Thermofluor-Based Optimization Strategy for the Stabilization of Recombinant Human Soluble Catechol-O-Methyltransferase
Abstract
1. Introduction
2. Results and Discussion
2.1. Stabilizer Effects on Recombinantly Biosynthesized hSCOMT
2.2. Buffer and Additive Screening Evaluation upon hSCOMTVal108-6His Thermal Stability
2.3. Influence of the Storage Temperature and Formulation Validation for hSCOMTVal108-6His Pure Samples
3. Materials and Methods
3.1. Chemicals
3.2. Recombinant hSBCOMT Biosynthesis and Recuperation
3.3. Purification of hSCOMTVal108-6His by Immobilized Metal Affinity Chromatography
3.4. hSCOMT Stabilization Studies
3.5. Thermal Shift Assay (TSA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niesen, F.H.; Berglund, H.; Vedadi, M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007, 2, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Tilgmann, C.; Ulmanen, I. Purification methods of mammalian catechol-O-methyltransferases. J. Chromatogr. B Biomed. Sci. Appl. 1996, 684, 147–161. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, P.; Zhao, D.F.; Gonzalez, F.J.; Fan, Y.F.; Xia, Y.L.; Ge, G.B.; Yang, L. Analytical methodologies for sensing catechol-O-methyltransferase activity and their applications. J. Pharm. Anal. 2021, 11, 15–27. [Google Scholar] [CrossRef]
- Leibly, D.J.; Nguyen, T.N.; Kao, L.T.; Hewitt, S.N.; Barrett, L.K.; Van Voorhis, W.C. Stabilizing Additives Added during Cell Lysis Aid in the Solubilization of Recombinant Proteins. PLoS ONE 2012, 7, e52482. [Google Scholar] [CrossRef] [PubMed]
- Belluzo, S.; Boeris, V.; Farruggia, B.; Picó, G. Influence of stabilizers cosolutes on catalase conformation. Int. J. Biol. Macromol. 2011, 49, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, A.L.; Mincu, N.B.; Vasilca, S.; Apetrei, R.; Stan, D.; Zorilă, B.; Stan, D. The influence of sugar-protein complexes on the thermostability of C-reactive protein (CRP). Sci. Rep. 2021, 11, 13017. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Lerbret, A.; Bordat, P.; Affouard, F.; Guinet, Y.; Hédoux, A.; Paccou, L.; Prévost, D.; Descamps, M. Influence of homologous disaccharides on the hydrogen-bond network of water: Complementary Raman scattering experiments and molecular dynamics simulations. Carbohydr. Res. 2005, 340, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Bruyas, J.F.; Sanson, J.P.; Battut, I.; Fiéni, F.; Tainturier, D. Comparison of the cryoprotectant properties of glycerol and ethylene glycol for early (day 6) equine embryos. J. Reprod. Fertil. Suppl. 2000, 56, 549–560. [Google Scholar]
- Hajduk, E.; Fik, M. Application of glycerol to freezing bovine pancreas. Part 1. Effect of cryoprotector on activity of certain enzymes in frozen minced pancreas. Die Nahr. 1990, 34, 75–79. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.E.; Chavan, V.; Mukherjee, K. Identification and glycerol-induced correction of misfolding mutations in the X-linked mental retardation gene CASK. PLoS ONE 2014, 9, e88276. [Google Scholar] [CrossRef]
- Khan, M.V.; Ishtikhar, M.; Rabbani, G.; Zaman, M.; Abdelhameed, A.S.; Khan, R.H. Polyols (Glycerol and Ethylene glycol) mediated amorphous aggregate inhibition and secondary structure restoration of metalloproteinase-conalbumin (ovotransferrin). Int. J. Biol. Macromol. 2017, 94, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hui, R.; Sun, C.; Gu, X.; Luo, M.; Zheng, X. Soluble expression, purification, and stabilization of a pro-apoptotic human protein, CARP. Protein Expr. Purif. 2006, 45, 329–334. [Google Scholar] [CrossRef]
- Scharnagl, C.; Reif, M.; Friedrich, J. Stability of proteins: Temperature, pressure and the role of the solvent. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2005, 1749, 187–213. [Google Scholar] [CrossRef]
- Rabbani, G.; Ahmad, E.; Khan, M.V.; Ashraf, M.T.; Bhat, R.; Khan, R.H. Impact of structural stability of cold adapted Candida antarctica lipase B (CaLB): In relation to pH, chemical and thermal denaturation. RSC Adv. 2015, 5, 20115–20131. [Google Scholar] [CrossRef]
- Rabbani, G.; Ahmad, E.; Zaidi, N.; Fatima, S.; Khan, R.H. pH-Induced molten globule state of Rhizopus niveus lipase is more resistant against thermal and chemical denaturation than its native state. Cell Biochem. Biophys. 2012, 62, 487–499. [Google Scholar] [CrossRef]
- Schellman, J.A. Temperature, stability, and the hydrophobic interaction. Biophys. J. 1997, 73, 2960–2964. [Google Scholar] [CrossRef]
- Senisterra, G.A.; Finerty, P.J., Jr. High throughput methods of assessing protein stability and aggregation. Mol. Biosyst. 2009, 5, 217–223. [Google Scholar] [CrossRef]
- Pantoliano, M.W.; Petrella, E.C.; Kwasnoski, J.D.; Lobanov, V.S.; Myslik, J.; Graf, E.; Carver, T.; Asel, E.; Springer, B.A.; Lane, P.; et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 2001, 6, 429–440. [Google Scholar] [CrossRef]
- Boivin, S.; Kozak, S.; Meijers, R. Optimization of protein purification and characterization using Thermofluor screens. Protein Expr. Purif. 2013, 91, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, U.B.; Hallberg, B.M.; Detitta, G.T.; Dekker, N.; Nordlund, P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 2006, 357, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Kaur, J.; Ahmad, E.; Khan, R.H.; Jain, S.K. Structural characteristics of thermostable immunogenic outer membrane protein from Salmonella enterica serovar Typhi. Appl. Microbiol. Biotechnol. 2014, 98, 2533–2543. [Google Scholar] [CrossRef]
- Kozak, S.; Lercher, L.; Karanth, M.N.; Meijers, R.; Carlomagno, T.; Boivin, S. Optimization of protein samples for NMR using thermal shift assays. J. Biomol. NMR 2016, 64, 281–289. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Sousa, Â.; Pedro, A.Q.; Romão, M.J.; Queiroz, J.A.; Gallardo, E.; Passarinha, L.A. Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation. Int. J. Mol. Sci. 2022, 23, 7264. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Vicente, P.; Gonçalves, A.M.; Barroca-Ferreira, J.; Silvestre, S.M.; Romão, M.J.; Queiroz, J.A.; Gallardo, E.; Passarinha, L.A. Unveiling the biopathway for the design of novel COMT inhibitors. Drug Discov. Today 2022, 27, 103328. [Google Scholar] [CrossRef]
- Di Salvo, C.; Barreca, D.; Laganà, G.; di Bella, M.; Tellone, E.; Ficarra, S.; Bellocco, E. Myelin basic protein: Structural characterization of spherulites formation and preventive action of trehalose. Int. J. Biol. Macromol. 2013, 57, 63–68. [Google Scholar] [CrossRef]
- Nunes, V.S.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Assessment of COMT isolation by HIC using a dual salt system and low temperature. Biomed. Chromatogr. BMC 2010, 24, 858–862. [Google Scholar] [CrossRef]
- Santos, F.M.; Pedro, A.Q.; Soares, R.F.; Martins, R.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Performance of hydrophobic interaction ligands for human membrane-bound catechol-O-methyltransferase purification. J. Sep. Sci. 2013, 36, 1693–1702. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Correia, F.F.; Santos, F.M.; Espírito-Santo, G.; Gonçalves, A.M.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Biosynthesis and purification of histidine-tagged human soluble catechol-O-methyltransferase. J. Chem. Technol. Biotechnol. 2016, 91, 3035–3044. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Role of trehalose in moisture-induced aggregation of bovine serum albumin. Eur. J. Pharm. Biopharm. 2008, 69, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.Q.; Rf, S.; Oppolzer, D.J.; Santos, F.M.; La, R.; Gonçalves, A.M.; Bonifácio, M.J.; Queiroz, J.A.; Gallardo, E.; Passarinha, L.A. An Improved HPLC Method for Quantification of Metanephrine with Coulometric Detection. J. Chromatogr. Sep. Tech. 2014, 5, 17–24. [Google Scholar]
- Ratnayake, S.; Dias, I.H.; Lattman, E.; Griffiths, H.R. Stabilising cysteinyl thiol oxidation and nitrosation for proteomic analysis. J. Proteom. 2013, 92, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, F.; Sung, D.; Haswell, K.M.; Tsin, A.; Ghosh, D. Thiol-dependent antioxidant activity of interphotoreceptor retinoid-binding protein. Exp. Eye Res. 2014, 120, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Alliegro, M.C. Effects of dithiothreitol on protein activity unrelated to thiol-disulfide exchange: For consideration in the analysis of protein function with Cleland’s reagent. Anal. Biochem. 2000, 282, 102–106. [Google Scholar] [CrossRef]
- Kiritsi, M.N.; Fragoulis, E.G.; Sideris, D.C. Essential cysteine residues for human RNase κ catalytic activity. FEBS J. 2012, 279, 1318–1326. [Google Scholar] [CrossRef]
- Kamal, M.Z.; Ahmad, S.; Rao, N.M. Stabilizing effect of polyols is sensitive to inherent stability of protein. Biophys. Chem. 2011, 156, 68–71. [Google Scholar] [CrossRef]
- Rutherford, K.; Le Trong, I.; Stenkamp, R.E.; Parson, W.W. Crystal structures of human 108V and 108M catechol O-methyltransferase. J. Mol. Biol. 2008, 380, 120–130. [Google Scholar] [CrossRef]
- Otrelo-Cardoso, A.R.; Schwuchow, V.; Rodrigues, D.; Cabrita, E.J.; Leimkühler, S.; Romão, M.J.; Santos-Silva, T. Biochemical, stabilization and crystallization studies on a molecular chaperone (PaoD) involved in the maturation of molybdoenzymes. PLoS ONE 2014, 9, e87295. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, L.; Han, Y.; Jiang, P.; Wei, H. Crystallization control of thermal stability and morphology of hen egg white lysozyme crystals by ionic liquids. J. Agric. Food Chem. 2010, 58, 5444–5448. [Google Scholar] [CrossRef]
- Passarinha, L.A.; Bonifácio, M.J.; Soares-da-Silva, P.; Queiroz, J.A. A new approach on the purification of recombinant human soluble catechol-O-methyltransferase from an Escherichia coli extract using hydrophobic interaction chromatography. J. Chromatogr. A 2008, 1177, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.R.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Analysis of hSCOMT adsorption in bioaffinity chromatography with immobilized amino acids: The influence of pH and ionic strength. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, E.; Okazaki, K.; Isaji, M.; Takeda, K. Crystal structures of the apo and holo form of rat catechol-O-methyltransferase. J. Struct. Biol. 2009, 165, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Bonifácio, M.J.; Soares-da-Silva, P.; Carrondo, M.A.; Archer, M. Crystallization and preliminary X-ray diffraction studies of a catechol-O-methyltransferase/inhibitor complex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2005, 61, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, S.P.; Tew, D.J. The citrate ion increases the conformational stability of alpha(1)-antitrypsin. Biochim. Et Biophys. Acta 2000, 1481, 11–17. [Google Scholar] [CrossRef]
- Bellavia, G.; Paccou, L.; Guinet, Y.; Hédoux, A. How does glycerol enhance the bioprotective properties of trehalose? Insight from protein-solvent dynamics. J. Phys. Chem. B 2014, 118, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, M.P. Chapter 10 Maintaining Protein Stability. In Methods in Enzymology; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 121–127. [Google Scholar]
- Tarver, C.L.; Yuan, Q.; Pusey, M.L. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. [Google Scholar] [CrossRef]
- Sanfelice, D.; Temussi, P.A. Cold denaturation as a tool to measure protein stability. Biophys. Chem. 2016, 208, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Hemar, Y.; Li, N.; Zhou, P. Glycerol induced stability enhancement and conformational changes of β-lactoglobulin. Food Chem. 2020, 308, 125596. [Google Scholar] [CrossRef]
- Chen, X.; Bhandari, B.; Zhou, P. Insight into the effect of glycerol on stability of globular proteins in high protein model system. Food Chem. 2019, 278, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Ertan, H.; Poljak, A.; Bridge, W.J. Evaluating Enzymatic Productivity—The Missing Link to Enzyme Utility. Int. J. Mol. Sci. 2022, 23, 6908. [Google Scholar] [CrossRef]



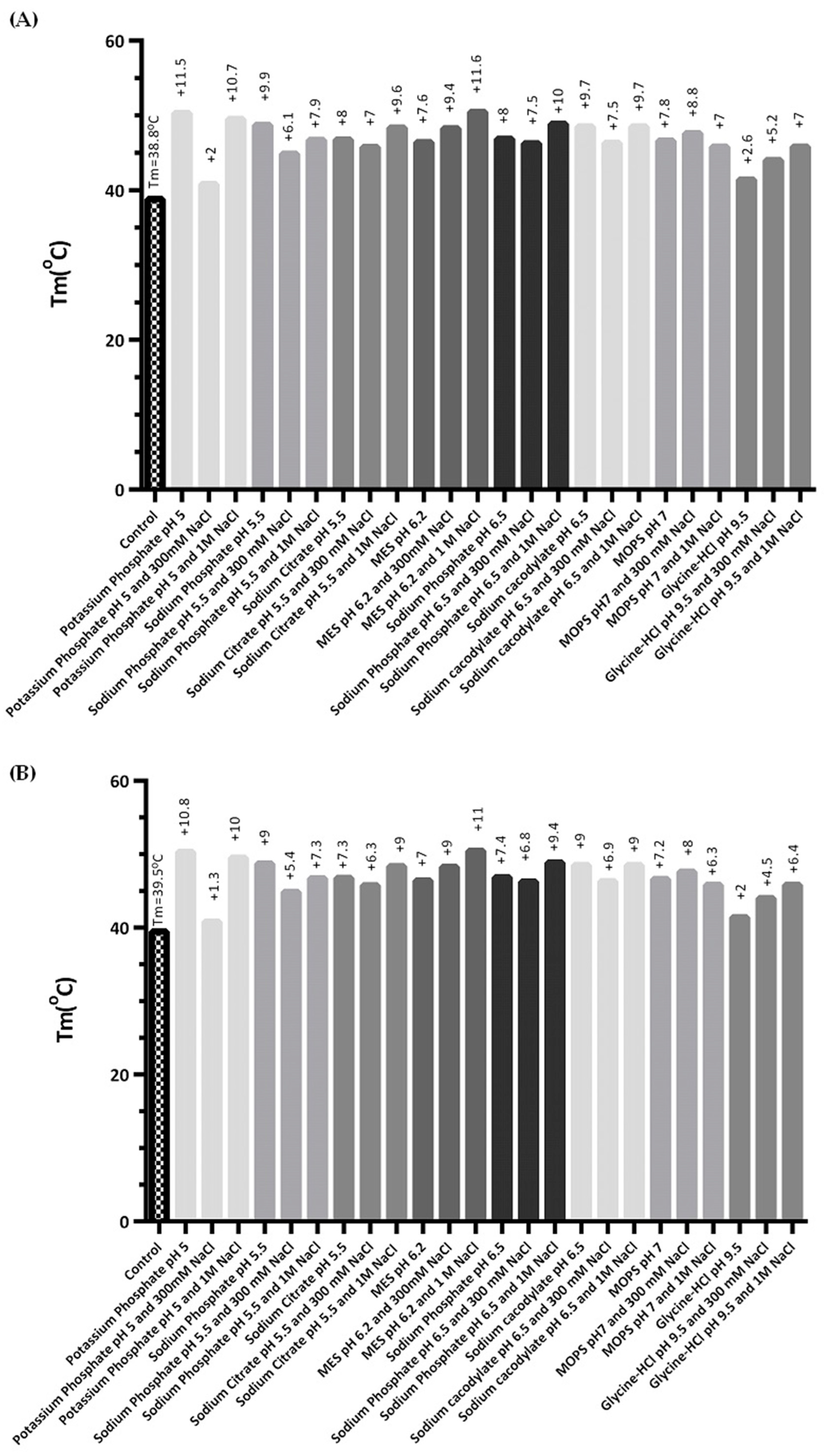

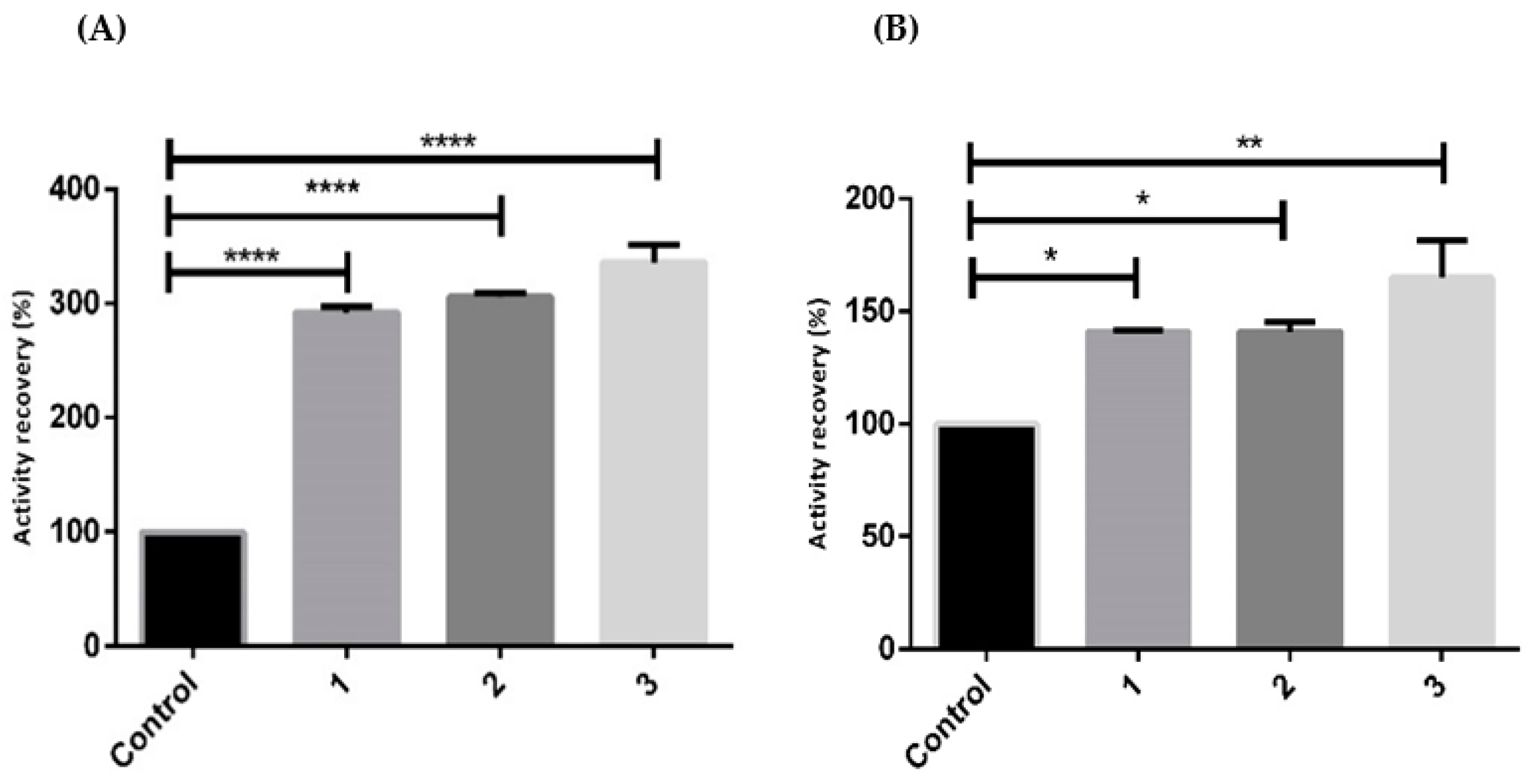
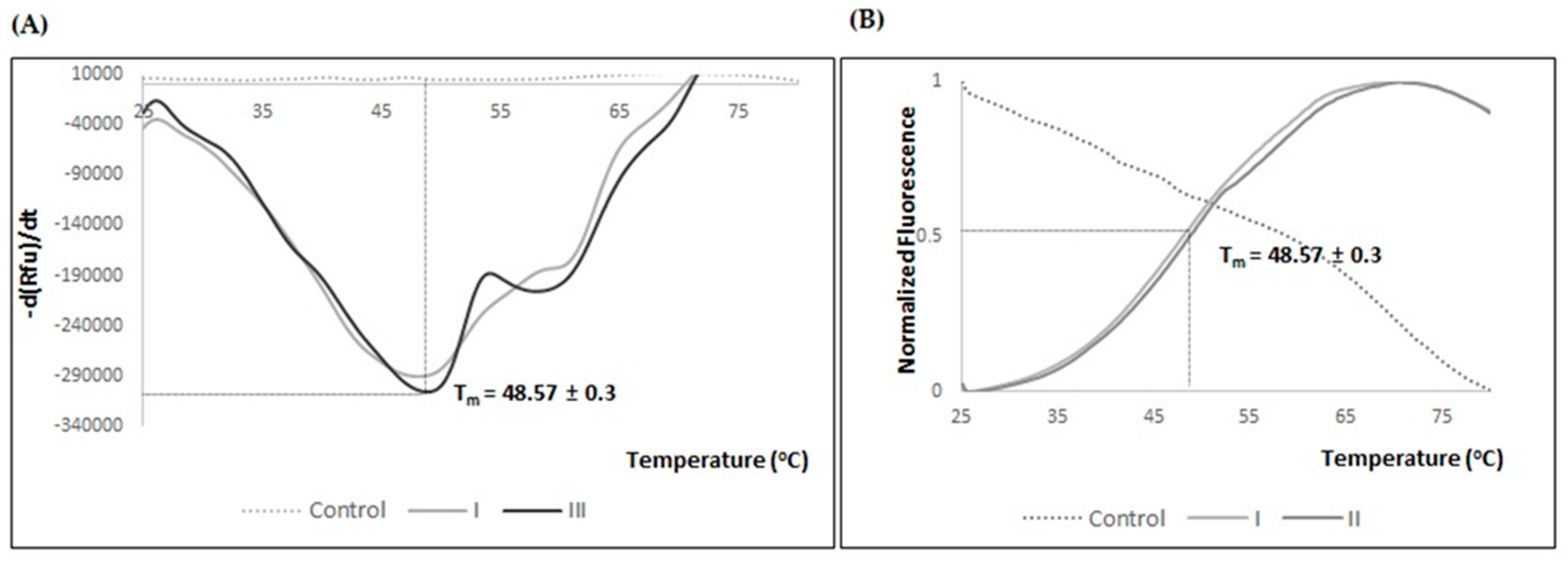

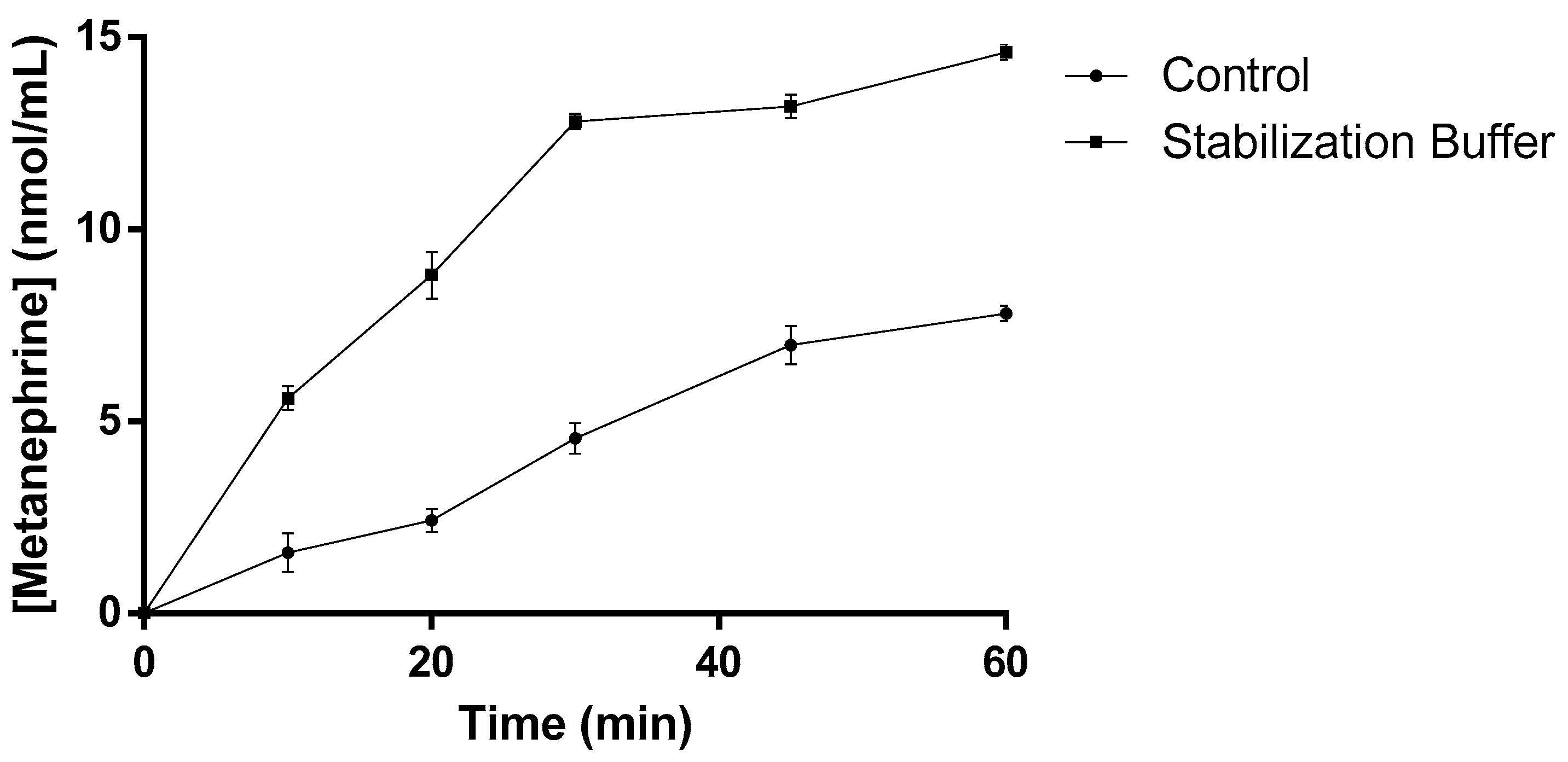
| Buffer | Additive (%(v/v)) | Storage Temperature (°C) | Tm (°C) |
|---|---|---|---|
| A | - - | 4 °C | 44.2 ± 1.0 |
| −80 °C | 37.6 ± 0.2 | ||
| 30% glycerol 30% glycerol | 4 °C | 40.6 ± 0.2 | |
| −80 °C | 40.4 ± 0.4 | ||
| B | - - | 4 °C | 43.1 ± 0.8 |
| −80 °C | 36.8 ± 0.5 | ||
| 30% glycerol 30% glycerol | 4 °C | 44.1 ± 0.6 | |
| −80 °C | 41.6 ± 0.3 |
| Stabilizer | Feature | Concentration Range |
|---|---|---|
| Cysteine | Stabilization of disulfide bonds | 75 to 500 mM |
| Trehalose | Thermal stabilizers/promote protein folding and refolding | 10 to 350 mM |
| Glycerol | Cryo-protector | 5 to 50% |
| [C4mim]Cl | Thermal stabilizer/aggregation behavior | 10 mM * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.M.; Pedro, A.Q.; Oliveira, D.M.; Oliveira, A.E.; Santos, M.F.A.; Correia, M.A.S.; Queiroz, J.A.; Gallardo, E.; Romão, M.J.; Passarinha, L.A. Thermofluor-Based Optimization Strategy for the Stabilization of Recombinant Human Soluble Catechol-O-Methyltransferase. Int. J. Mol. Sci. 2022, 23, 12298. https://doi.org/10.3390/ijms232012298
Gonçalves AM, Pedro AQ, Oliveira DM, Oliveira AE, Santos MFA, Correia MAS, Queiroz JA, Gallardo E, Romão MJ, Passarinha LA. Thermofluor-Based Optimization Strategy for the Stabilization of Recombinant Human Soluble Catechol-O-Methyltransferase. International Journal of Molecular Sciences. 2022; 23(20):12298. https://doi.org/10.3390/ijms232012298
Chicago/Turabian StyleGonçalves, Ana M., Augusto Q. Pedro, Diana M. Oliveira, Adriana E. Oliveira, Marino F. A. Santos, Márcia A. S. Correia, João A. Queiroz, Eugénia Gallardo, Maria J. Romão, and Luís A. Passarinha. 2022. "Thermofluor-Based Optimization Strategy for the Stabilization of Recombinant Human Soluble Catechol-O-Methyltransferase" International Journal of Molecular Sciences 23, no. 20: 12298. https://doi.org/10.3390/ijms232012298
APA StyleGonçalves, A. M., Pedro, A. Q., Oliveira, D. M., Oliveira, A. E., Santos, M. F. A., Correia, M. A. S., Queiroz, J. A., Gallardo, E., Romão, M. J., & Passarinha, L. A. (2022). Thermofluor-Based Optimization Strategy for the Stabilization of Recombinant Human Soluble Catechol-O-Methyltransferase. International Journal of Molecular Sciences, 23(20), 12298. https://doi.org/10.3390/ijms232012298








