Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea
Abstract
:1. Introduction
2. Results
2.1. Presentation of Data
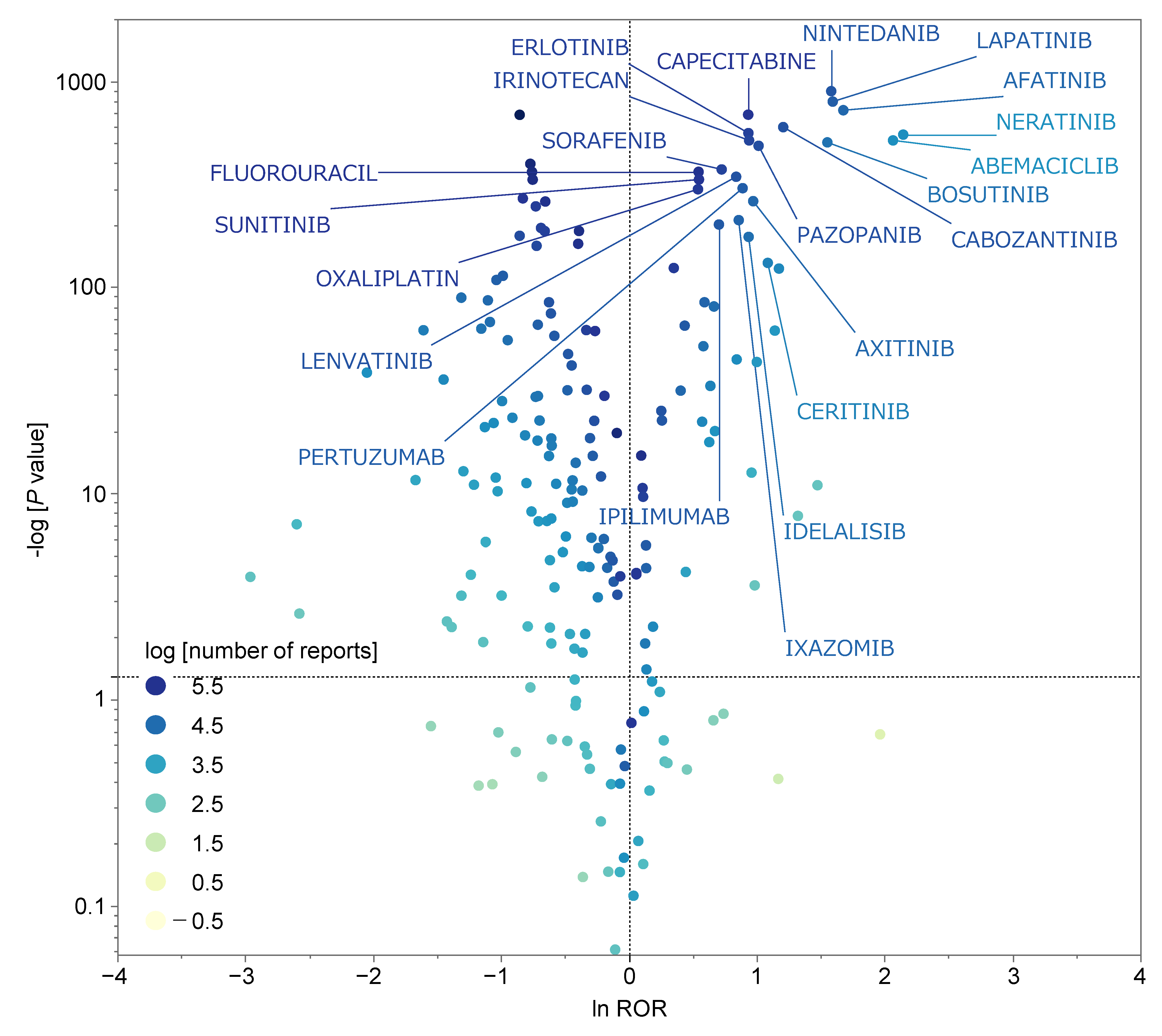
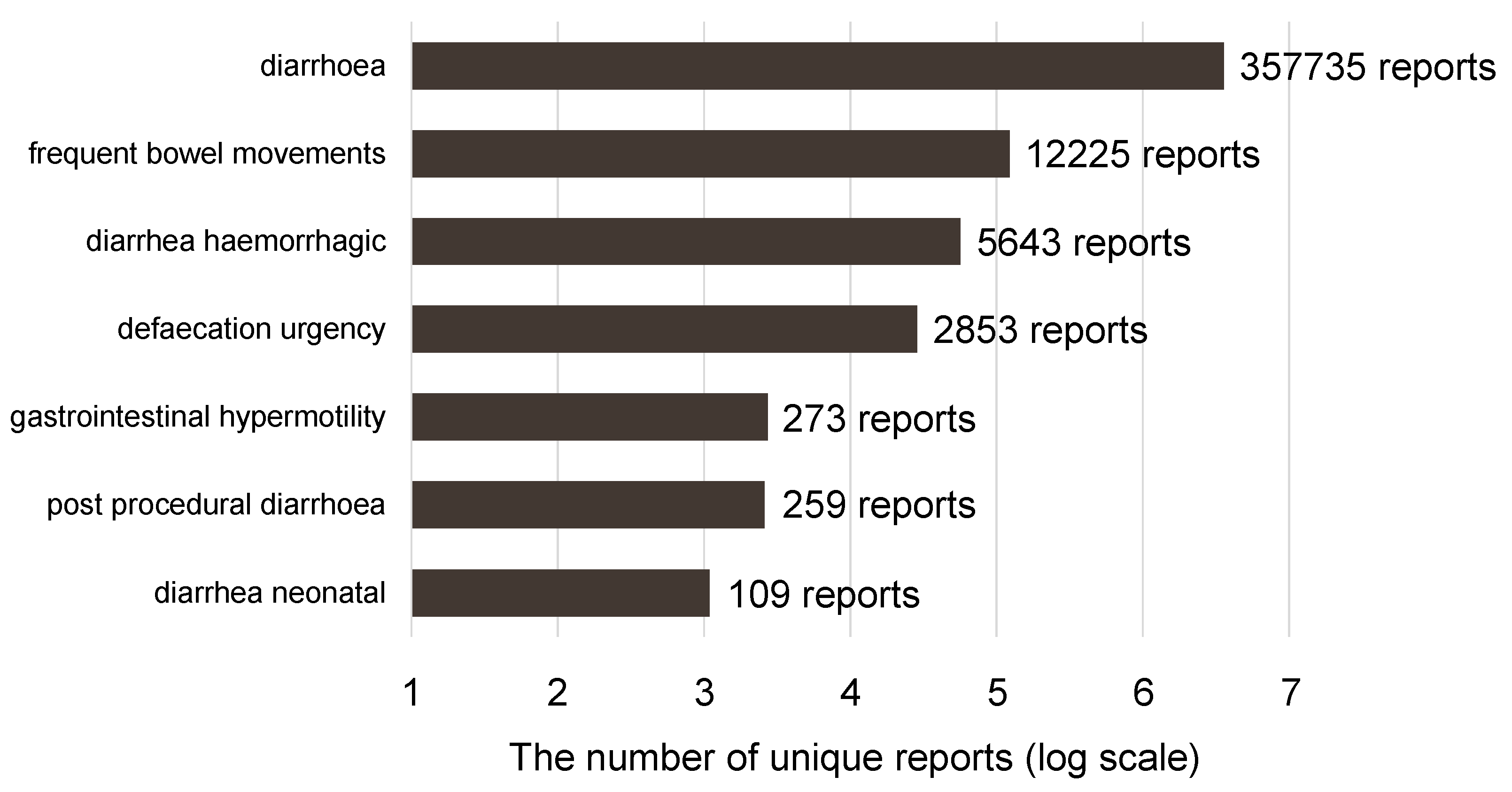
2.2. Diarrhea-Inducing Antineoplastic Agents
| Antineoplastic Agents | ROR (95% CI) | p-Value | Number of Report | ATC Code | ATC Name |
|---|---|---|---|---|---|
| Neratinib | 8.56 (8.03–9.12) | <0.0001 | 8136 | L01EH | HER2 tyrosine kinase inhibitors |
| Abemaciclib | 7.90 (7.41–8.43) | <0.0001 | 8400 | L01EF | CDK inhibitors |
| Afatinib | 5.35 (5.17–5.55) | <0.0001 | 38,698 | L01EB | EGFR tyrosine kinase inhibitors |
| Lapatinib | 4.93 (4.78–5.08) | <0.0001 | 57,315 | L01EH | HER2 tyrosine kinase inhibitors |
| Nintedanib | 4.87 (4.74–5.00) | <0.0001 | 75,028 | L01EX | Other protein kinase inhibitors |
| Bosutinib | 4.73 (4.51–4.95) | <0.0001 | 24,978 | L01EA | BCR-ABL tyrosine kinase inhibitors |
| Dacomitinib | 4.38 (3.09–6.19) | <0.0001 | 465 | L01EB | EGFR tyrosine kinase inhibitors |
| Diarrhea | Non—Diarrhea | |
|---|---|---|
| Reports with the suspected medicine | a | c |
| All other reports | b | d |
2.3. Univariate Analysis of Antineoplastic Agents Suspected of Inducing Diarrhea
2.4. Multivariate Analysis of Antineoplastic Agents Suspected of Inducing Diarrhea
3. Discussion
Limitations
4. Materials and Methods
4.1. Database Information
4.2. Terminology of ADE
4.3. Creation of Data Analysis Table
4.4. Reporting Odds Ratio (ROR) Calculations
4.5. Construction of Scatter Plot
4.6. MIE Activity Prediction Using QSAR Toxicity Predictor
4.7. Multivariate Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chassany, O.; Michaux, A.; Bergmann, J.F. Drug-induced diarrhoea. Drug Saf. 2000, 22, 53–72. [Google Scholar] [CrossRef]
- Saltz, L.B.; Douillard, J.Y.; Pirotta, N.; Alakl, M.; Gruia, G.; Awad, L.; Elfring, G.L.; Locker, P.K.; Miller, L.L. Irinotecan plus fluorouracil/leucovorin for metastatic colorectal cancer: A new sur-vival standard. Oncologist 2001, 6, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shaughnessy, J.; Miles, D.; Vukelja, S.; Moiseyenko, V.; Ayoub, J.-P.; Cervantes, G.; Fumoleau, P.; Jones, S.; Lui, W.-Y.; Mauriac, L.; et al. Superior survival with capecitabine plus docetaxel combination therapy in an-thracycline-pretreated patients with advanced breast cancer: Phase III trial results. J. Clin. Oncol. 2002, 20, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Norman, A.R.; Cunningham, D.; Tait, D.; Ross, P.J.; Iveson, T.; Hill, M.; Hickish, T.; Lofts, F.; Jodrell, D.; et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann. Oncol. 2005, 16, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crino, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar]
- Fuchs, C.S.; Marshall, J.; Mitchell, E.; Wierzbicki, R.; Ganju, V.; Jeffery, M.; Schulz, J.; Richards, D.; Soufi-Mahjoubi, R.; Wang, B.; et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropy-rimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C Study. J. Clin. Oncol. 2007, 25, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Kohne, C.-H.; Lang, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Faculty Opinions recommendation of Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2014, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with ad-vanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Tredan, O.; Chen, S.-C.; Manso, L.; et al. Faculty Opinions recommendation of MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2019, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Pariente, E.A. Drug-induced enteropathies. Gastroenterol. Clin. Biol. 1982, 6, 16–18. [Google Scholar]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, M.W.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef]
- Allen, T.E.; Goodman, J.M.; Gutsell, S.; Russell, P.J. Defining molecular initiating events in the adverse outcome pathway framework for risk assessment. Chem. Res. Toxicol. 2014, 27, 2100–2112. [Google Scholar] [CrossRef]
- Gadaleta, D.; Manganelli, S.; Roncaglioni, A.; Toma, C.; Benfenati, E.; Mombelli, E. QSAR modeling of ToxCast assays relevant to the molecular initiating events of AOPs leading to hepatic steatosis. J. Chem. Inf. Model. 2018, 58, 1501–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.P.; Nelson, L.A.; Williams, J.L.; Kemp, C.J.; Erwin, C.R.; Warner, B.W. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J. Surg. Res. 2002, 105, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, I.; López-Tarruella, S.; Márquez-Rodas, I.; Jerez, Y.; Marin, M. Neratinib for the treatment of HER2-positive early stage breast cancer. Expert. Rev. Anticancer. Ther. 2017, 17, 669–679. [Google Scholar] [CrossRef]
- Fan, L.; Iseki, S. Immunohistochemical localization of vascular endothelial growth factor in the endocrine glands of the rat. Arch. Histol. Cytol. 1998, 61, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kamba, T.; Tam, B.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C.; et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvas-culature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef] [Green Version]
- Van Sebille, Y.Z.; Gibson, R.J.; Wardill, H.R.; Bowen, J.M. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: Chloride secretion as a mechanistic hypothesis. Cancer Treat. Rev. 2015, 41, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Secombe, K.R.; Van Sebille, Y.Z.A.; Mayo, B.J.; Coller, J.K.; Gibson, R.J.; Bowen, J.M. Diarrhea Induced by Small Molecule Tyrosine Kinase Inhibitors Compared With Chemotherapy: Potential Role of the Microbiome. Integr. Cancer Ther. 2020, 19, 1534735420928493. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E. Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol. Cell Endocrinol. 2012, 357, 4–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Kominea, A.; Vandoros, G.; Skyiotis, G.P.; Andricopoulos, P.; Varakis, I.; Sotiropoulou-Bonikou, G.; Papavassiliou, A.G. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur. J. Cancer 2003, 39, 1251–1258. [Google Scholar] [CrossRef]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor be-ta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Hoy, E.; Xu, D.-Z.; Lu, Q.; Feinman, R.; Deitch, E.A. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am. J. Physiol. Liver Physiol. 2005, 288, G466–G472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mifeprex (Mifepristone) Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/mifeprex-mifepristone-information#:~:text=Mifeprex%20%28mifepristone%29%20is%20used%2C%20together%20with%20another%20medication,data%20and%20information%20submitted%20by%20the%20drug%20manufacturer (accessed on 17 August 2022).
- Rajaram, R.D.; Brisken, C. Paracrine signaling by progesterone. Mol. Cell Endocrinol. 2012, 357, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salame, G.; Fourrier-Reglat, A.; Haramburu, F.; Beguad, B.; Moore, N.; Asso-ciation Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salame, G.; Fourrier-Reglat, A.; Haramburu, F.; Beguad, B.; Moore, N.; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Evaluation of an au-tomated method to decrease false-positive signals induced by co-prescriptions in spontaneous reporting databases. Phar-macoepidemiol. Drug Saf. 2014, 23, 186–194. [Google Scholar]
- Maeda, R. 5. JADER from Pharmacovigilance Point of View. Jpn. J. Pharmacoepidemiol. Eekigaku 2014, 19, 51–56. [Google Scholar] [CrossRef]
- Lumini, A.; Nanni, L. Convolutional neural networks for ATC classification. Curr. Pharm. Des. 2018, 24, 4007–4012. [Google Scholar] [CrossRef] [PubMed]
- FDA Adverse Event Reporting System (FAERS). Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers (accessed on 17 August 2022).
- Watanabe, H.; Matsushita, Y.; Watanabe, A.; Maeda, T.; Nukui, K.; Ogawa, Y.; Sawa, J.; Maeda, H. Early detection of important safety information. Recent methods for signal detection. Jpn. J. Biomet. 2004, 25, 37–60. [Google Scholar] [CrossRef]
- Ohyama, K.; Sugiura, M. Evaluation of the association between topical prostaglandin F2α analogs and asthma using the JADER database: Comparison with β-blockers. Yakugaku Zasshi 2018, 138, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.-J. Selection of differentially expressed genes in microarray data analysis. Pharm. J. 2007, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of factors associated with hiccups based on the Japanese Adverse Drug Event Report Database. PLoS ONE 2017, 12, e0172057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toriumi, S.; Kobayashi, A.; Uesawa, Y. Comprehensive study of the risk factors for medication-related osteonecrosis of the jaw based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals 2020, 13, 467. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Matsui, R.; Uesawa, Y. Evaluation of the expression profile of irinotecan-induced diarrhea in patients with colorectal cancer. Pharmaceuticals 2021, 14, 377. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Comprehensive Analysis of Chemotherapeutic Agents That Induce Infectious Neutro-penia. Pharmaceuticals 2021, 14, 681. [Google Scholar] [CrossRef]
- Kurosaki, K.; Wu, R.; Uesawa, Y. A Toxicity Prediction Tool for Potential Agonist/Antagonist Activities in Molecular Initiating Events Based on Chemical Structures. Int. J. Mol. Sci. 2020, 21, 7853. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| ROR | 95% CI | p-Value | ROR | p-Value | ||
| Lower | Upper | |||||
| GR antagonist | 5.342 | 1.54 | 18.59 | 0.0024 | — | — |
| PR antagonist | 4.750 | 2.08 | 10.84 | <0.0001 | 0.779 | 0.0002 |
| VDR agonist | 4.537 | 1.65 | 12.47 | 0.0011 | — | — |
| TGFb antagonist | 4.071 | 1.67 | 9.95 | 0.0008 | — | — |
| ERb antagonist | 3.919 | 1.60 | 9.58 | 0.0012 | — | — |
| ARfull antagonist | 3.831 | 1.39 | 10.58 | 0.0043 | — | — |
| ARfulls antagonist | 3.619 | 1.48 | 8.86 | 0.0023 | — | — |
| ERR agonist | 3.619 | 1.48 | 8.86 | 0.0023 | — | — |
| Shh antagonist | 3.537 | 1.37 | 9.13 | 0.0045 | — | — |
| PPARd agonist | 3.273 | 1.50 | 7.14 | 0.0017 | — | — |
| PPARg antagonist | 3.250 | 1.26 | 8.41 | 0.0082 | — | — |
| PPARg agonist | 3.119 | 1.37 | 7.12 | 0.0040 | — | — |
| Arom antagonist | 3.113 | 1.20 | 8.06 | 0.0111 | — | — |
| ERlbd antagonist | 3.083 | 1.31 | 7.27 | 0.0058 | — | — |
| ERR agonist | 3.079 | 1.25 | 7.57 | 0.0082 | — | — |
| PPARd antagonist | 2.885 | 1.26 | 6.60 | 0.0074 | — | — |
| ARlbd agonist | 2.714 | 1.10 | 6.69 | 0.0190 | — | — |
| PXR agonist | 2.565 | 1.24 | 5.32 | 0.0082 | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okunaka, M.; Kano, D.; Uesawa, Y. Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. Int. J. Mol. Sci. 2022, 23, 12407. https://doi.org/10.3390/ijms232012407
Okunaka M, Kano D, Uesawa Y. Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. International Journal of Molecular Sciences. 2022; 23(20):12407. https://doi.org/10.3390/ijms232012407
Chicago/Turabian StyleOkunaka, Mashiro, Daisuke Kano, and Yoshihiro Uesawa. 2022. "Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea" International Journal of Molecular Sciences 23, no. 20: 12407. https://doi.org/10.3390/ijms232012407
APA StyleOkunaka, M., Kano, D., & Uesawa, Y. (2022). Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. International Journal of Molecular Sciences, 23(20), 12407. https://doi.org/10.3390/ijms232012407







