Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing
Abstract
1. Introduction
2. Results
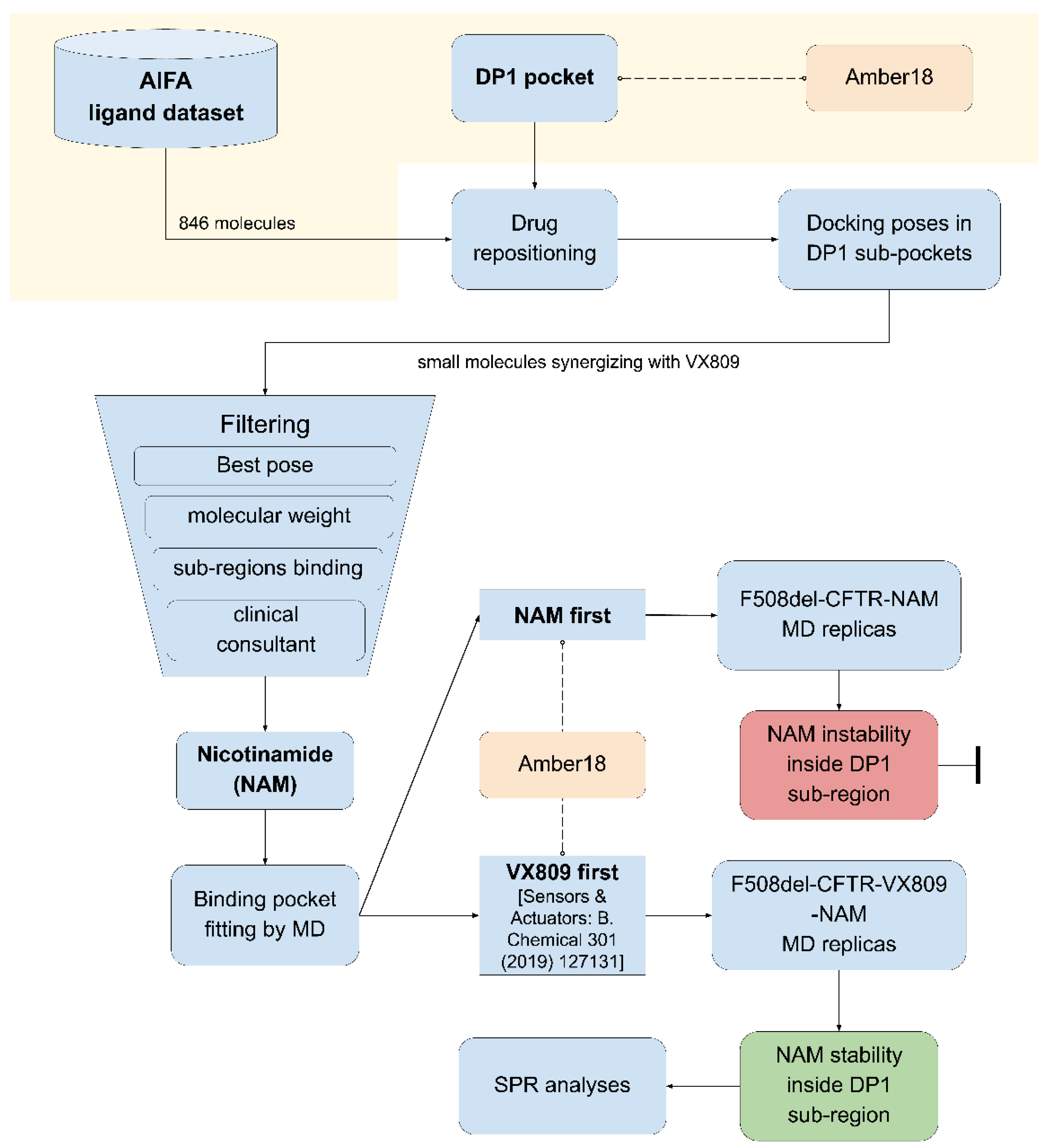
2.1. Computational Drug Repositioning Pipeline to Identify Small Molecules Able to Interact with the Lumacaftor-Binding Region of F508del-CFTR
2.2. Clinical Assessment and Selection of the Repositioned Small Compounds
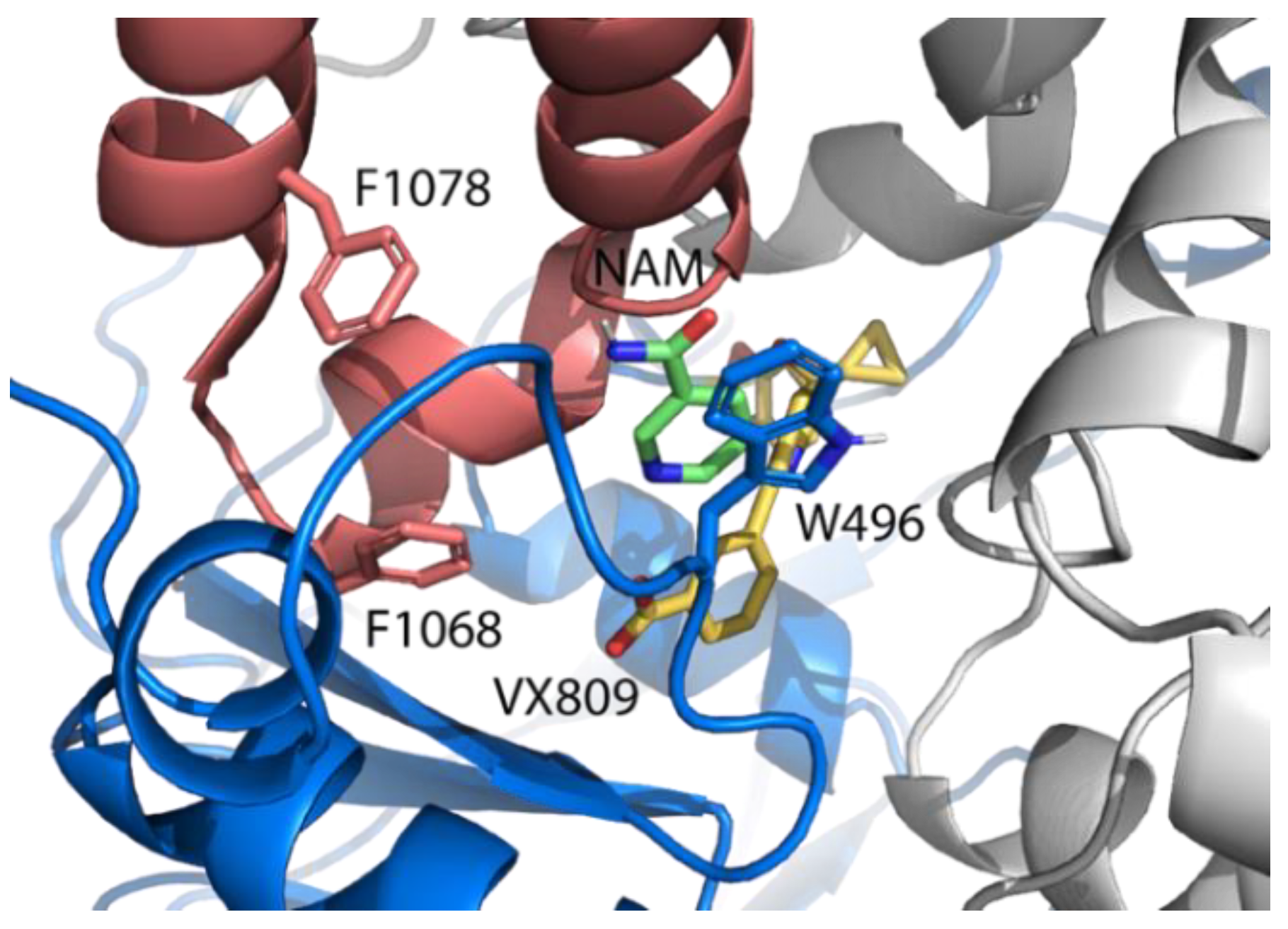
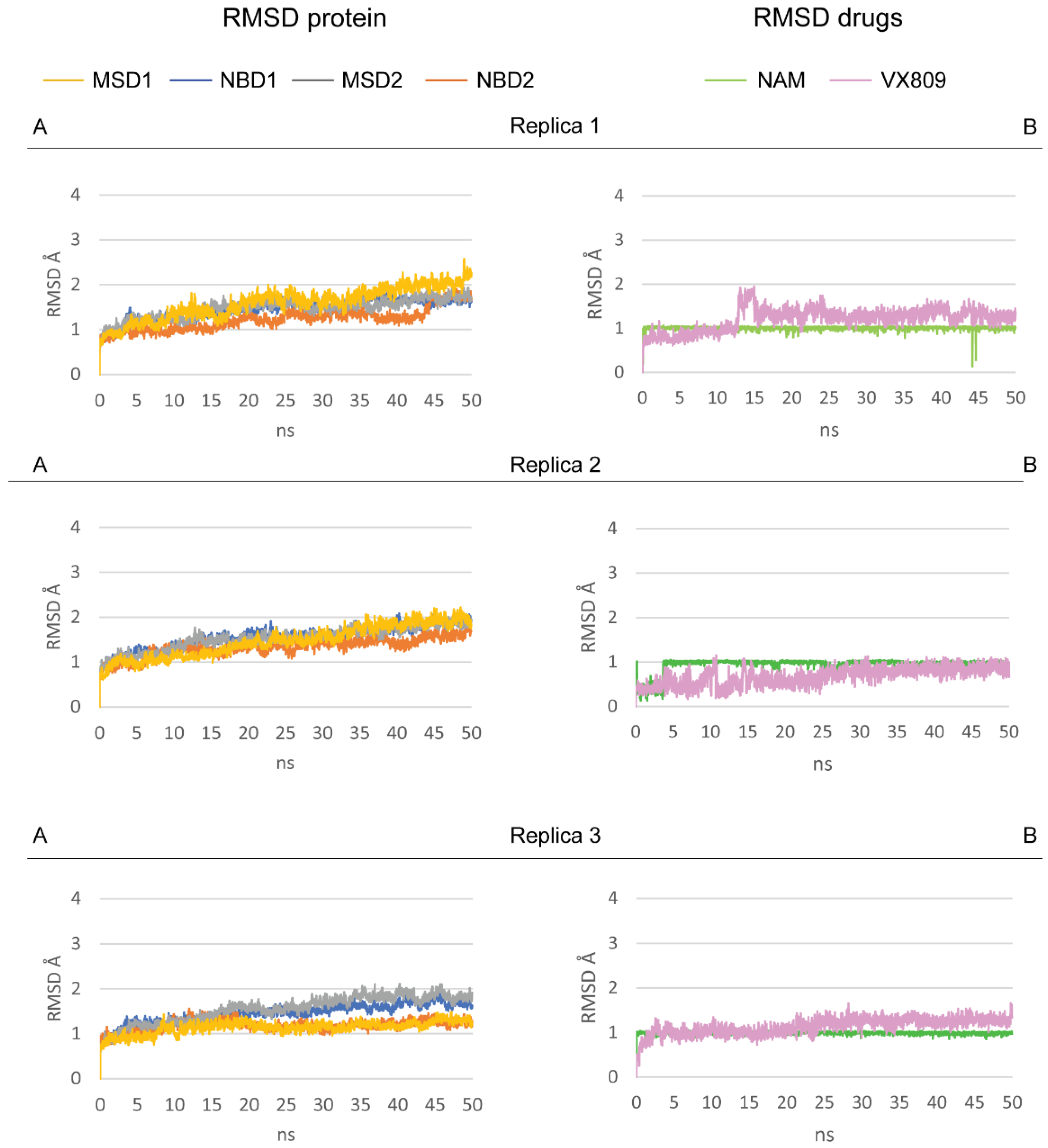
2.3. Pocket Fitting Evaluation for Lumacaftor and NAM: Which Molecule Binds First?
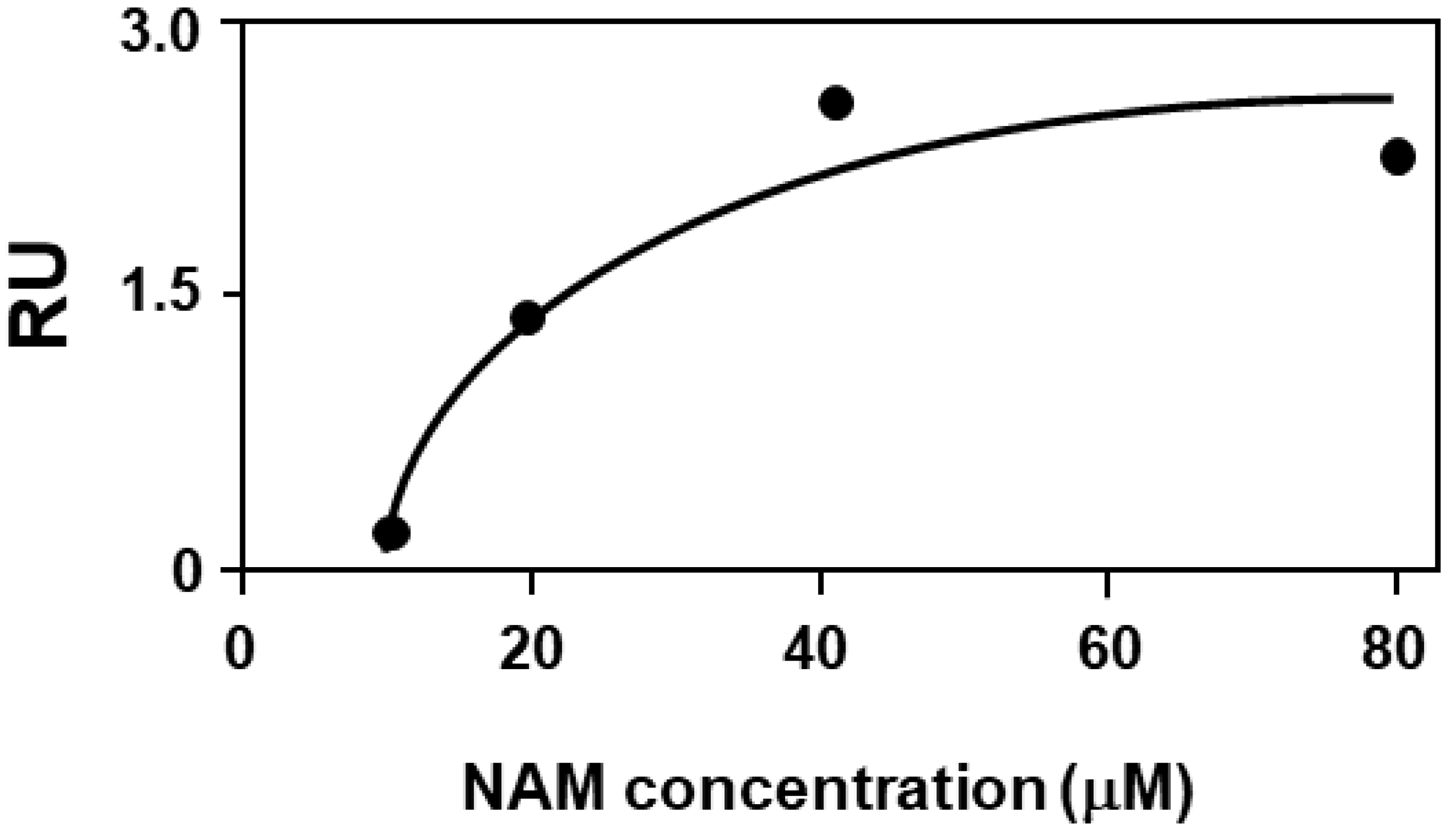
2.4. Biochemical Validation: Binding Assay of NAM to F508del-CFTR by Surface Plasmon Resonance
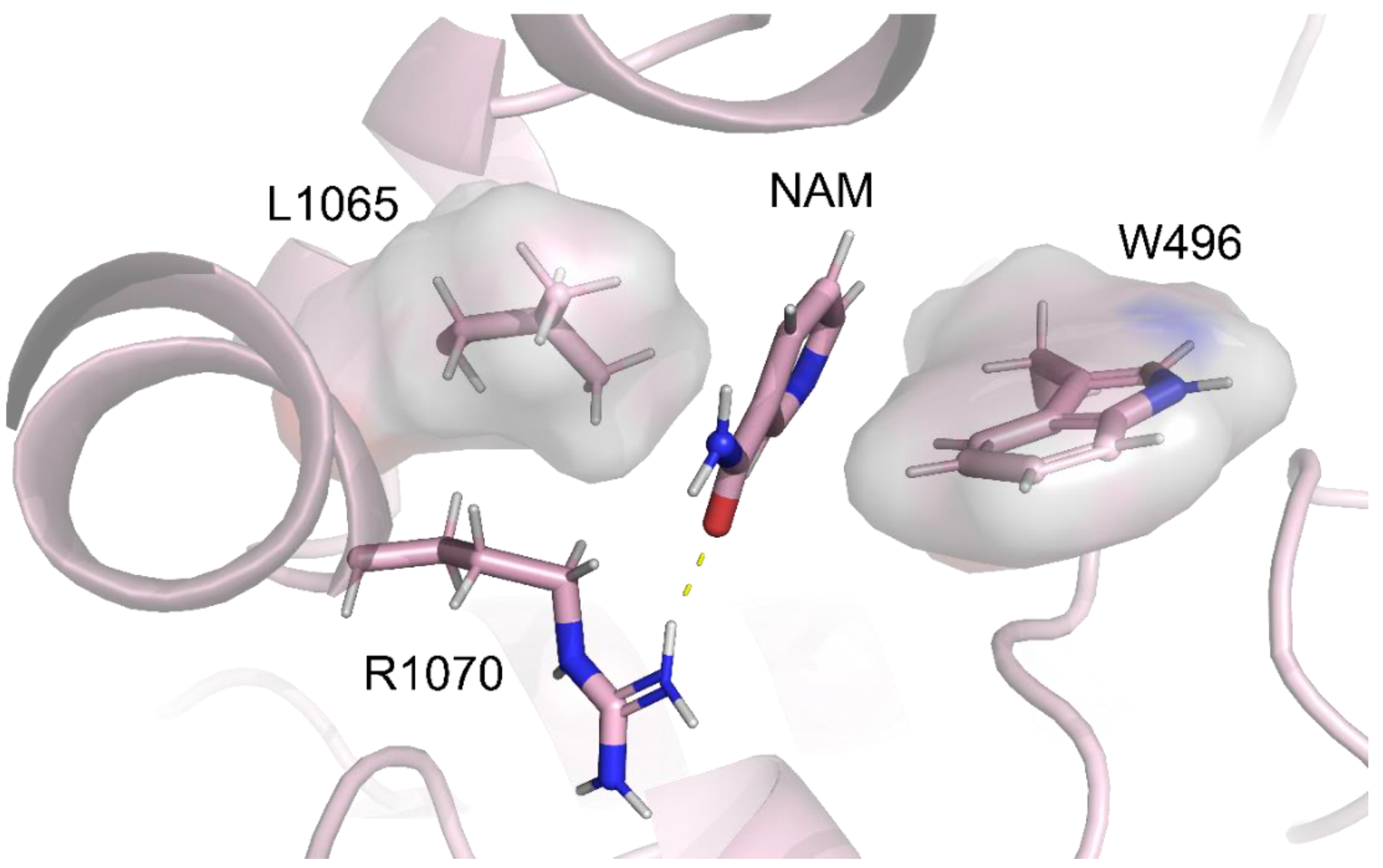
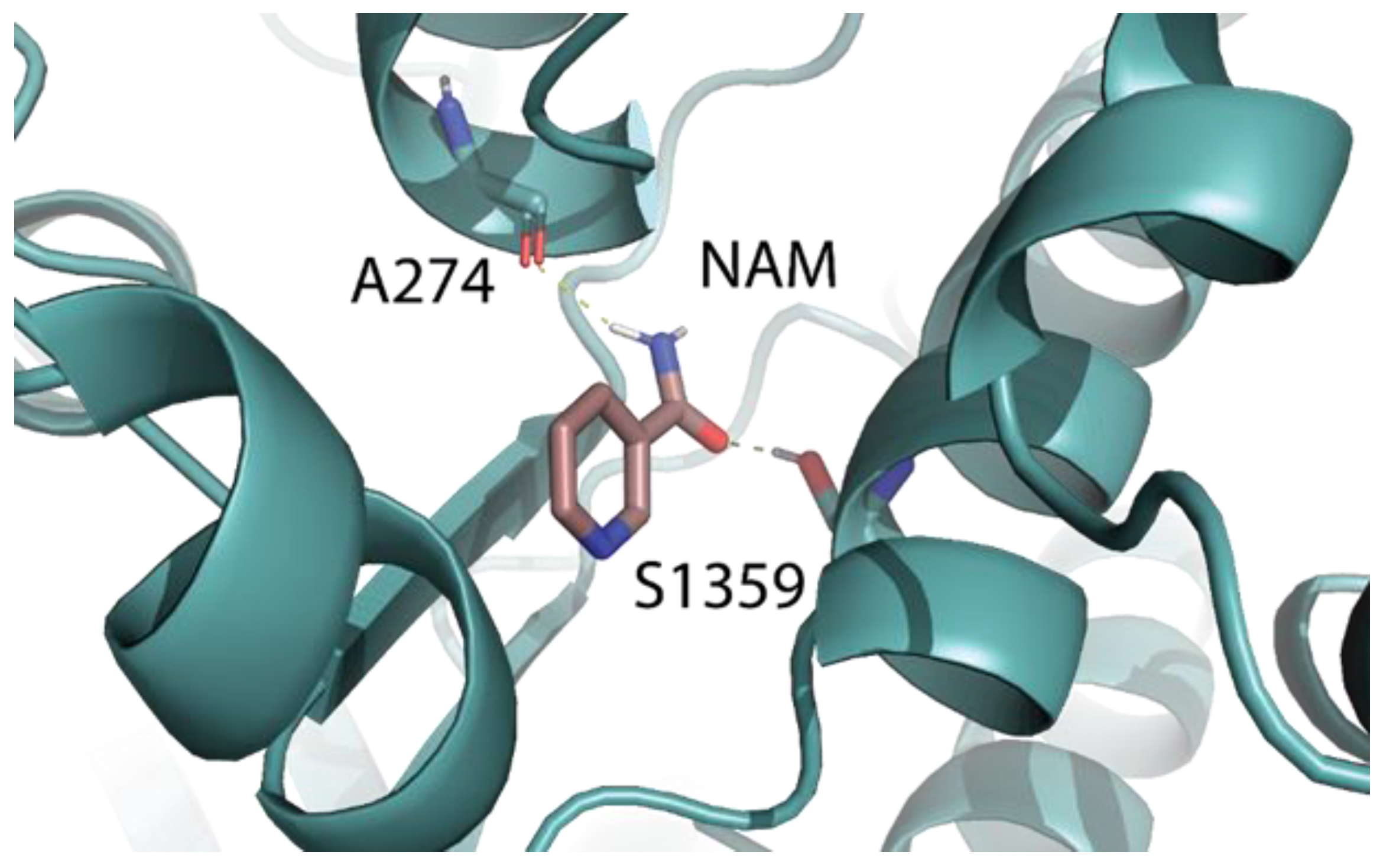
2.5. Identification of the Binding Pocket of NAM
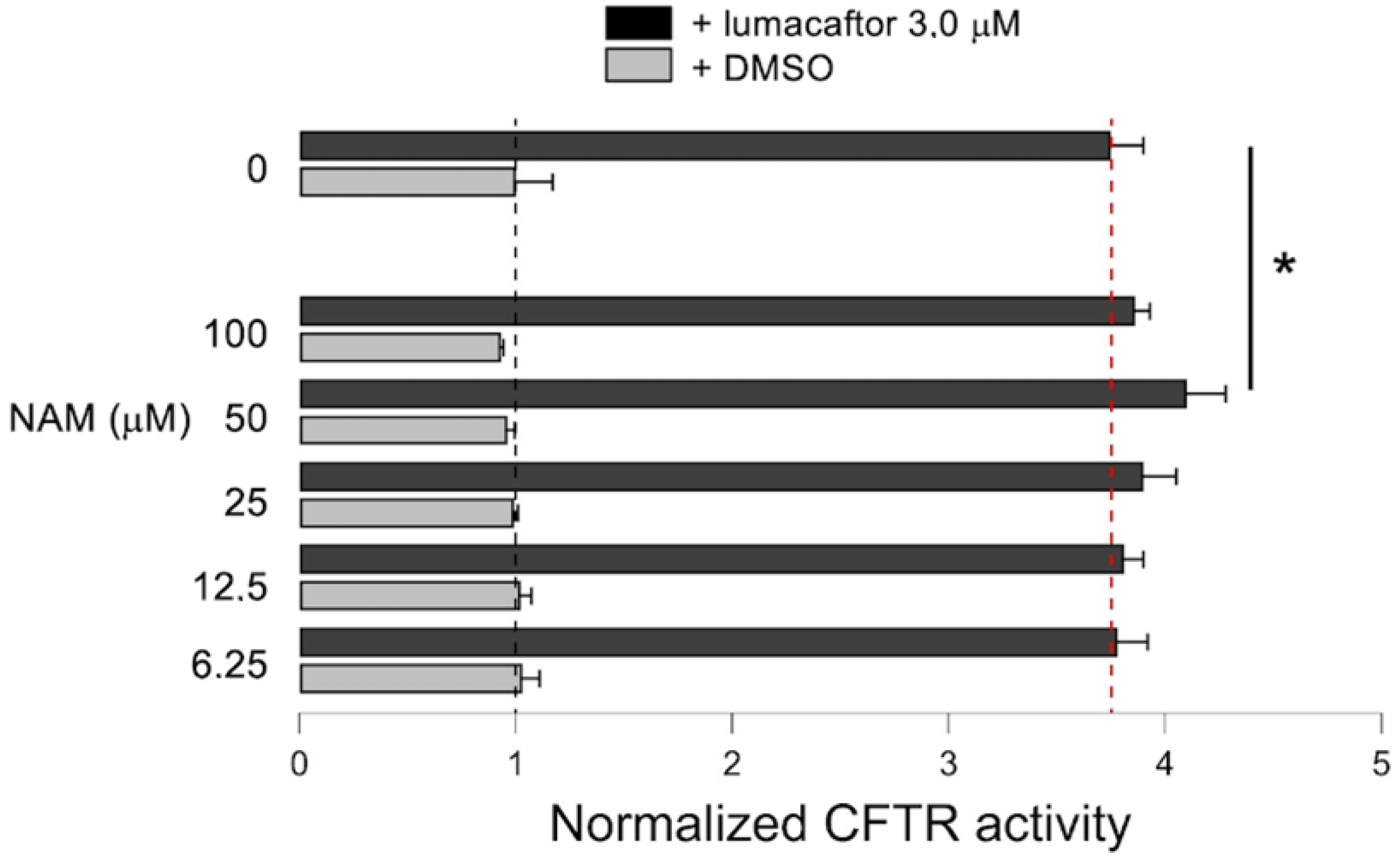
2.6. Effect of NAM-Lumacaftor Co-Treatment on Mutant CFTR Rescue
3. Discussion
4. Materials and Methods
4.1. Drug Repositioning Pipeline
4.2. AIFA Ligand Dataset
4.3. Molecular Docking Analysis
4.4. Molecular Dynamic Simulations
4.5. Data Curation
4.6. Reagents
4.7. Surface Plasmon Resonance
4.8. YFP-Based Assay for CFTR Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From Cftr Biology toward Combinatorial Pharmacotherapy: Expanded Classification of Cystic Fibrosis Mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Rivas Caldas, R.; Boisrame, S. Upper Aero-Digestive Contamination by Pseudomonas Aeruginosa and Implications in Cystic Fibrosis. J. Cyst. Fibros. 2015, 14, 6–15. [Google Scholar] [CrossRef]
- Scott-Ward, T.S.; Amaral, M.D. Deletion of Phe508 in the First Nucleotide-Binding Domain of the Cystic Fibrosis Transmembrane Conductance Regulator Increases Its Affinity for the Heat Shock Cognate 70 Chaperone. FEBS J. 2009, 276, 7097–7109. [Google Scholar] [CrossRef]
- Amaral, M.D.; Farinha, C.M. Rescuing Mutant Cftr: A Multi-Task Approach to a Better Outcome in Treating Cystic Fibrosis. Curr. Pharm. Des. 2013, 19, 3497–3508. [Google Scholar] [CrossRef]
- Rogan, M.P.; DStoltz, A.; Hornick, D.B. Cystic Fibrosis Transmembrane Conductance Regulator Intracellular Processing, Trafficking, and Opportunities for Mutation-Specific Treatment. Chest 2011, 139, 1480–1490. [Google Scholar] [CrossRef]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of Cystic Fibrosis: Cftr Mutation Classifications toward Genotype-Based Cf Therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Bierlaagh, M.C.; Muilwijk, D.; Beekman, J.M.; van der Ent, C.K. A New Era for People with Cystic Fibrosis. Eur. J. Pediatr. 2021, 180, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, D.; Villella, A.; Bastos, C.; King, R.; Finley, D.; Kelly, J.W.; Morimoto, R.I.; Hartl, F.U.; Munoz, B.; Lee, P.S.; et al. Amplifiers Co-Translationally Enhance Cftr Biosynthesis Via Pcbp1-Mediated Regulation of Cftr Mrna. J. Cyst. Fibros. 2020, 19, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Ponzano, S.; GNigrelli; Fregonese, L.; Eichler, I.; Bertozzi, F.; Bandiera, T.; Galietta, L.J.V.; Papaluca, M. A European Regulatory Perspective on Cystic Fibrosis: Current Treatments, Trends in Drug Development and Translational Challenges for Cftr Modulators. Eur. Respir. Rev. 2018, 27, 170124. [Google Scholar] [CrossRef]
- Habib, A.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A Systematic Review of the Clinical Efficacy and Safety of Cftr Modulators in Cystic Fibrosis. Sci. Rep. 2019, 9, 7234. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, K.; Chen, J. Mechanism of Cftr Correction by Type I Folding Correctors. Cell 2022, 185, 158–168.e11. [Google Scholar] [CrossRef] [PubMed]
- Baatallah, N.; Elbahnsi, A.; Mornon, J.P.; Chevalier, B.; Pranke, I.; Servel, N.; Zelli, R.; Decout, J.L.; Edelman, A.; Sermet-Gaudelus, I.; et al. Pharmacological Chaperones Improve Intra-Domain Stability and Inter-Domain Assembly Via Distinct Binding Sites to Rescue Misfolded Cftr. Cell. Mol. Life Sci. 2021, 78, 7813–7829. [Google Scholar] [CrossRef]
- Bitam, S.; Elbahnsi, A.; Creste, G.; Pranke, I.; Chevalier, B.; Berhal, F.; Hoffmann, B.; Servel, N.; Baatalah, N.; Tondelier, D.; et al. New Insights into Structure and Function of Bis-Phosphinic Acid Derivatives and Implications for Cftr Modulation. Sci. Rep. 2021, 11, 6842. [Google Scholar] [CrossRef] [PubMed]
- Haggie, P.M.; Phuan, P.W.; Tan, J.A.; Xu, H.; Avramescu, R.G.; Perdomo, D.; Zlock, L.; Nielson, D.W.; Finkbeiner, W.E.; Lukacs, G.L.; et al. Correctors and Potentiators Rescue Function of the Truncated W1282x-Cystic Fibrosis Transmembrane Regulator (Cftr) Translation Product. J. Biol. Chem. 2017, 292, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; MKleinman, E.; Miller, D.T.; Neuberg, D.S.; Giobbie-Hurder, A.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.; Snyder, B.D.; et al. Clinical Trial of a Farnesyltransferase Inhibitor in Children with Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16666–16671. [Google Scholar] [CrossRef] [PubMed]
- Roessler, H.I.; Knoers, N.; van Haelst, M.M.; van Haaften, G. Drug Repurposing for Rare Diseases. Trends Pharmacol. Sci. 2021, 42, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Polamreddy, P.; Gattu, N. The Drug Repurposing Landscape from 2012 to 2017: Evolution, Challenges, and Possible Solutions. Drug Discov. Today 2019, 24, 789–795. [Google Scholar] [CrossRef]
- Farghali, H.; Canova, N.K.; Arora, M. The Potential Applications of Artificial Intelligence in Drug Discovery and Development. Physiol. Res. 2021, 70 (Suppl. S4), S715–S722. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Meenakshisundaram, S.; Manickam, M.; Sankaranarayanan, M. A Medicinal Chemistry Perspective of Drug Repositioning: Recent Advances and Challenges in Drug Discovery. Eur. J. Med. Chem. 2020, 195, 112275. [Google Scholar] [CrossRef]
- Park, K. A Review of Computational Drug Repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef]
- Lara-Ramirez, E.E.; Lopez-Cedillo, J.C.; Nogueda-Torres, B.; Kashif, M.; Garcia-Perez, C.; Bocanegra-Garcia, V.; Agusti, R.; Uhrig, M.L.; Rivera, G. An in Vitro and in Vivo Evaluation of New Potential Trans-Sialidase Inhibitors of Trypanosoma Cruzi Predicted by a Computational Drug Repositioning Method. Eur. J. Med. Chem. 2017, 132, 249–261. [Google Scholar] [CrossRef]
- Newman, S.P. Delivering Drugs to the Lungs: The History of Repurposing in the Treatment of Respiratory Diseases. Adv. Drug Deliv. Rev. 2018, 133, 5–18. [Google Scholar] [CrossRef]
- Anderson, S.D.; Daviskas, E.; Brannan, J.D.; Chan, H.K. Repurposing Excipients as Active Inhalation Agents: The Mannitol Story. Adv. Drug Deliv. Rev. 2018, 133, 45–56. [Google Scholar] [CrossRef]
- Orro, A.; Uggeri, M.; Rusnati, M.; Urbinati, C.; Pedemonte, N.; Pesce, E.; Moscatelli, M.; Padoan, R.; Cichero, E.; Fossa, P.; et al. In Silico Drug Repositioning on F508del-Cftr: A Proof-of-Concept Study on the Aifa Library. Eur. J. Med. Chem. 2021, 213, 113186. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.A.; Turck, D. Cystic Fibrosis: Nutritional Consequences and Management. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Portal, C.; Gouyer, V.; Leonard, R.; Husson, M.O.; Gottrand, F.; Desseyn, J.L. Long-Term Dietary (N-3) Polyunsaturated Fatty Acids Show Benefits to the Lungs of Cftr F508del Mice. PLoS ONE 2018, 13, e0197808. [Google Scholar] [CrossRef]
- Fangous, M.S.; Lazzouni, I.; Alexandre, Y.; Gouriou, S.; Boisrame, S.; Vallet, S.; le Bihan, J.; Ramel, S.; Hery-Arnaud, G.; le Berre, R. Prevalence and Dynamics of Lactobacillus Sp. In the Lower Respiratory Tract of Patients with Cystic Fibrosis. Res. Microbiol. 2018, 169, 222–226. [Google Scholar] [CrossRef]
- Sagel, S.D.; Khan, U.; Jain, R.; Graff, G.; Daines, C.L.; Dunitz, J.M.; Borowitz, D.; Orenstein, D.M.; Abdulhamid, I.; Noe, J.; et al. Effects of an Antioxidant-Enriched Multivitamin in Cystic Fibrosis. A Randomized, Controlled, Multicenter Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 639–647. [Google Scholar] [CrossRef]
- D’Ursi, P.; Uggeri, M.; Urbinati, C.; Millo, E.; Paiardi, G.; Milanesi, L.; Ford, R.C.; Clews, J.; Meng, X.; Bergese, P.; et al. Exploitation of a Novel Biosensor Based on the Full-Length Human F508de1-Cftr with Computational Studies, Biochemical and Biological Assays for the Characterization of a New Lumacaftor/Tezacaftor Analogue. Sens. Actuators B-Chem. 2019, 301, 127131. [Google Scholar] [CrossRef]
- Hudson, R.P.; Dawson, J.E.; Chong, P.A.; Yang, Z.; Millen, L.; Thomas, P.J.; Brouillette, C.G.; Forman-Kay, J.D. Direct Binding of the Corrector Vx-809 to Human Cftr Nbd1: Evidence of an Allosteric Coupling between the Binding Site and the Nbd1:Cl4 Interface. Mol. Pharmacol. 2017, 92, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Okiyoneda, T.; Veit, G.; Dekkers, J.F.; Bagdany, M.; Soya, N.; Xu, H.; Roldan, A.; Verkman, A.S.; Kurth, M.; Simon, A.; et al. Mechanism-Based Corrector Combination Restores Deltaf508-Cftr Folding and Function. Nat. Chem. Biol. 2013, 9, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, M.K.; Tricco, A.C.; Tashkandi, M.; Mamdani, M.; Straus, S.E.; Ba Hammam, A.S. Sodium Oxybate for Narcolepsy with Cataplexy: Systematic Review and Meta-Analysis. J. Clin. Sleep Med. 2012, 8, 451–458. [Google Scholar] [CrossRef]
- Keating, G.M. Sodium Oxybate: A Review of Its Use in Alcohol Withdrawal Syndrome and in the Maintenance of Abstinence in Alcohol Dependence. Clin. Drug Investig. 2014, 34, 63–80. [Google Scholar] [CrossRef]
- Kahaly, G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef]
- Truitt, C.; Hoff, W.D.; Deole, R. Health Functionalities of Betaine in Patients with Homocystinuria. Front. Nutr. 2021, 8, 690359. [Google Scholar] [CrossRef]
- Kasimer, R.N.; Langman, C.B. Adult Complications of Nephropathic Cystinosis: A Systematic Review. Pediatr. Nephrol. 2021, 36, 223–236. [Google Scholar] [CrossRef]
- Besouw, M.; Masereeuw, R.; van den Heuvel, L.; Levtchenko, E. Cysteamine: An Old Drug with New Potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef]
- Devereux, G.; Steele, S.; Griffiths, K.; Devlin, E.; Fraser-Pitt, D.; Cotton, S.; Norrie, J.; Chrystyn, H.; O’Neil, D. An Open-Label Investigation of the Pharmacokinetics and Tolerability of Oral Cysteamine in Adults with Cystic Fibrosis. Clin. Drug Investig. 2016, 36, 605–612. [Google Scholar] [CrossRef]
- Tosco, A.; de Gregorio, F.; Esposito, S.; de Stefano, D.; Sana, I.; Ferrari, E.; Sepe, A.; Salvadori, L.; Buonpensiero, P.; di Pasqua, A.; et al. A Novel Treatment of Cystic Fibrosis Acting on-Target: Cysteamine Plus Epigallocatechin Gallate for the Autophagy-Dependent Rescue of Class Ii-Mutated Cftr. Cell Death Differ. 2016, 23, 1380–1393. [Google Scholar] [CrossRef]
- De Stefano, D.; Villella, V.R.; Esposito, S.; Tosco, A.; Sepe, A.; de Gregorio, F.; Salvadori, L.; Grassia, R.; Leone, C.A.; de Rosa, G.; et al. Restoration of Cftr Function in Patients with Cystic Fibrosis Carrying the F508del-Cftr Mutation. Autophagy 2014, 10, 2053–2074. [Google Scholar] [CrossRef]
- Devereux, G.; Wrolstad, D.; Bourke, S.J.; Daines, C.L.; Doe, S.; Dougherty, R.; Franco, R.; Innes, A.; Kopp, B.T.; Lascano, J.; et al. Oral Cysteamine as an Adjunct Treatment in Cystic Fibrosis Pulmonary Exacerbations: An Exploratory Randomized Clinical Trial. PLoS ONE 2020, 15, e0242945. [Google Scholar] [CrossRef]
- Villella, V.R.; Esposito, S.; Maiuri, M.C.; Raia, V.; Kroemer, G.; Maiuri, L. Towards a Rational Combination Therapy of Cystic Fibrosis: How Cystamine Restores the Stability of Mutant Cftr. Autophagy 2013, 9, 1431–1434. [Google Scholar] [CrossRef]
- Ferrari, E.; Monzani, R.; Villella, V.R.; Esposito, S.; Saluzzo, F.; Rossin, F.; D’Eletto, M.; Tosco, A.; de Gregorio, F.; Izzo, V.; et al. Cysteamine Re-Establishes the Clearance of Pseudomonas Aeruginosa by Macrophages Bearing the Cystic Fibrosis-Relevant F508del-Cftr Mutation. Cell Death Dis. 2017, 8, e2544. [Google Scholar] [CrossRef]
- Charrier, C.; Rodger, C.; Robertson, J.; Kowalczuk, A.; Shand, N.; Fraser-Pitt, D.; Mercer, D.; O’Neil, D. Cysteamine (Lynovex(R)), a Novel Mucoactive Antimicrobial & Antibiofilm Agent for the Treatment of Cystic Fibrosis. Orphanet. J. Rare Dis. 2014, 9, 189. [Google Scholar]
- Maiuri, L.; Raia, V.; Kroemer, G. Strategies for the Etiological Therapy of Cystic Fibrosis. Cell Death Differ. 2017, 24, 1825–1844. [Google Scholar] [CrossRef]
- Tomati, V.; Caci, E.; Ferrera, L.; Pesce, E.; Sondo, E.; Cholon, D.M.; Quinney, N.L.; Boyles, S.E.; Armirotti, A.; Ravazzolo, R.; et al. Thymosin Alpha-1 Does Not Correct F508del-Cftr in Cystic Fibrosis Airway Epithelia. JCI Insight 2018, 3, e98699. [Google Scholar] [CrossRef]
- Armirotti, A.; Tomati, V.; Matthes, E.; Veit, G.; Cholon, D.M.; Phuan, P.W.; Braccia, C.; Guidone, D.; Gentzsch, M.; Lukacs, G.L.; et al. Bioactive Thymosin Alpha-1 Does Not Influence F508del-Cftr Maturation and Activity. Sci. Rep. 2019, 9, 10310. [Google Scholar] [CrossRef]
- Rolfe, H.M. A Review of Nicotinamide: Treatment of Skin Diseases and Potential Side Effects. J. Cosmet. Dermatol. 2014, 13, 324–328. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsieh, N.K.; Liou, H.L.; Chen, H.I. Niacinamide Mitigated the Acute Lung Injury Induced by Phorbol Myristate Acetate in Isolated Rat’s Lungs. J. Biomed. Sci. 2012, 19, 27. [Google Scholar] [CrossRef]
- Odera, M.; Furuta, T.; Sohma, Y.; Sakurai, M. Molecular Dynamics Simulation Study on the Structural Instability of the Most Common Cystic Fibrosis-Associated Mutant Deltaf508-Cftr. Biophys. Physicobiol. 2018, 15, 33–44. [Google Scholar] [CrossRef]
- Liu, K.; Watanabe, E.; Kokubo, H. Exploring the Stability of Ligand Binding Modes to Proteins by Molecular Dynamics Simulations. J. Comput. Aided Mol. Des. 2017, 31, 201–211. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. Plip: Fully Automated Protein-Ligand Interaction Profiler. Nucl. Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
- Prins, S.; Corradi, V.; Sheppard, D.N.; Tieleman, D.P.; Vergani, P. Can Two Wrongs Make a Right? F508del-Cftr Ion Channel Rescue by Second-Site Mutations in Its Transmembrane Domains. J. Biol. Chem. 2022, 298, 101615. [Google Scholar] [CrossRef]
- Rusnati, M.; Sala, D.; Orro, A.; Bugatti, A.; Trombetti, G.; Cichero, E.; Urbinati, C.; di Somma, M.; Millo, E.; Galietta, L.J.V.; et al. Speeding up the Identification of Cystic Fibrosis Transmembrane Conductance Regulator-Targeted Drugs: An Approach Based on Bioinformatics Strategies and Surface Plasmon Resonance. Molecules 2018, 23, 120. [Google Scholar] [CrossRef]
- Rusnati, M.; D’Ursi, P.; Pedemonte, N.; Urbinati, C.; Ford, R.C.; Cichero, E.; Uggeri, M.; Orro, A.; Fossa, P. Recent Strategic Advances in Cftr Drug Discovery: An Overview. Int. J. Mol. Sci. 2020, 21, 2407. [Google Scholar] [CrossRef]
- Parodi, A.; Righetti, G.; Pesce, E.; Salis, A.; Tasso, B.; Urbinati, C.; Tomati, V.; Damonte, G.; Rusnati, M.; Pedemonte, N.; et al. Discovery of Novel Vx-809 Hybrid Derivatives as F508del-Cftr Correctors by Molecular Modeling, Chemical Synthesis and Biological Assays. Eur. J. Med. Chem. 2020, 208, 112833. [Google Scholar] [CrossRef]
- Slater, O.; Miller, B.; Kontoyianni, M. Decoding Protein-Protein Interactions: An Overview. Curr. Top. Med. Chem. 2020, 20, 855–882. [Google Scholar] [CrossRef]
- Laselva, O.; Guerra, L.; Castellani, S.; Favia, M.; di Gioia, S.; Conese, M. Small-Molecule Drugs for Cystic Fibrosis: Where Are We Now? Pulm. Pharmacol. Ther. 2022, 72, 102098. [Google Scholar] [CrossRef]
- Martelli, G.; Giacomini, D. Antibacterial and Antioxidant Activities for Natural and Synthetic Dual-Active Compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef]
- Gaskin, K.J. Nutritional Care in Children with Cystic Fibrosis: Are Our Patients Becoming Better? Eur. J. Clin. Nutr. 2013, 67, 558–564. [Google Scholar] [CrossRef]
- Yang, H.; Ma, T. F508del-Cystic Fibrosis Transmembrane Regulator Correctors for Treatment of Cystic Fibrosis: A Patent Review. Expert Opin. Ther. Patents 2015, 25, 991–1002. [Google Scholar] [CrossRef]
- Dey, I.; Shah, K.; Bradbury, N.A. Natural Compounds as Therapeutic Agents in the Treatment Cystic Fibrosis. J. Genet. Syndr. Gene Ther. 2016, 7, 284. [Google Scholar] [CrossRef]
- Esposito, S.; Testa, I.; Zani, E.M.; Cunico, D.; Torelli, L.; Grandinetti, R.; Fainardi, V.; Pisi, G.; Principi, N. Probiotics Administration in Cystic Fibrosis: What Is the Evidence? Nutrients 2022, 14, 3160. [Google Scholar] [CrossRef]
- Gur, M.; Zuckerman-Levin, N.; Masarweh, K.; Hanna, M.; Laghi, L.; Marazzato, M.; Levanon, S.; Hakim, F.; Bar-Yoseph, R.; Wilschanski, M.; et al. The Effect of Probiotic Administration on Metabolomics and Glucose Metabolism in Cf Patients. Pediatr. Pulmonol. 2022, 57, 2335–2343. [Google Scholar] [CrossRef]
- Scambi, C.; de Franceschi, L.; Guarini, P.; Poli, F.; Siciliano, A.; Pattini, P.; Biondani, A.; la Verde, V.; Bortolami, O.; Turrini, F.; et al. Preliminary Evidence for Cell Membrane Amelioration in Children with Cystic Fibrosis by 5-Mthf and Vitamin B12 Supplementation: A Single Arm Trial. PLoS ONE 2009, 4, e4782. [Google Scholar] [CrossRef]
- Nowak, J.K.; Krzyzanowska-Jankowska, P.; Drzymala-Czyz, S.; Gozdzik-Spychalska, J.; Wojsyk-Banaszak, I.; Skorupa, W.; Sapiejka, E.; Miskiewicz-Chotnicka, A.; Brylak, J.; Zielinska-Psuja, B.; et al. Fat-Soluble Vitamins in Standard vs. Liposomal Form Enriched with Vitamin K2 in Cystic Fibrosis: A Randomized Multi-Center Trial. J. Clin. Med. 2022, 11, 462. [Google Scholar] [CrossRef]
- Turowski, J.B.; Pietrofesa, R.A.; Lawson, J.A.; Christofidou-Solomidou, M.; Hadjiliadis, D. Flaxseed Modulates Inflammatory and Oxidative Stress Biomarkers in Cystic Fibrosis: A Pilot Study. BMC Complement. Altern. Med. 2015, 15, 148. [Google Scholar] [CrossRef]
- Soares, V.E.M.; do Carmo, T.I.T.; Anjos, F.D.; Wruck, J.; de Oliveira Maciel, S.F.V.; Bagatini, M.D.; de Resende, E.S.D.T. Role of Inflammation and Oxidative Stress in Tissue Damage Associated with Cystic Fibrosis: Cape as a Future Therapeutic Strategy. Mol. Cell Biochem. 2022, 477, 39–51. [Google Scholar] [CrossRef]
- Carbone, A.; Montalbano, A.; Spano, V.; Musante, I.; Galietta, L.J.V.; Barraja, P. Furocoumarins as Multi-Target Agents in the Treatment of Cystic Fibrosis. Eur. J. Med. Chem. 2019, 180, 283–290. [Google Scholar] [CrossRef]
- Sohma, Y.; Yu, Y.C.; Hwang, T.C. Curcumin and Genistein: The Combined Effects on Disease-Associated Cftr Mutants and Their Clinical Implications. Curr. Pharm. Des. 2013, 19, 3521–3528. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.; Fairbourn, N.; Mylavarapu, C.; Dbeis, A.; Bowman, T.; Chandrashekar, A.; Banayat, T.; Hodges, C.A.; Al-Nakkash, L. Consuming Genistein Improves Survival Rates in the Absence of Laxative in Deltaf508-Cf Female Mice. Nutrients 2018, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, G.; Jakobkiewicz-Banecka, J.; Gabig-Ciminska, M.; Piotrowska, E.; Narajczyk, M.; Kloska, A.; Malinowska, M.; Dziedzic, D.; Golebiewska, I.; Moskot, M.; et al. Genistein: A Natural Isoflavone with a Potential for Treatment of Genetic Diseases. Biochem. Soc. Trans. 2010, 38, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised Clinical Trial to Determine the Safety of Quercetin Supplementation in Patients with Chronic Obstructive Pulmonary Disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Belchamber, K.B.R.; Donnelly, L.E. Targeting Defective Pulmonary Innate Immunity-A New Therapeutic Option? Pharmacol. Ther. 2020, 9, 107500. [Google Scholar] [CrossRef]
- Moran, O.; Galietta, L.J.; Zegarra-Moran, O. Binding Site of Activators of the Cystic Fibrosis Transmembrane Conductance Regulator in the Nucleotide Binding Domains. Cell Mol. Life Sci. 2005, 62, 446–460. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Sui, Y.; Yang, S.; Luan, J.; Wang, X.; Ma, T.; Yang, H. Potentiation of Mutant Cftr Cl- Channel Currents by the Naturally Occurring Stilbene Compound Resveratrol. Pharmazie 2013, 68, 877–881. [Google Scholar]
- Pyle, L.C.; Fulton, J.C.; Sloane, P.A.; Backer, K.; Mazur, M.; Prasain, J.; Barnes, S.; Clancy, J.P.; Rowe, S.M. Activation of the Cystic Fibrosis Transmembrane Conductance Regulator by the Flavonoid Quercetin: Potential Use as a Biomarker of Deltaf508 Cystic Fibrosis Transmembrane Conductance Regulator Rescue. Am. J. Respir. Cell Mol. Biol. 2010, 43, 607–616. [Google Scholar] [CrossRef]
- Centko, R.M.; Carlile, G.W.; Barne, I.; Patrick, B.O.; Blagojevic, P.; Thomas, D.Y.; Andersen, R.J. Combination of Selective Parp3 and Parp16 Inhibitory Analogues of Latonduine a Corrects F508del-Cftr Trafficking. ACS Omega 2020, 5, 25593–25604. [Google Scholar] [CrossRef]
- Dekkers, J.F.; van Mourik, P.; Vonk, A.M.; Kruisselbrink, E.; Berkers, G.; Groot, K.M.de.; Janssens, H.M.; Bronsveld, I.; van der Ent, C.K.; de Jonge, H.R.; et al. Potentiator Synergy in Rectal Organoids Carrying S1251n, G551d, or F508del Cftr Mutations. J. Cyst. Fibros. 2016, 15, 568–578. [Google Scholar] [CrossRef]
- Wang, W.; Hong, J.S.; Rab, A.; Sorscher, E.J.; Kirk, K.L. Robust Stimulation of W1282x-Cftr Channel Activity by a Combination of Allosteric Modulators. PLoS ONE 2016, 11, e0152232. [Google Scholar] [CrossRef]
- Cho, D.Y.; Zhang, S.; Lazrak, A.; Grayson, J.W.; Garcia, J.A.P.; Skinner, D.F.; Lim, D.J.; Mackey, C.; Banks, C.; Matalon, S.; et al. Resveratrol and Ivacaftor Are Additive G551d Cftr-Channel Potentiators: Therapeutic Implications for Cystic Fibrosis Sinus Disease. Int. Forum Allergy Rhinol. 2019, 9, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Pesce, E.; Gorrieri, G.; Sirci, F.; Napolitano, F.; Carrella, D.; Caci, E.; Tomati, V.; Zegarra-Moran, O.; di Bernardo, D.; Galietta, L.J. Evaluation of a Systems Biology Approach to Identify Pharmacological Correctors of the Mutant Cftr Chloride Channel. J. Cyst. Fibros. 2016, 15, 425–435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, B.; Zhang, Y. Teaching an Old Dog New Tricks: Drug Discovery by Repositioning Natural Products and Their Derivatives. Drug Discov. Today 2022, 27, 1936–1944. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Fievez, L.; Ucakar, B.; Neyrinck, A.M.; Fillee, C.; Huaux, F.; Delzenne, N.M.; Bureau, F.; Vanbever, R. Nicotinamide Enhances Apoptosis of G(M)-Csf-Treated Neutrophils and Attenuates Endotoxin-Induced Airway Inflammation in Mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L354–L361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stutts, M.J.; Gabriel, S.E.; Price, E.M.; Sarkadi, B.; Olsen, J.C.; Boucher, R.C. Pyridine Nucleotide Redox Potential Modulates Cystic Fibrosis Transmembrane Conductance Regulator Cl- Conductance. J. Biol. Chem. 1994, 269, 8667–8674. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Schmidt, A.; Li, Q.; Nuvaga, E.; Barrett, T.; Bridges, R.J.; Feranchak, A.P.; Brautigam, C.A.; Thomas, P.J. Requirements for Efficient Correction of Deltaf508 Cftr Revealed by Analyses of Evolved Sequences. Cell 2012, 148, 164–174. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules 2020, 10, 687. [Google Scholar] [CrossRef]
- Wang, F.; Zeltwanger, S.; Yang, I.C.; Nairn, A.C.; Hwang, T.C. Actions of Genistein on Cystic Fibrosis Transmembrane Conductance Regulator Channel Gating. Evidence for Two Binding Sites with Opposite Effects. J. Gen. Physiol. 1998, 111, 477–490. [Google Scholar] [CrossRef]
- Laselva, O.; Qureshi, Z.; Zeng, Z.W.; Petrotchenko, E.V.; Ramjeesingh, M.; Hamilton, C.M.; Huan, L.J.; Borchers, C.H.; Pomes, R.; Young, R.; et al. Identification of Binding Sites for Ivacaftor on the Cystic Fibrosis Transmembrane Conductance Regulator. iScience 2021, 24, 102542. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, C.A.; Zeitlin, P.L.; Bratcher, P.E. Elexacaftor Is a Cftr Potentiator and Acts Synergistically with Ivacaftor During Acute and Chronic Treatment. Sci. Rep. 2021, 11, 19810. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Autodock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. A new model for calculating atomic charges in molecules. Tetrahedron 1978, 19, 3181–3184. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; TDarden; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A., Jr.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14sb: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99sb. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Dickson, C.J.; Madej, B.D.; Skjevik, A.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, L.; Rimington, T.; Cant, N.; Ford, R.C. Expression and Purification of the Cystic Fibrosis Transmembrane Conductance Regulator Protein in Saccharomyces Cerevisiae. J. Vis. Exp. 2012, 10, 3860. [Google Scholar] [CrossRef]
- Pollock, N.; Cant, N.; Rimington, T.; Ford, R.C. Purification of the Cystic Fibrosis Transmembrane Conductance Regulator Protein Expressed in Saccharomyces Cerevisiae. J. Vis. Exp. 2014, 10, 51447. [Google Scholar] [CrossRef] [PubMed]
- Trutnau, H.H. New Multi-Step Kinetics Using Common Affinity Biosensors Saves Time and Sample at Full Access to Kinetics and Concentration. J. Biotechnol. 2006, 124, 191–195. [Google Scholar] [CrossRef]
- Pedemonte, N.; Bertozzi, F.; Caci, E.; Sorana, F.; di Fruscia, P.; Tomati, V.; Ferrera, L.; Rodriguez-Gimeno, A.; Berti, F.; Pesce, E.; et al. Discovery of a Picomolar Potency Pharmacological Corrector of the Mutant Cftr Chloride Channel. Sci. Adv. 2020, 6, eaay9669. [Google Scholar] [CrossRef]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.; Verkman, A.S. Small-Molecule Correctors of Defective Deltaf508-Cftr Cellular Processing Identified by High-Throughput Screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Sondo, E.; Tomati, V.; Caci, E.; Esposito, A.I.; Pfeffer, U.; Pedemonte, N.; Galietta, L.J. Rescue of the Mutant Cftr Chloride Channel by Pharmacological Correctors and Low Temperature Analyzed by Gene Expression Profiling. Am. J. Physiol. Cell Physiol. 2011, 301, C872–C885. [Google Scholar] [CrossRef] [PubMed]
- Sondo, E.; Falchi, F.; Caci, E.; Ferrera, L.; Giacomini, E.; Pesce, E.; Tomati, V.; Bertozzi, S.M.; Goldoni, L.; Armirotti, A.; et al. Pharmacological Inhibition of the Ubiquitin Ligase Rnf5 Rescues F508del-Cftr in Cystic Fibrosis Airway Epithelia. Cell Chem. Biol. 2018, 25, 891–905.e8. [Google Scholar] [CrossRef] [PubMed]

| Molecule Name | Molecular Weight (g/mol) | Docking Score (Kcal/mol) | 2D Structure |
|---|---|---|---|
| Cysteamine | 77.15 | −2.7 |  |
| Sodium oxybate | 104.17 | −4.4 | 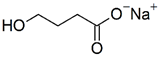 |
| Methimazole | 114.17 | −3.6 |  |
| Glycine betaine | 118.16 | −3.9 | 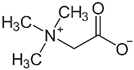 |
| Nicotinamide | 122.13 | −5.4 | 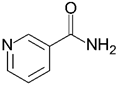 |
| Lumacaftor | L171 (ICL1) | L173 (ICL1) | I177 (ICL1) | F494 (NBD1) | K1060 (ICL4) | K1063 (ICL4) | D1341 (NBD2) |
| 5.05 Å | 3.95 Å | 4.92 Å | 5.55 Å | 4.45 Å | 4.11 Å | 5.14 Å | |
| NAM | W496 (NBD1) | L1065 (ICL4) | |||||
| 5.31 Å | 4.53 Å |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fossa, P.; Uggeri, M.; Orro, A.; Urbinati, C.; Rondina, A.; Milanesi, M.; Pedemonte, N.; Pesce, E.; Padoan, R.; Ford, R.C.; et al. Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing. Int. J. Mol. Sci. 2022, 23, 12274. https://doi.org/10.3390/ijms232012274
Fossa P, Uggeri M, Orro A, Urbinati C, Rondina A, Milanesi M, Pedemonte N, Pesce E, Padoan R, Ford RC, et al. Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing. International Journal of Molecular Sciences. 2022; 23(20):12274. https://doi.org/10.3390/ijms232012274
Chicago/Turabian StyleFossa, Paola, Matteo Uggeri, Alessandro Orro, Chiara Urbinati, Alessandro Rondina, Maria Milanesi, Nicoletta Pedemonte, Emanuela Pesce, Rita Padoan, Robert C. Ford, and et al. 2022. "Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing" International Journal of Molecular Sciences 23, no. 20: 12274. https://doi.org/10.3390/ijms232012274
APA StyleFossa, P., Uggeri, M., Orro, A., Urbinati, C., Rondina, A., Milanesi, M., Pedemonte, N., Pesce, E., Padoan, R., Ford, R. C., Meng, X., Rusnati, M., & D’Ursi, P. (2022). Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing. International Journal of Molecular Sciences, 23(20), 12274. https://doi.org/10.3390/ijms232012274









