [CrossRef] [PubMed]
- Herring, A.; Münster, Y.; Akkaya, T.; Moghaddam, S.; Deinsberger, K.; Meyer, J.; Zahel, J.; Sanchez-Mendoza, E.; Wang, Y.; Hermann, D.M.; et al. Kallikrein-8 inhibition attenuates Alzheimer’s disease pathology in mice. Alzheimer Dement. 2016, 12, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Magnen, M.; Gueugnon, F.; Guillon, A.; Baranek, T.; Thibault, V.V.; Petit-Courty, A.; Veer, S.J.; Harris, J.; Humbles, A.A.; Si-Tahar, M.; et al. Kallikrein-Related Peptidase 5 Contributes to H3N2 Influenza Virus Infection in Human Lungs. J. Virol. 2017, 91, e00421-17. [Google Scholar] [CrossRef] [PubMed]
- Yousef, G.M.; Pasic, M.; Diamandis, E.P. Highlight: The 5th International Symposium on Kallikreins and Kallikrein-Related Peptidases. Biol. Chem. 2014, 395, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Papachristopoulou, G.; Tsapralis, N.; Michaelidou, K.; Ardavanis-Loukeris, G.; Griniatsos, I.; Scorilas, A.; Talieri, M. Human kallikrein-related peptidase 12 (KLK12) splice variants expression in breast cancer and their clinical impact. Clin. Biochem. 2018, 58, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Roseweir, A.K.; Park, J.H.; Hoorn, S.T.; Powell, A.G.; Aherne, S.; Roxburgh, C.S.; McMillan, D.C.; Horgan, P.G.; Ryan, E.; Sheahan, K.; et al. Histological phenotypic subtypes predict recurrence risk and response to adjuvant chemotherapy in patients with stage III colorectal cancer. J. Pathol. Clin. Res. 2020, 6, 283–296. [Google Scholar] [CrossRef]
- Kimura, A.; Kihara, T.; Ohkura, R.; Ogiwara, K.; Takahashi, T. Localization of bradykinin B (2) receptor in the follicles of porcine ovary and increased expression of matrix metalloproteinase-3 and -20 in cultured granulosa cells by bradykinin treatment. Biol. Reprod. 2001, 65, 1462–1470. [Google Scholar] [CrossRef]
- Schanstra, J.P.; Neau, E.; Drogoz, P.; Gomez, M.A.A.; Novoa, J.M.L.; Calise, D.; Pecher, C.; Bader, M.; Girolami, J.P.; Bascands, J.L. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J. Clin. Investig. 2002, 110, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zaczynska, E.; Gabra, B.H.; Sirois, P. Bradykinin stimulates MMP-2 production in guinea pig tracheal smooth muscle cells. Inflammation 2003, 27, 307–315. [Google Scholar] [CrossRef]
- Ehrenfeld, P.; Conejeros, I.; Pavicic, M.F.; Matus, C.E.; Gonzalez, C.B.; Quest, A.F.G.; Bhoola, K.D.; Poblete, M.T.; Burgos, R.A.; Figueroa, C.D. Activation of kinin B1 receptor increases the release of metalloproteases-2 and -9 from both estrogen-sensitive and-insensitive breast cancer cells. Cancer Lett. 2011, 301, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Yang, X.; Crosson, C.E. Bradykinin activation of extracellular signal-regulated kinases in human trabecular meshwork cells. Exp. Eye Res. 2011, 92, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Blaber, S.I.; Li, W.; Scarisbrick, I.A.; Blaber, M. Activation profiles of human kallikrein-related peptidases by matrix metalloproteinases. Biol. Chem. 2013, 394, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Sabir, N.; Hussain, T.; Mangi, M.H.; Zhao, D.; Zhou, X. Matrix metalloproteinases: Expression, regulation and role in the immunopathology of tuberculosis. Cell Prolif. 2019, 52, e12649. [Google Scholar] [CrossRef] [PubMed]
- Hmama, Z.; Pena-Diaz, S.; Joseph, S.; Av-Gay, Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 2015, 264, 220–232. [Google Scholar] [CrossRef]
- Guerardel, Y.; Maes, E.; Briken, V.; Chirat, F.; Leroy, Y.; Locht, C.; Strecker, G.; Kremer, L. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: Novel structural features and apoptosis-inducing properties. J. Biol. Chem. 2003, 278, 36637–36651. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Kurihara, H.; Morita, Y.S.; Kinoshita, T.; Mauri, L.; Prinetti, A.; Sonnino, S.; Yokoyama, N.; Ogawa, H.; Takamori, K.; et al. Lipoarabinomannan binding to lactosylceramide in lipid rafts is essential for the phagocytosis of mycobacteria by human neutrophils. Sci. Signal 2016, 9, ra101. [Google Scholar] [CrossRef]
- Boom, W.H.; Schaible, U.E.; Achkar, J.M. The knowns and unknowns of latent Mycobacterium tuberculosis infection. J. Clin. Investig. 2021, 131, e136222. [Google Scholar] [CrossRef]
- Elkington, P.T.G.; Emerson, J.E.; Lopez-Pascua, L.D.C.; O’Kane, C.M.; Horncastle, D.E.; Boyle, J.J.; Friedland, J.S. Mycobacterium tuberculosis Up-Regulates Matrix Metalloproteinase-1 Secretion from Human Airway Epithelial Cells via a p38 MAPK Switch. J. Immunol. 2005, 175, 5333–5340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Moideen, K.; Viswanathan, V.; Shruthi, B.S.; Sivakumar, S.; Menon, P.A.; Kornfeld, H.; Babu, S. Elevated levels of matrix metalloproteinases reflect severity and extent of disease in tuberculosis-diabetes co-morbidity and are predominantly reversed following standard antituberculosis or metformin treatment. BMC Infect. Dis. 2018, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; Magni, R.; Zaidi, F.; Araujo, R.; Saini, N.; Harpole, M.; Coronel, J.; Kirwan, D.E.; Steinberg, H.; Gilman, R.H.; et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci. Transl. Med. 2017, 9, eaal2807. [Google Scholar] [CrossRef]
- Plumlee, C.R.; Duffy, F.J.; Gern, B.H.; Delahaye, J.L.; Cohen, S.B.; Stoltzfus, C.R.; Rustad, T.R.; Hansen, S.G.; Axthelm, M.K.; Picker, L.J.; et al. Ultra-low Dose Aerosol Infection of Mice with Mycobacterium tuberculosis More Closely Models Human Tuberculosis. Cell Host Microbe 2021, 29, 68–82. [Google Scholar] [CrossRef]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mercer, B.A.; Kolesnikova, N.; Sonett, J.; D’Armiento, J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J. Biol. Chem. 2004, 279, 17690–17696. [Google Scholar] [CrossRef]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Izzo, A.A.; Izzo, L.S.; Kasimos, J.; Majka, S. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis 2004, 84, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pando, R.; Orozco, H.; Arriaga, K.; Pavön, L.; Rook, G. Treatment with BB-94, a broad spectrum inhibitor of zinc-dependent metalloproteinases, causes deviation of the cytokine profile towards type-2 in experimental pulmonary tuberculosis in Balb/c mice. Int. J. Exp. Pathol. 2000, 81, 199–209. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hawn, T.R.; Matheson, A.I.; Maley, S.N.; Vandal, O. Host-directed therapeutics for tuberculosis: Can we harness the host? Microbiol. Mol. Biol. 2013, 7, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Zimmerman, M.D.; Chen, K.; Huang, L.; Fu, D.; Kaya, F.; Rakhilin, N.; Nazarova, E.V.; Bu, P.; et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog. 2018, 14, e1006974. [Google Scholar] [CrossRef]
- Lou, J.; Wang, Y.; Zhang, Z.; Qiu, W. Activation of MMPs in Macrophages by Mycobacterium tuberculosis via the miR-223-BMAL1 Signaling Pathway. J. Cell Biochem. 2017, 118, 4804–4812. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.B.; Bayless, K.J.; Davis, G.E. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J. Cell Sci. 2005, 118, 2325–2340. [Google Scholar] [CrossRef]
- Rivera-Marrero, C.A.; Schuyler, W.; Roser, S.; Ritzenthaler, J.D.; Newburn, S.A.; Roman, J.M. Tuberculosis induction of matrix metalloproteinase-9: The role of mannose and receptor-mediated mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L546–L555. [Google Scholar] [CrossRef] [PubMed]
- Rand, L.; Green, J.A.; Saraiva, L.; Friedland, J.S.; Elkington, P. Matrix metalloproteinase–1 is regulated in tuberculosis by a p38 MAPK–dependent, p–aminosalicylic acid–sensitive signaling cascade. J. Immunol. 2009, 182, 5865–5872. [Google Scholar] [CrossRef] [PubMed]
- Moores, R.C.; Brilha, S.; Schutgens, F. Epigenetic regulation of matrix metalloproteinase-1 and -3 expression in mycobacterium tuberculosis infection. Front. Immunol. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.T.; Green, J.A.; Emerson, J.E. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am. J. Respir. Cell Mol. Biol. 2007, 37, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Nigou, J.; Herrmann, J.L. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 2003, 278, 5513–5516. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.S.; Voelker, D.R.; McCormack, F.X. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydratelectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 1999, 163, 312–321. [Google Scholar]
- Schlesinger, L.S.; Hull, S.R.; Kaufman, T.M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 1994, 152, 4070–4079. [Google Scholar]
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections-a comparativeanalysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Horst, M.; Karamchand, L.; Bauer, W.S.; Nel, A.J.M.; Blackburn, J.M.; Wright, D.W. The cyanobacterial lectin, microvirin-N, enhances the specificity and sensitivity of lipoarabinomannan-based TB diagnostic tests. Analyst 2021, 146, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Torrelles, J.B. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty026. [Google Scholar] [CrossRef]
- Chan, C.E.; Götze, S.; Seah, G.T.; Seeberger, P.H.; Tukvadze, N.; Wenk, M.R.; Hanson, B.J.; MacAry, P.A. The diagnostic targeting of a carbohydrate virulence factor from M. tuberculosis. Sci. Rep. 2015, 5, 10281. [Google Scholar] [CrossRef] [PubMed]
- Sia, I.G.; Wieland, M.L. Current concepts in the management of tuberculosis. Mayo Clin. Proc. 2011, 86, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef] [PubMed]

| Role | AUC 1 | Sensitivity (%) | 95% CI 2 (%) | Specificity (%) | 95% CI (%) | Cutoff Value (ng/mL) |
|---|---|---|---|---|---|---|
| Identification of LTBI and healthy cattle | 0.92 | 81.93 | 72.30–88.73 | 80.00 | 49.02–96.45 | >16.49 |
| Identification of active bTB and LTBI cattle | 0.94 | 100.00 | 81.57–100.00 | 85.54 | 76.41–91.53 | >41.55 |
| Identification of active TB and healthy humans | 0.87 | 70.00 | 48.10–85.45 | 95.00 | 76.39–99.74 | >11.09 |
| Gene Name | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
| KLK12 | CAGCCAGACTCTCTGGTTCC | TCCAGCCCCTAGCTAACAGA |
| MMP-1 | TTCCCCAAATCCCATCCAGC | ACCCGAATGTAGAACCTGCC |
| MMP-9 | TACTGGGCGTTAGGGACAGA | TAACGCACAGACCCCCTCTA |
| B1R | AGCACCAGCTGCTCATCTACC | GACCGGCGAAAGAGGATATAGAC |
| B2R | CAGCACCTTCCTGGATACGCTGCATC | CACCTCCCAAGACTTCTTTCGGAAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qu, M.; Liu, Y.; Wang, H.; Dong, Y.; Zhou, X. KLK12 Regulates MMP-1 and MMP-9 via Bradykinin Receptors: Biomarkers for Differentiating Latent and Active Bovine Tuberculosis. Int. J. Mol. Sci. 2022, 23, 12257. https://doi.org/10.3390/ijms232012257
Wang Y, Qu M, Liu Y, Wang H, Dong Y, Zhou X. KLK12 Regulates MMP-1 and MMP-9 via Bradykinin Receptors: Biomarkers for Differentiating Latent and Active Bovine Tuberculosis. International Journal of Molecular Sciences. 2022; 23(20):12257. https://doi.org/10.3390/ijms232012257
Chicago/Turabian StyleWang, Yuanzhi, Mengjin Qu, Yiduo Liu, Haoran Wang, Yuhui Dong, and Xiangmei Zhou. 2022. "KLK12 Regulates MMP-1 and MMP-9 via Bradykinin Receptors: Biomarkers for Differentiating Latent and Active Bovine Tuberculosis" International Journal of Molecular Sciences 23, no. 20: 12257. https://doi.org/10.3390/ijms232012257
APA StyleWang, Y., Qu, M., Liu, Y., Wang, H., Dong, Y., & Zhou, X. (2022). KLK12 Regulates MMP-1 and MMP-9 via Bradykinin Receptors: Biomarkers for Differentiating Latent and Active Bovine Tuberculosis. International Journal of Molecular Sciences, 23(20), 12257. https://doi.org/10.3390/ijms232012257







