Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants
Abstract
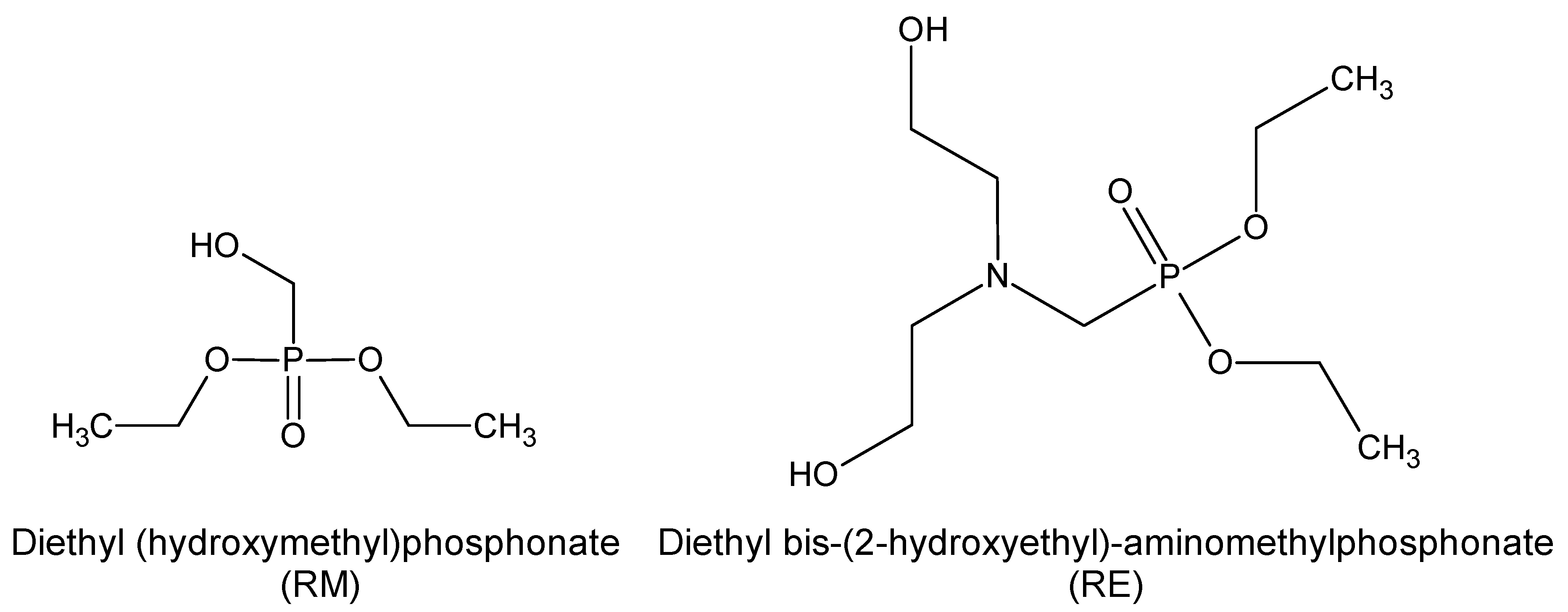
1. Introduction
2. Result and Discussion
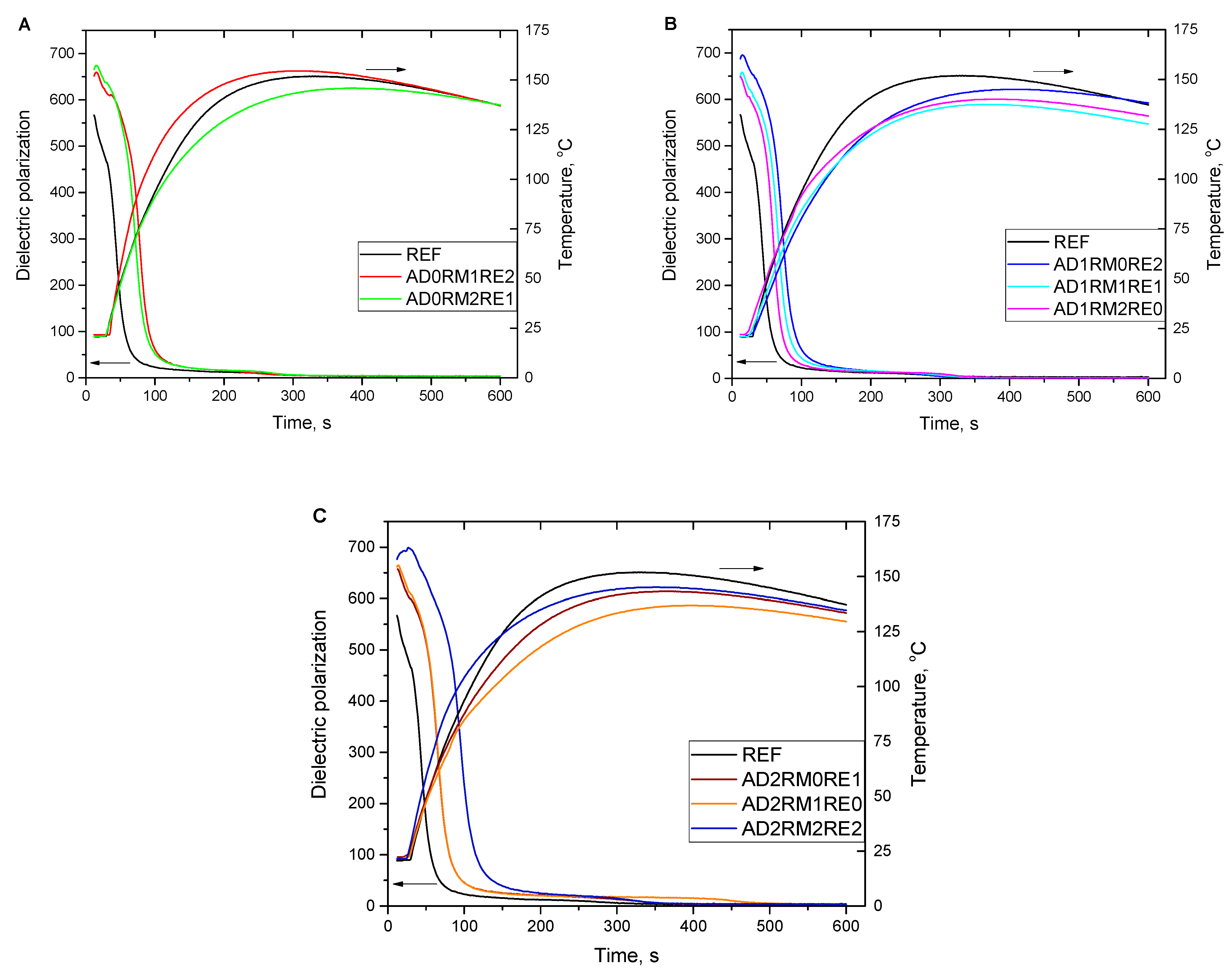
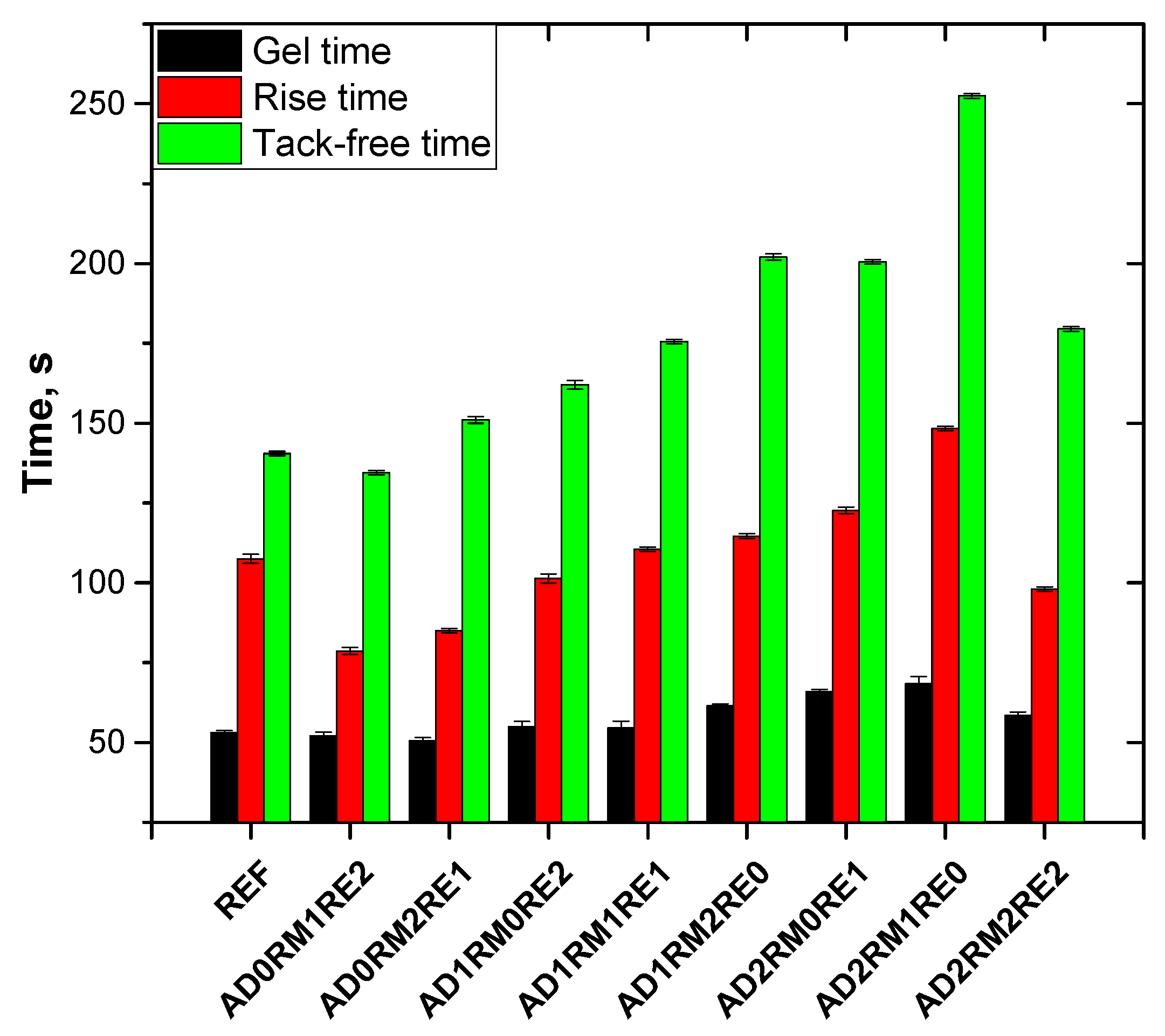
2.1. Analysis of the Foaming Process and Processing Times
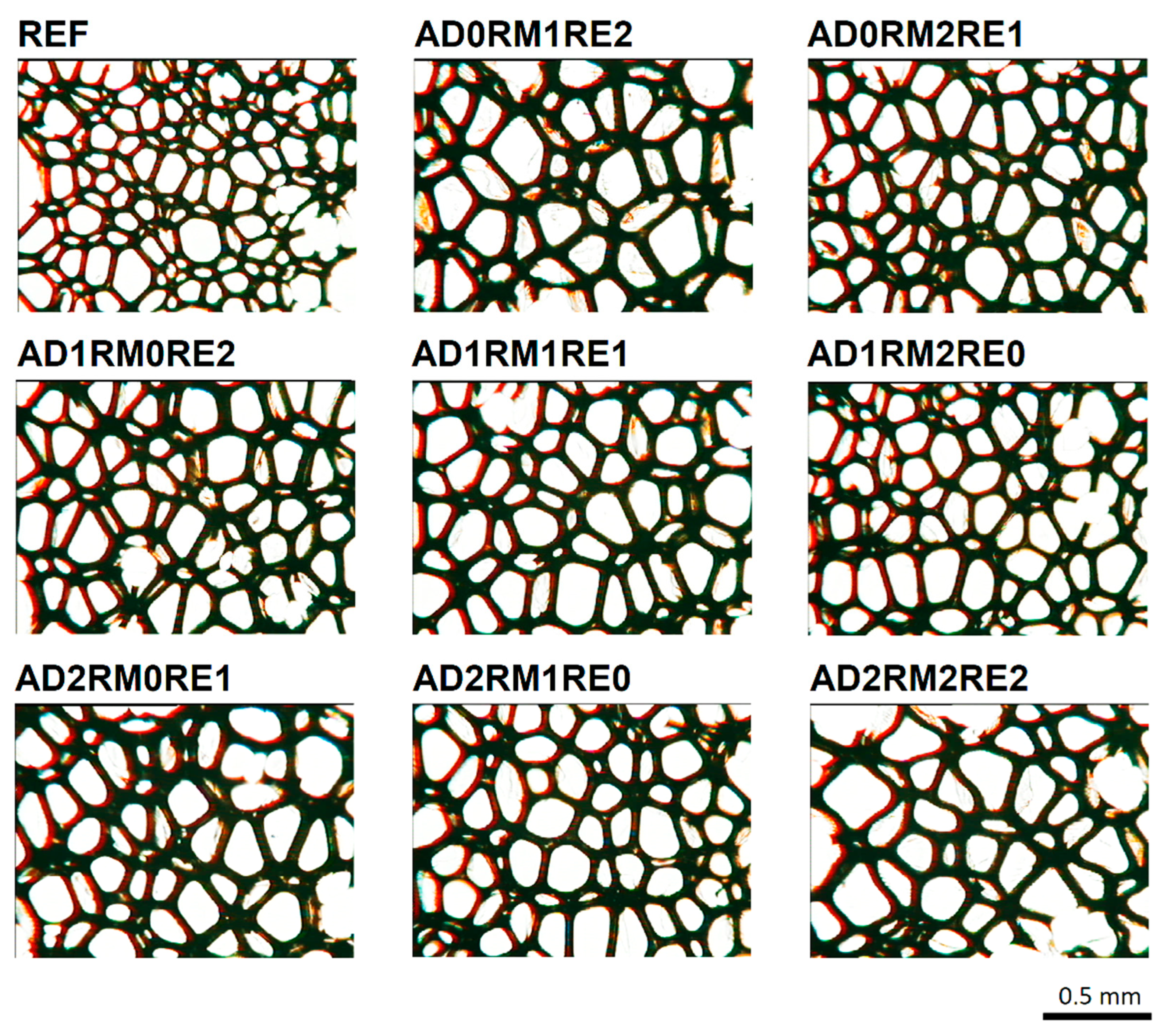
2.2. Analysis of the Cellular Structure
2.3. Analysis of Apparent Density and Thermal Conductivity
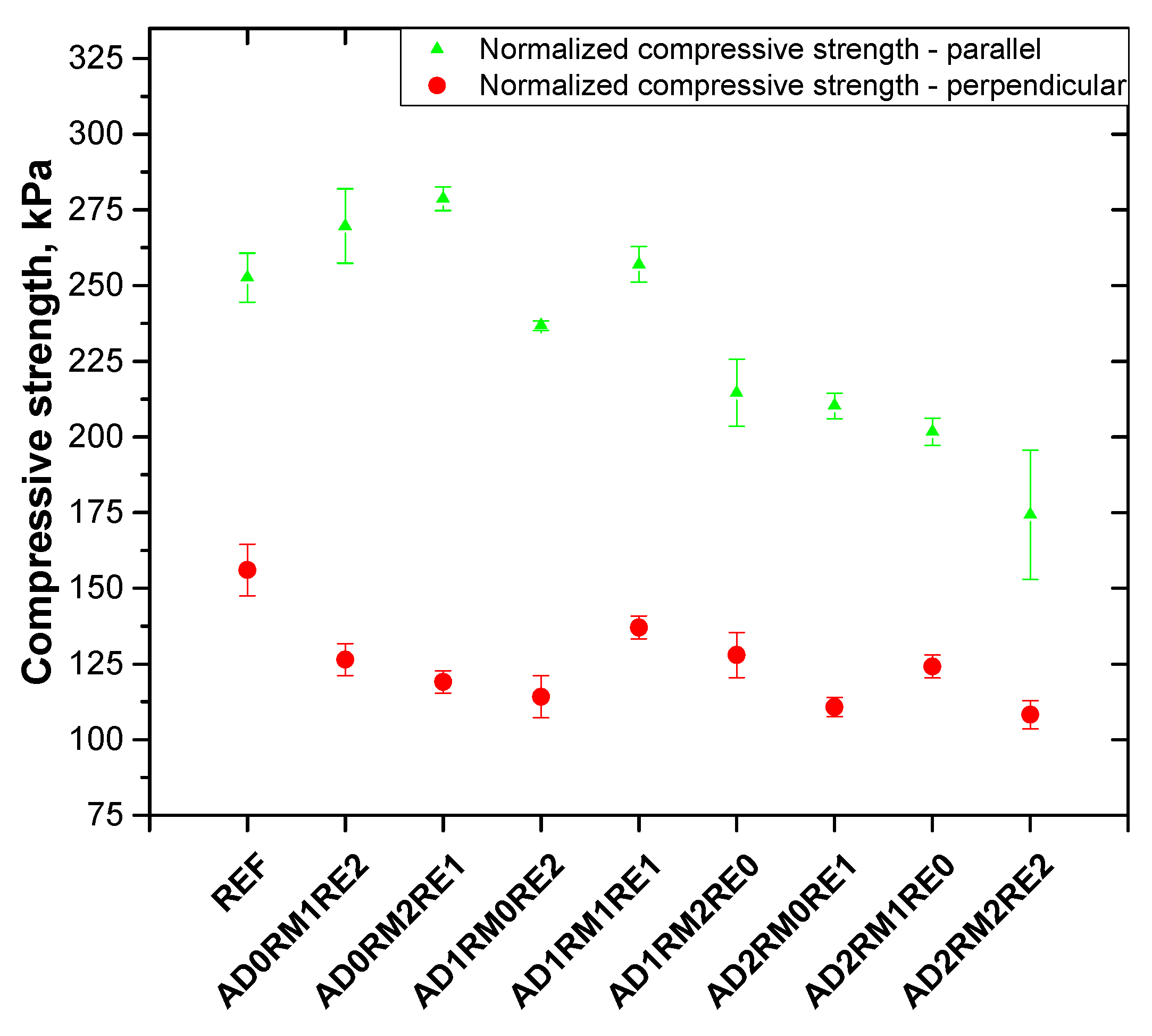
2.4. Analysis of the Mechanical Properties
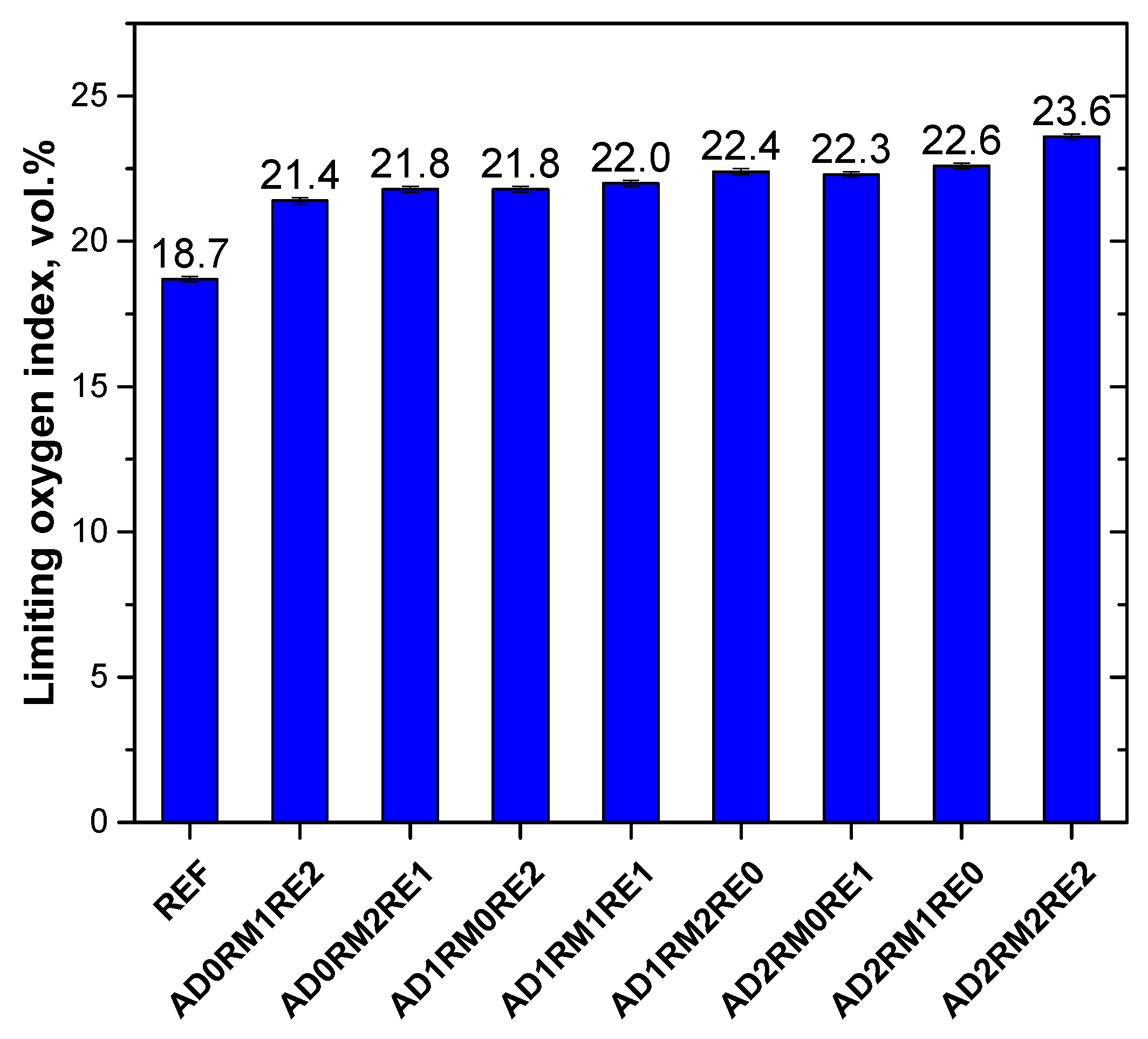
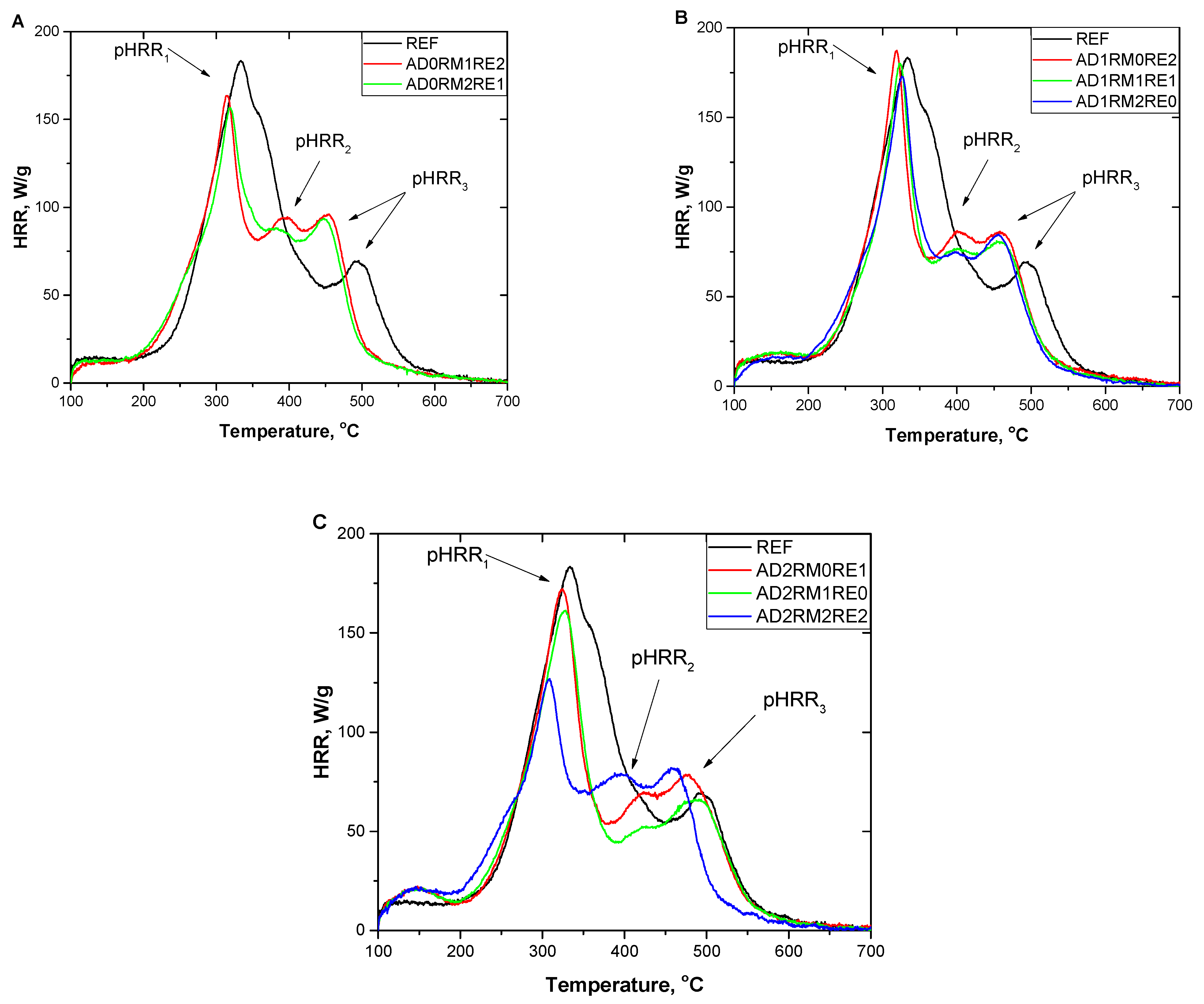
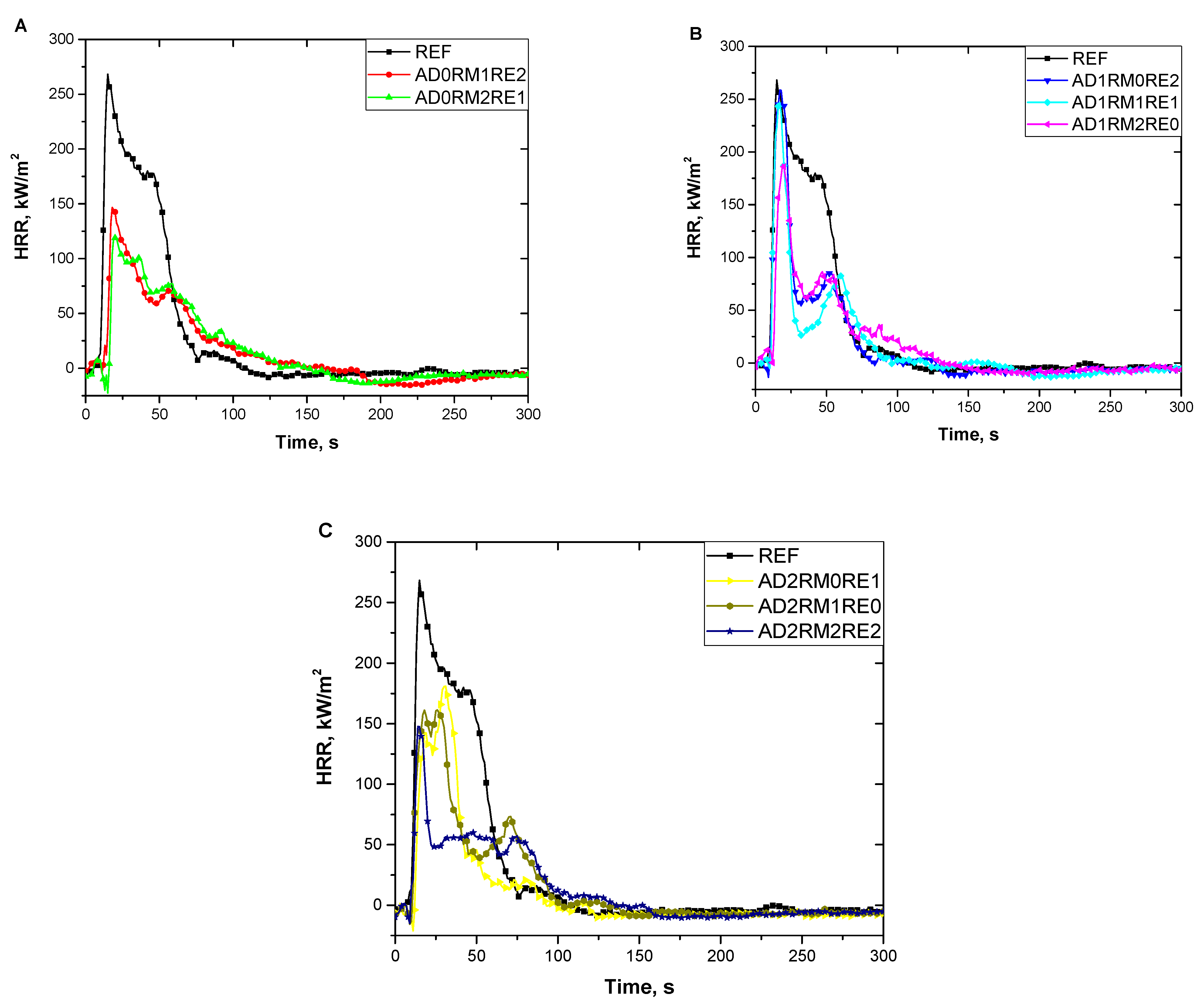
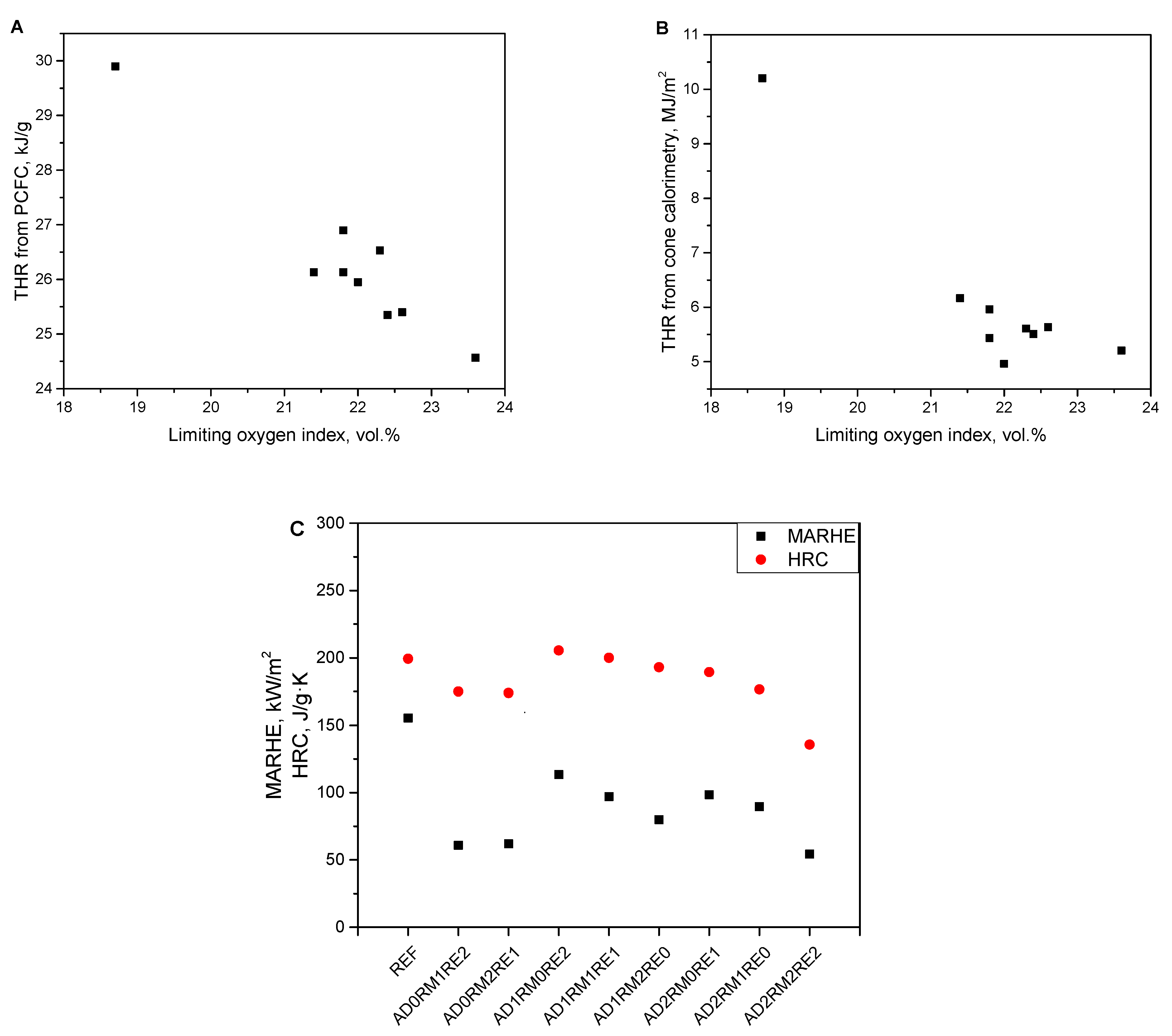
2.5. Analysis of Flammability
3. Materials and Methods
3.1. Materials
3.2. Manufacture of the Polyurethane Foams
3.3. Characterization of Rigid Polyurethane Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Casein/Apricot Filler in the Production of Flame-Retardant Polyurethane Composites. Materials 2021, 14, 3620. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Malewska, E.; Ryszkowska, J.; Kurańska, M.; Gloc, M.; Leszczyński, M.K.; Prociak, A. Vegetable Fillers and Rapeseed Oil-Based Polyol as Natural Raw Materials for the Production of Rigid Polyurethane Foams. Materials 2021, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K.; Nestler, D.; Tröltzsch, J.; Ireka, I.; Niedziela, D.; Steiner, K.; Kroll, L. Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation. Polymers 2020, 12, 105. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, silica, and ionic liquid as a novel flame retardant for rigid polyurethane foams with enhanced flame retardancy and mechanical properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- Gong, Q.; Qin, L.; Yang, L.; Liang, K.; Wang, N. Effect of flame retardants on mechanical and thermal properties of bio-based polyurethane rigid foams. RSC Adv. 2021, 11, 30860–30872. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.; Hodge, D.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.A.; Hull, T.R. Assessment of the fire toxicity of building insulation materials. Energy Build. 2011, 43, 498–506. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Kuang, R.; Zang, F.Y.; Zhang, A.Q.; Xie, H. Synthetic routes to flame retardant isocyanurate of rigid polyurethane foams. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479. [Google Scholar] [CrossRef]
- Kapatel, P.; Patel, R. Flame retardant waterborne polyurethanes: Synthesis, characterization, and evaluation of different properties. Biointerface Res. Appl. Chem. 2022, 12, 3198–3214. [Google Scholar] [CrossRef]
- Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.K.; Gupta, R.K. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C J. Carbon Res. 2019, 5, 13. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Zagożdżon, I.; Parcheta, P.; Datta, J. Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors. Materials 2021, 14, 2699. [Google Scholar] [CrossRef] [PubMed]
- Durganala, S.; Morgan, A.B.; Benin, V. Reactive flame retardants for polyurethanes: Current technology and new directions. In Proceedings of the Conference Proceedings—Fire and Materials 2011, 12th International Conference and Exhibition, San Francisco, CA, USA, 10–11 January 2011; pp. 343–354. [Google Scholar]
- Strąkowska, A.; Członka, S.; Konca, P.; Strzelec, K. New Flame Retardant Systems Based on Expanded Graphite for Rigid Polyurethane Foams. Appl. Sci. 2020, 10, 5817. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, C.; Fan, R.; Yuan, Y.; Xing, Y.; Ma, X. Halogen-free flame-retardant rigid polyurethane foam with a nitrogen-phosphorus flame retardant. J. Fire Sci. 2017, 35, 99–117. [Google Scholar] [CrossRef]
- Gu, L.; Ge, Z.; Huang, M.; Luo, Y. Halogen-free flame-retardant waterborne polyurethane with a novel cyclic structure of phosphorus-nitrogen synergistic flame retardant. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Salasinska, K.; Borucka, M.; Leszczyńska, M.; Zatorski, W.; Celiński, M.; Gajek, A.; Ryszkowska, J. Analysis of flammability and smoke emission of rigid polyurethane foams modified with nanoparticles and halogen-free fire retardants. J. Therm. Anal. Calorim. 2017, 130, 131–141. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Zhang, L. Synthesis of a green reactive flame-retardant polyether polyol and its application. J. Appl. Polym. Sci. 2021, 138, 50154. [Google Scholar] [CrossRef]
- Zarzyka, I. Foamed polyurethane plastics of reduced flammability. J. Appl. Polym. Sci. 2018, 135, 45748. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Velencoso, M.M.; Rodríguez, J.F.; Serrano, Á.; Carrero, M.J.; Ramos, M.J. Synthesis of aminophosphonate polyols and polyurethane foams with improved fire retardant properties. J. Appl. Polym. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Patel, R.H.; Shah, M.D.; Patel, H.B. Synthesis and characterization of structurally modified polyurethanes based on castor oil and phosphorus-containing polyol for flame-retardant coatings. Int. J. Polym. Anal. Charact. 2011, 16, 107–117. [Google Scholar] [CrossRef]
- Tang, G.; Liu, M.; Deng, D.; Zhao, R.; Liu, X.; Yang, Y.; Yang, S.; Liu, X. Phosphorus-containing soybean oil-derived polyols for flame-retardant and smoke-suppressant rigid polyurethane foams. Polym. Degrad. Stab. 2021, 191, 109701. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, P.; Zhou, Y.; Zhang, M. Preparation and characterization of tung oil-based flame retardant polyols. Chin. J. Chem. Eng. 2018, 26, 2664–2671. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, S.; Fan, H.; Chen, Y.; Yan, J. Flame retardancy and hydrolysis resistance of waterborne polyurethane bearing organophosphate moieties lateral chain. Prog. Org. Coat. 2015, 89, 170–180. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Wang, X.; Liu, Y. A phosphorous-based bi-functional flame retardant for rigid polyurethane foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Xia, L.; Liu, J.; Li, Z.; Wang, X.; Wang, P.; Wang, D.; Hu, X. Synthesis and flame retardant properties of new boron-containing polyurethane. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 560–568. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Chen, Y.; Wang, J.; Liu, X. Addition flame-retardant behaviors of expandable graphite and [bis(2-hydroxyethyl)amino]-methyl-phosphonic acid dimethyl ester in rigid polyurethane foams. Polym. Degrad. Stab. 2015, 122, 36–43. [Google Scholar] [CrossRef]
- Luo, Y.; Miao, Z.; Sun, T.; Zou, H.; Liang, M.; Zhou, S.; Chen, Y. Preparation and mechanism study of intrinsic hard segment flame-retardant polyurethane foam. J. Appl. Polym. Sci. 2021, 138, 49920. [Google Scholar] [CrossRef]
- Gomez, J.C.; Zakaria, R.; Aung, M.M.; Mokhtar, M.N.; Yunus, R. Synthesis and Characterization of Polyurethanes from Residual Palm Oil with High Poly-Unsaturated Fatty Acid Oils as Additive. Polymers 2021, 13, 4214. [Google Scholar] [CrossRef]
- Heinen, M.; Gerbase, A.E.; Petzhold, C.L. Vegetable oil-based rigid polyurethanes and phosphorylated flame-retardants derived from epoxydized soybean oil. Polym. Degrad. Stab. 2014, 108, 76–86. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-oil derived nonhalogenated reactive flame-retardant-based polyurethane foams with significant reduced heat release rate. J. Appl. Polym. Sci. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Uram, K.; Prociak, A.; Vevere, L.; Pomilovskis, R.; Cabulis, U.; Kirpluks, M. Natural Oil-Based Rigid Polyurethane Foam Thermal Insulation Applicable at Cryogenic Temperatures. Polymers 2021, 13, 4276. [Google Scholar] [CrossRef]
- Uram, K.; Leszczyńska, M.; Prociak, A.; Czajka, A.; Gloc, M.; Leszczyński, M.K.; Michałowski, S.; Ryszkowska, J. Polyurethane Composite Foams Synthesized Using Bio-Polyols and Cellulose Filler. Materials 2021, 14, 3474. [Google Scholar] [CrossRef]
- Prociak, A.; Szczepkowski, L.; Ryszkowska, J.; Kurańska, M.; Auguścik, M.; Malewska, E.; Gloc, M.; Michałowski, S. Influence of Chemical Structure of Petrochemical Polyol on Properties of Bio-polyurethane Foams. J. Polym. Environ. 2019, 27, 2360–2368. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Lazdiņa, B.; Misāne, M.; Vilsone, D. Biobased Polyurethanes from Rapeseed Oil Polyols: Structure, Mechanical and Thermal Properties. J. Polym. Environ. 2013, 21, 952–962. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpiuks, M. Water-blown polyurethane-polyisocyanurate foams based on bio-polyols with wood fibers. Polimery 2015, 61, 705–712. [Google Scholar] [CrossRef]
- Kirpluks, M.; Vanags, E.; Abolins, A.; Michałowski, S.; Fridrihsone, A.; Cabulis, U. High functionality bio-polyols from tall oil and rigid polyurethane foams formulated solely using bio-polyols. Materials 2020, 13, 1985. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of bio-polyols with different chemical structures on foaming of polyurethane systems and foam properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. Environmentally friendly polyurethane-polyisocyanurate foams for applications in the construction industry. Czas. Tech. 2015, 2014, 149–152. [Google Scholar] [CrossRef]
- Uram, K.; Prociak, A.; Kurańska, M. Influence of the chemical structure of rapeseed oil-based polyols on selected properties of polyurethane foams. Polimery 2020, 65, 698–707. [Google Scholar] [CrossRef]
- Kurańska, M.; Polaczek, K.; Auguscik-Krolikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams based on modified used cooking oil. Polimery 2020, 65, 216–225. [Google Scholar] [CrossRef]
- Andersons, J.; Kirpluks, M.; Stiebra, L.; Cabulis, U. Anisotropy of the stiffness and strength of rigid low-density closed-cell polyisocyanurate foams. Mater. Des. 2016, 92, 836–845. [Google Scholar] [CrossRef]
- Choe, K.H.; Lee, D.S.; Seo, W.J.; Kim, W.N. Properties of Rigid Polyurethane Foams with Blowing Agents and Catalysts. Polym. J. 2004, 36, 368–373. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Günther, M.; Lorenzetti, A.; Schartel, B. Fire phenomena of rigid polyurethane foams. Polymers 2018, 10, 1166. [Google Scholar] [CrossRef]
- Dębski, K.; Magiera, J.; Pielichowski, J. Wpływ struktury sztywnych pianek poliuretanowych spienianych poroforem węglowodorowym na wartość zastępczego współczynnika przewodnictwa ciepła. Polimery 2001, 46, 631–637. [Google Scholar]
- Kurańska, M.; Barczewski, M.; Uram, K.; Lewandowski, K.; Prociak, A.; Michałowski, S. Basalt waste management in the production of highly effective porous polyurethane composites for thermal insulating applications. Polym. Test. 2019, 76, 90–100. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Addition of anti-flaming agents in castor oil based rigid polyurethane foams: Studies on mechanical and flammable behaviour. Mater. Res. Express 2020, 7, 015333. [Google Scholar] [CrossRef]
- Hawkins, M.C.; O’Toole, B.; Jackovich, D. Cell morphology and mechanical properties of rigid polyurethane foam. J. Cell. Plast. 2005, 41, 267–285. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. Synthesis and application of new bio-polyols based on mustard oil for the production of selected polyurethane materials. Ind. Crops Prod. 2020, 155, 112831. [Google Scholar] [CrossRef]
- Bo, C.; Hu, L.; Jia, P.; Liang, B.; Zhou, J.; Zhou, Y. Structure and thermal properties of phosphorus-containing polyol synthesized from cardanol. RSC Adv. 2015, 5, 106651–106660. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Wang, S.; Qian, L.; Xin, F. The synergistic flame-retardant behaviors of pentaerythritol phosphate and expandable graphite in rigid polyurethane foams. Polym. Compos. 2018, 39, 329–336. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Qian, L.; Xu, B.; Xi, W. Addition flame-retardant effect of nonreactive phosphonate and expandable graphite in rigid polyurethane foams. J. Appl. Polym. Sci. 2018, 135, 45960. [Google Scholar] [CrossRef]
- Sykam, K.; Meka, K.K.R.; Donempudi, S. Intumescent Phosphorus and Triazole-Based Flame-Retardant Polyurethane Foams from Castor Oil. ACS Omega 2019, 4, 1086–1094. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. Investigation on flammability of rigid polyurethane foam-mineral fillers composite. Fire Mater. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- Zemła, M.; Prociak, A.; Michałowski, S. Bio-Based Rigid Polyurethane Foams Modified with Phosphorus Flame Retardants. Polymers 2021, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Li, L.; Chen, Y.; Xu, B.; Qiu, Y. Quickly self-extinguishing flame retardant behavior of rigid polyurethane foams linked with phosphaphenanthrene groups. Compos. Part B Eng. 2019, 175. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Y.Q.; Lin, Z.; Ma, X.; Qu, H.; Fan, R. Synthesis and characterization of bio-based intumescent flame retardant and its application in polyurethane. Fire Mater. 2020, 44, 814–824. [Google Scholar] [CrossRef]
- Schartel, B.; Pawlowski, K.H.; Lyon, R.E. Pyrolysis combustion flow calorimeter: A tool to assess flame retarded PC/ABS materials? Thermochim. Acta 2007, 462, 1–14. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, D.Y.; Liang, W.J.; Li, F.; Wang, J.S.; Liu, Y.Q. Bi-phase flame-retardant actions of water-blown rigid polyurethane foam containing diethyl-N,N-bis(2-hydroxyethyl) phosphoramide and expandable graphite. J. Anal. Appl. Pyrolysis 2017, 124, 247–255. [Google Scholar] [CrossRef]
- Szczotok, A.M.; Madsen, D.; Serrano, A.; Carmona, M.; Van Hees, P.; Rodriguez, J.F.; Kjøniksen, A.L. Flame retardancy of rigid polyurethane foams containing thermoregulating microcapsules with phosphazene-based monomers. J. Mater. Sci. 2021, 56, 1172–1188. [Google Scholar] [CrossRef]
- Gosselin, R.; Rodrigue, D. Cell morphology analysis of high density polymer foams. Polym. Test. 2005, 24, 1027–1035. [Google Scholar] [CrossRef]

| Foam Symbol | Number of Cells per 1 mm2 | Average Cross-Sectional Area of Cell·103, mm2 | Anisotropy Index |
|---|---|---|---|
| REF | 53 ± 4 | 9.27 ± 0.59 | 1.10 ± 0.04 |
| AD0RM1RE2 | 31 ± 1 | 13.65 ± 0.69 | 1.18 ± 0.05 |
| AD0RM2RE1 | 36 ± 2 | 12.39 ± 0.93 | 1.21 ± 0.01 |
| AD1RM0RE2 | 39 ± 2 | 11.73 ± 0.72 | 1.17 ± 0.06 |
| AD1RM1RE1 | 31 ± 2 | 14.72 ± 0.81 | 1.18 ± 0.06 |
| AD1RM2RE0 | 40 ± 2 | 11.77 ± 0.86 | 1.16 ± 0.05 |
| AD2RM0RE1 | 32 ± 2 | 13.15 ± 0.87 | 1.14 ± 0.04 |
| AD2RM1RE0 | 37 ± 3 | 11.55 ± 0.86 | 1.11 ± 0.07 |
| AD2RM2RE2 | 31 ± 2 | 14.25 ± 0.63 | 1.05 ± 0.04 |
| Foam Symbol | Number of Cells per 1 mm2 | Average Cross-Sectional Area of Cell·103, mm2 | Anisotropy Index |
|---|---|---|---|
| REF | 66 ± 3 | 6.95 ± 0.73 | 0.94 ± 0.04 |
| AD0RM1RE2 | 48 ± 3 | 9.76 ± 0.88 | 0.86 ± 0.01 |
| AD0RM2RE1 | 47 ± 3 | 9.43 ± 0.28 | 0.88 ± 0.03 |
| AD1RM0RE2 | 56 ± 3 | 8.27 ± 0.69 | 0.91 ± 0.01 |
| AD1RM1RE1 | 51 ± 2 | 8.30 ± 0.90 | 0.88 ± 0.04 |
| AD1RM2RE0 | 52 ± 2 | 8.58 ± 0.40 | 0.88 ± 0.03 |
| AD2RM0RE1 | 51 ± 3 | 8.24 ± 0.83 | 0.91 ± 0.03 |
| AD2RM1RE0 | 50 ± 3 | 8.27 ± 0.71 | 0.87 ± 0.03 |
| AD2RM2RE2 | 48 ± 3 | 8.70 ± 0.77 | 0.90 ± 0.03 |
| Foam Symbol | Content of Closed Cell, % | CD, Number of Cells/mm3 |
|---|---|---|
| REF | 90.8 ± 0.7 | 432 ± 7 |
| AD0RM1RE2 | 88.8 ± 0.6 | 216 ± 3 |
| AD0RM2RE1 | 86.3 ± 0.7 | 244 ± 4 |
| AD1RM0RE2 | 86.0 ± 0.7 | 295 ± 4 |
| AD1RM1RE1 | 86.1 ± 0.4 | 254 ± 5 |
| AD1RM2RE0 | 86.4 ± 0.6 | 287 ± 3 |
| AD2RM0RE1 | 88.4 ± 1.3 | 227 ± 3 |
| AD2RM1RE0 | 89.2 ± 0.8 | 263 ± 4 |
| AD2RM2RE2 | 87.9 ± 0.9 | 212 ± 3 |
| Foam Symbol | Apparent Density, kg/m3 | Thermal Conductivity Coefficient, mW/m·K | Content of Closed Cell, % |
|---|---|---|---|
| REF | 36.8 ± 0.4 | 24.36 ± 0.08 | 90.8 ± 0.7 |
| AD0RM1RE2 | 35.5 ± 0.4 | 25.82 ± 0.83 | 88.8 ± 0.6 |
| AD0RM2RE1 | 35.1 ± 1.0 | 25.37 ± 0.41 | 86.3 ± 0.7 |
| AD1RM0RE2 | 36.8 ± 0.5 | 25.24 ± 0.01 | 86.0 ± 0.7 |
| AD1RM1RE1 | 37.3 ± 0.8 | 24.74 ± 0.06 | 86.1 ± 0.4 |
| AD1RM2RE0 | 38.4 ± 0.1 | 24.38 ± 0.16 | 86.4 ± 0.6 |
| AD2RM0RE1 | 39.4 ± 0.1 | 25.02 ± 0.24 | 88.4 ± 1.3 |
| AD2RM1RE0 | 40.4 ± 0.5 | 24.89 ± 0.06 | 89.2 ± 0.8 |
| AD2RM2RE2 | 39.7 ± 0.1 | 24.94 ± 0.20 | 87.9 ± 0.9 |
| Foam Symbol | Parallel | Perpendicular | Brittleness, % | ||
|---|---|---|---|---|---|
| Compressive Strength, kPa | Modulus, MPa | Compressive Strength, kPa | Modulus, MPa | ||
| REF | 212.6 ± 6.8 | 5.17 ± 0.34 | 131.3 ± 7.2 | 3.33 ± 0.16 | 3.04 ± 0.23 |
| AD0RM1RE2 | 209.4 ± 9.6 | 5.15 ± 0.32 | 98.2 ± 4.1 | 2.41 ± 0.05 | 4.90 ± 0.43 |
| AD0RM2RE1 | 211.2 ± 3.0 | 4.93 ± 0.17 | 90.3 ± 2.8 | 2.41 ± 0.11 | 5.91 ± 0.31 |
| AD1RM0RE2 | 199.2 ± 1.3 | 4.91 ± 0.17 | 96.0 ± 5.9 | 2.53 ± 0.14 | 6.06 ± 0.50 |
| AD1RM1RE1 | 221.9 ± 5.1 | 5.00 ± 0.06 | 118.3 ± 3.3 | 2.84 ± 0.17 | 5.29 ± 0.24 |
| AD1RM2RE0 | 197.0 ± 10.1 | 4.72 ± 0.12 | 117.5 ± 6.8 | 2.74 ± 0.10 | 4.22 ± 0.33 |
| AD2RM0RE1 | 203.5 ± 4.1 | 4.81 ± 0.05 | 107.2 ± 3.1 | 2.63 ± 0.07 | 3.35 ± 0.32 |
| AD2RM1RE0 | 205.5 ± 4.6 | 5.00 ± 0.13 | 126.4 ± 3.8 | 3.01 ± 0.05 | 3.70 ± 0.22 |
| AD2RM2RE2 | 171.7 ± 21.1 | 4.61 ± 0.64 | 106.7 ± 4.6 | 2.25 ± 0.16 | 3.93 ± 0.44 |
| Foam Symbol | THR, kJ/g | HRC, J/g·K | Temp1, °C | pHRR1, W/g | Temp2, °C | pHRR2, W/g | Temp3, °C | pHRR3, W/g |
|---|---|---|---|---|---|---|---|---|
| REF | 29.9 ± 0.3 | 199 ± 3 | 334 ± 2 | 182.0 ± 1.7 | - | - | 488 ± 7 | 67.2 ± 6.2 |
| AD0RM1RE2 | 26.2 ± 0.5 | 175 ± 5 | 317 ± 2 | 160.4 ± 4.3 | 392 ± 3 | 91.6 ± 5.3 | 450 ± 2 | 94.3 ± 3.1 |
| AD0RM2RE1 | 26.1 ± 0.2 | 174 ± 7 | 319 ± 1 | 158.9 ± 6.3 | 378 ± 3 | 89.8 ± 3.0 | 448 ± 1 | 93.2 ± 1.0 |
| AD1RM0RE2 | 26.9 ± 0.6 | 206 ± 1 | 320 ± 1 | 187.9 ± 1.7 | 401 ± 1 | 89.7 ± 3.1 | 456 ± 2 | 90.3 ± 3.4 |
| AD1RM1RE1 | 26.0 ± 0.1 | 200 ± 6 | 323 ± 1 | 182.2 ± 3.4 | 392 ± 5 | 77.1 ± 0.7 | 456 ± 2 | 81.8 ± 1.8 |
| AD1RM2RE0 | 25.4 ± 0.4 | 193 ± 8 | 324 ± 1 | 176.4 ± 5.2 | 401 ± 3 | 72.6 ± 3.4 | 457 ± 5 | 82.3 ± 2.9 |
| AD2RM0RE1 | 26.5 ± 0.4 | 189 ± 3 | 325 ± 2 | 172.9 ± 1.8 | 426 ± 2 | 68.6 ± 1.1 | 477 ± 2 | 77.1 ± 2.0 |
| AD2RM1RE0 | 25.4 ± 0.7 | 177 ± 4 | 327 ± 3 | 162.8 ± 2.7 | 426 ± 1 | 53.3 ± 1.6 | 486 ± 2 | 65.8 ± 1.5 |
| AD2RM2RE2 | 24.6 ± 0.3 | 136 ± 5 | 308 ± 1 | 124.0 ± 4.0 | 394 ± 1 | 81.8 ± 3.3 | 458 ± 2 | 84.6 ± 2.6 |
| Foam Symbol | TTI, s | THR, MJ/m2 | pHRR, kW/m2 | Av-HRR, kW/m2 | MARHE, kW/m2 | Av-EHC, MJ/kg |
|---|---|---|---|---|---|---|
| REF | 4 ± 1 | 10.2 ± 1.1 | 268.7 ± 10.7 | 31.3 ± 3.9 | 156 ± 4 | 12.8 ± 0.6 |
| AD0RM1RE2 | 3 ± 1 | 6.2 ± 0.4 | 155.8 ± 12.9 | 13.6 ± 4.5 | 61 ± 9 | 8.1 ± 0.2 |
| AD0RM2RE1 | 3 ± 1 | 5.4 ± 0.9 | 128.3 ± 8.8 | 14.1 ± 2.9 | 62 ± 6 | 7.1 ± 0.7 |
| AD1RM0RE2 | 4 ± 1 | 6.0 ± 0.3 | 262.3 ± 4.8 | 15.4 ± 1.0 | 113 ± 3 | 6.2 ± 0.3 |
| AD1RM1RE1 | 4 ± 1 | 5.0 ± 0.8 | 237.5 ± 13.4 | 12.7 ± 3.4 | 97 ± 5 | 5.1 ± 0.5 |
| AD1RM2RE0 | 5 ± 1 | 5.5 ± 0.7 | 201.2 ± 13.6 | 14.4 ± 3.1 | 80 ± 2 | 7.1 ± 0.1 |
| AD2RM0RE1 | 3 ± 1 | 5.6 ± 0.6 | 190.2 ± 8.9 | 14.8 ± 2.9 | 98 ± 6 | 5.9 ± 0.3 |
| AD2RM1RE0 | 3 ± 1 | 5.6 ± 0.5 | 162.7 ± 8.0 | 14.9 ± 1.7 | 90 ± 5 | 5.1 ± 0.8 |
| AD2RM2RE2 | 2 ± 1 | 5.2 ± 0.2 | 139.6 ± 11.4 | 13.7 ± 1.1 | 54 ± 1 | 5.8 ± 0.4 |
| Foam Symbol | TSR, m2/m2 | TSP, m2 | Av-COY, kg/kg | Av-CO2Y, kg/kg | CO/CO2 Weight Ratio | Residue, % |
|---|---|---|---|---|---|---|
| REF | 501 ± 39 | 4.43 ± 0.34 | 0.60 ± 0.08 | 3.87 ± 0.38 | 0.155 ± 0.004 | 30.7 ± 3.2 |
| AD0RM1RE2 | 315 ± 44 | 2.79 ± 0.39 | 0.64 ± 0.04 | 3.70 ± 0.33 | 0.173 ± 0.012 | 37.0 ± 4.1 |
| AD0RM2RE1 | 360 ± 36 | 3.18 ± 0.31 | 0.68 ± 0.08 | 3.65 ± 0.42 | 0.187 ± 0.007 | 35.3 ± 8.9 |
| AD1RM0RE2 | 519 ± 41 | 4.59 ± 0.36 | 0.91 ± 0.06 | 3.29 ± 0.40 | 0.278 ± 0.018 | 26.8 ± 4.2 |
| AD1RM1RE1 | 476 ± 28 | 4.21 ± 0.24 | 0.99 ± 0.21 | 3.48 ± 0.71 | 0.285 ± 0.016 | 42.7 ± 0.3 |
| AD1RM2RE0 | 598 ± 31 | 5.29 ± 0.27 | 1.11 ± 0.17 | 3.31 ± 0.38 | 0.336 ± 0.015 | 30.5 ± 5.2 |
| AD2RM0RE1 | 818 ± 32 | 7.23 ± 0.28 | 1.15 ± 0.08 | 2.57 ± 0.20 | 0.448 ± 0.008 | 19.1 ± 3.9 |
| AD2RM1RE0 | 875 ± 32 | 8.11 ± 0.67 | 1.25 ± 0.11 | 2.75 ± 0.28 | 0.455 ± 0.007 | 17.0 ± 6.9 |
| AD2RM2RE2 | 453 ± 24 | 4.01 ± 0.21 | 0.98 ± 0.04 | 3.16 ± 0.32 | 0.311 ± 0.017 | 29.9 ± 8.5 |
| Foam Symbol | RF-551, g | 1.6Hex, g | Polycat® 218, g | L-6915, g | Water, g | PMDI, g | AD, g | RM, g | RE, g |
|---|---|---|---|---|---|---|---|---|---|
| REF | 60 | 40 | 1.5 | 1.5 | 3.23 | 148.00 | 0 | 0 | 0 |
| AD0RM1RE2 | 30 | 144.73 | 0 | 10 | 20 | ||||
| AD0RM2RE1 | 30 | 140.27 | 0 | 20 | 10 | ||||
| AD1RM0RE2 | 40 | 148.80 | 10 | 0 | 20 | ||||
| AD1RM1RE1 | 40 | 144.33 | 10 | 10 | 10 | ||||
| AD1RM2RE0 | 40 | 139.87 | 10 | 20 | 0 | ||||
| AD2RM0RE1 | 50 | 148.40 | 20 | 0 | 10 | ||||
| AD2RM1RE0 | 50 | 143.94 | 20 | 10 | 0 | ||||
| AD2RM2RE2 | 20 | 140.67 | 20 | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemła, M.; Prociak, A.; Michałowski, S.; Cabulis, U.; Kirpluks, M.; Simakovs, K. Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants. Int. J. Mol. Sci. 2022, 23, 12386. https://doi.org/10.3390/ijms232012386
Zemła M, Prociak A, Michałowski S, Cabulis U, Kirpluks M, Simakovs K. Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants. International Journal of Molecular Sciences. 2022; 23(20):12386. https://doi.org/10.3390/ijms232012386
Chicago/Turabian StyleZemła, Marcin, Aleksander Prociak, Sławomir Michałowski, Ugis Cabulis, Mikelis Kirpluks, and Kirils Simakovs. 2022. "Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants" International Journal of Molecular Sciences 23, no. 20: 12386. https://doi.org/10.3390/ijms232012386
APA StyleZemła, M., Prociak, A., Michałowski, S., Cabulis, U., Kirpluks, M., & Simakovs, K. (2022). Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants. International Journal of Molecular Sciences, 23(20), 12386. https://doi.org/10.3390/ijms232012386









