Transcriptomic Analysis in Marine Medaka Gill Reveals That the Hypo-Osmotic Stress Could Alter the Immune Response via the IL17 Signaling Pathway
Abstract
1. Introduction
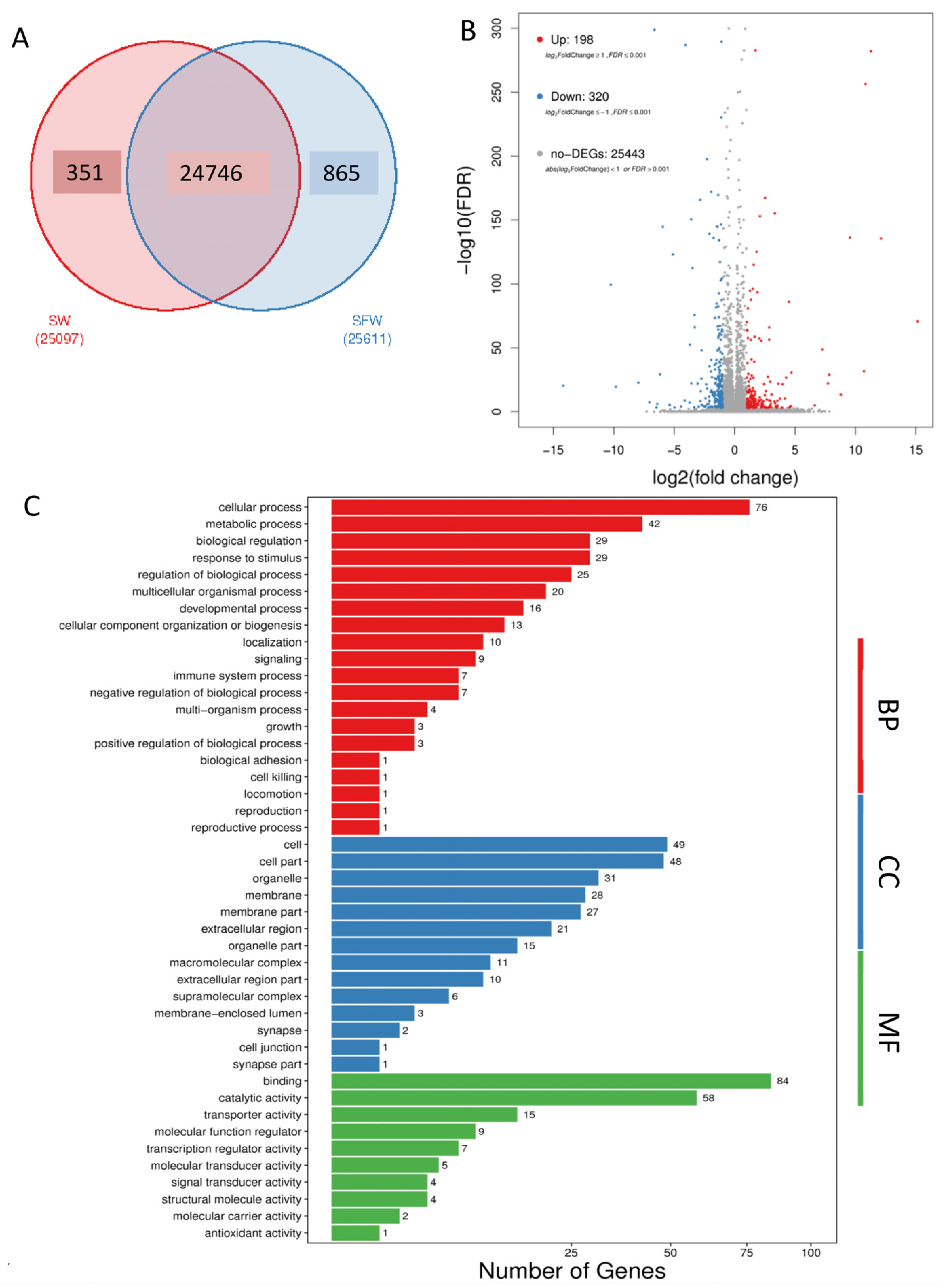
2. Results
3. Discussion
4. Materials and Methods
4.1. Fish Maintenance and Experimental Setup
4.2. Fish Sampling
4.3. Library Construction and Illumina RNA-seq
4.4. Reverse Transcription and Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Mizuhira, V.; Amakawa, T.; Yamashina, S.; Shirai, N.; Utida, S. Electron microscopic studies on the localization of sodium ions and sodium-potassium-activated adenosinetriphosphatase in chloride cells of eel gills. Exp. Cell Res. 1970, 59, 346–348. [Google Scholar] [CrossRef]
- Wong, C.K.C.; Chan, D.K.O. Effects of cortisol on chloride cells in the gill epithelium of Japanese eel, Anguilla japonica. J. Endocrinol. 2001, 168, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, B.Y.; Han, J.; Jeong, C.B.; Hwang, D.S.; Lee, M.C.; Kang, H.M.; Kim, D.H.; Lee, D.; Kim, J.; et al. The genome of the marine medaka Oryzias melastigma. Mol. Ecol. Resour. 2018, 18, 656–665. [Google Scholar] [CrossRef]
- Lai, K.P.; Li, J.W.; Wang, S.Y.; Chiu, J.M.Y.; Tse, A.; Lau, K.; Lok, S.; Au, D.W.T.; Tse, W.K.F.; Wong, C.K.C.; et al. Tissue-specific transcriptome assemblies of the marine medaka Oryzias melastigma and comparative analysis with the freshwater medaka Oryzias latipes. BMC Genom. 2015, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Gong, Z.; Tse, W.K.F. Zebrafish as the toxicant screening model: Transgenic and omics approaches. Aquat. Toxicol. 2021, 234, 105813. [Google Scholar] [CrossRef]
- Liang, P.; Saqib, H.S.A.; Lin, Z.; Zheng, R.; Qiu, Y.; Xie, Y.; Ma, D.; Shen, Y. RNA-seq analyses of Marine Medaka (Oryzias melastigma) reveals salinity responsive transcriptomes in the gills and livers. Aquat. Toxicol. Amst. Neth. 2021, 240, 105970. [Google Scholar] [CrossRef] [PubMed]
- Kultz, D. The combinatorial nature of osmosensing in fishes. Physiol. Bethesda Md. 2012, 27, 259–275. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Au, D.W.T.; Wong, C.K.C. Effect of osmotic shrinkage and hormones on the expression of Na+/H+ exchanger-1, Na+/K+/2Cl(-) cotransporter and Na+/K+-ATPase in gill pavement cells of freshwater adapted Japanese eel, Anguilla japonica. J. Exp. Biol. 2007, 210, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.K.F.; Chow, S.C.; Wong, C.K.C. The cloning of eel osmotic stress transcription factor and the regulation of its expression in primary gill cell culture. J. Exp. Biol. 2008, 211, 1964–1968. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Lai, K.P.; Takei, Y. Medaka osmotic stress transcription factor 1b (Ostf1b/TSC22D3-2) triggers hyperosmotic responses of different ion transporters in medaka gill and human embryonic kidney cells via the JNK signalling pathway. Int. J. Biochem. Cell Biol. 2011, 43, 1764–1775. [Google Scholar] [CrossRef]
- Chow, S.C.; Wong, C.K.C. Regulatory function of hyperosmotic stress-induced signaling cascades in the expression of transcription factors and osmolyte transporters in freshwater Japanese eel primary gill cell culture. J. Exp. Biol. 2011, 214, 1264–1270. [Google Scholar] [CrossRef][Green Version]
- Tse, W.K.F.; Au, D.W.T.; Wong, C.K.C. Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem. Biophys. Res. Commun. 2006, 346, 1181–1190. [Google Scholar] [CrossRef]
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review. Int. J. Mol. Sci. 2018, 19, 245. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.M.S.; Taverne-Thiele, J.J.; Barnes, A.C.; van Muiswinkel, W.B.; Ellis, A.E.; Rombout, J.H.W.M. The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax L.) in a Photobacterium damselae ssp. piscicida bacterin: An ontogenetic study. Fish Shellfish Immunol. 2001, 11, 65–74. [Google Scholar] [CrossRef]
- Grove, S.; Johansen, R.; Reitan, L.J.; Press, C.M. Immune- and enzyme histochemical characterisation of leukocyte populations within lymphoid and mucosal tissues of Atlantic halibut (Hippoglossus hippoglossus). Fish Shellfish Immunol. 2006, 20, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Mulero, I.; Sepulcre, M.P.; Roca, F.J.; Meseguer, J.; García-Ayala, A.; Mulero, V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev. Comp. Immunol. 2008, 32, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Zhu, P.; Boncan, D.A.T.; Yang, L.; Leung, C.C.T.; Ho, J.C.H.; Lin, X.; Chan, T.F.; Kong, R.Y.C.; Tse, W.K.F. Integrated Omics Approaches Revealed the Osmotic Stress-Responsive Genes and Microbiota in Gill of Marine Medaka. mSystems 2022, 7, e0004722. [Google Scholar] [CrossRef] [PubMed]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Roberts, S.D.; Powell, M.D. Comparative ionic flux and gill mucous cell histochemistry: Effects of salinity and disease status in Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 525–537. [Google Scholar] [CrossRef]
- Ringo, E.; Wesmajervi, M.S.; Bendiksen, H.R.; Berg, A.; Olsen, R.E.; Johnsen, T.; Mikkelsen, H.; Seppola, M.; Strom, E.; Holzapfel, W. Identification and characterization of Carnobacteria isolated from fish intestine. Syst. Appl. Microbiol. 2001, 24, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Laiz-Carrion, R.; Del Rio, M.P.; Meseguer, J.; Mancera, J.M.; Esteban, M.A. Salinity influences the humoral immune parameters of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2005, 18, 255–261. [Google Scholar] [CrossRef]
- Taylor, J.F.; Needham, M.P.; North, B.P.; Morgan, A.; Thompson, K.; Migaud, H. The influence of ploidy on saltwater adaptation, acute stress response and immune function following seawater transfer in non-smolting rainbow trout. Gen. Comp. Endocrinol. 2007, 152, 314–325. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Sun, J.; Zhang, H.M.; Lai, K.P.; Gu, J.; Qiu, J.W.; Wong, C.K.C. iTRAQ-based quantitative proteomic analysis reveals acute hypo-osmotic responsive proteins in the gills of the Japanese eel (Anguilla japonica). J. Proteom. 2014, 105, 133–143. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, H.; Cao, Q.; Yin, S.; Lai, K.P.; Tse, W.K.F.; Wong, C.K.C.; Shi, H. Identification of immune-related genes in gill cells of Japanese eels (Anguilla japonica) in adaptation to water salinity changes. Fish Shellfish Immunol. 2018, 73, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ma, A.; Huang, Z.; Wang, X.; Liu, Z.; Xia, D.; Yang, S.; Zhao, T. Comparative transcriptomic analysis reveals mechanisms of divergence in osmotic regulation of the turbot Scophthalmus maximus. Fish Physiol. Biochem. 2020, 46, 1519–1536. [Google Scholar] [CrossRef]
- Lai, K.P.; Li, J.W.; Gu, J.; Chan, T.F.; Tse, W.K.; Wong, C.K. Transcriptomic analysis reveals specific osmoregulatory adaptive responses in gill mitochondria-rich cells and pavement cells of the Japanese eel. BMC Genom. 2015, 16, 1072. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Xu, P. Gills full-length transcriptomic analysis of osmoregulatory adaptive responses to salinity stress in Coilia nasus. Ecotoxicol. Env. Saf. 2021, 226, 112848. [Google Scholar] [CrossRef]
- Takahashi, H.; Prunet, P.; Kitahashi, T.; Kajimura, S.; Hirano, T.; Grau, E.G.; Sakamoto, T. Prolactin receptor and proliferating/apoptotic cells in esophagus of the Mozambique tilapia (Oreochromis mossambicus) in fresh water and in seawater. Gen. Comp. Endocrinol. 2007, 152, 326–331. [Google Scholar] [CrossRef]
- Ching, B.; Chen, X.L.; Yong, J.H.; Wilson, J.M.; Hiong, K.C.; Sim, E.W.; Wong, W.P.; Lam, S.H.; Chew, S.F.; Ip, Y.K. Increases in apoptosis, caspase activity and expression of p53 and bax, and the transition between two types of mitochondrion-rich cells, in the gills of the climbing perch, Anabas testudineus, during a progressive acclimation from freshwater to seawater. Front. Physiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Xiao, S.; Wong, N.K.; Li, J.; Lin, Y.; Zhang, Y.; Ma, H.; Mo, R.; Zhang, Y.; Yu, Z. Analysis of in situ Transcriptomes Reveals Divergent Adaptive Response to Hyper- and Hypo-Salinity in the Hong Kong Oyster, Crassostrea hongkongensis. Front. Physiol. 2018, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.N.; Corkins, M.E.; Li, J.C.; Singh, K.; Parsons, S.; Tucey, T.M.; Sorkaç, A.; Huang, H.; Dimitriadi, M.; Sinclair, D.A.; et al. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 2016, 154, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qin, H.; Du, W.; Shen, Y.W.; Lee, W.H.; Riggs, A.D.; Liu, C.P. Inhibition of S-phase kinase-associated protein 2 (Skp2) reprograms and converts diabetogenic T cells to Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9493–9498. [Google Scholar] [CrossRef]
- Huang, H.; Tindall, D.J. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Et. Biophys. Acta 2011, 1813, 1961–1964. [Google Scholar] [CrossRef]
- Ke, Z.B.; Cai, H.; Wu, Y.P.; Lin, Y.Z.; Li, X.D.; Huang, J.B.; Sun, X.L.; Zheng, Q.S.; Xue, X.Y.; Wei, Y.; et al. Identification of key genes and pathways in benign prostatic hyperplasia. J. Cell Physiol. 2019, 234, 19942–19950. [Google Scholar] [CrossRef]
- Brennan, R.S.; Galvez, F.; Whitehead, A. Reciprocal osmotic challenges reveal mechanisms of divergence in phenotypic plasticity in the killifish Fundulus heteroclitus. J. Exp. Biol. 2015, 218, 1212–1222. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Wu, G.; Zang, Z.; Zhang, J.Q.; Li, X.; Tao, J.; Shen, M.; Liu, H. FOXO1 mediates hypoxia-induced G0/G1 arrest in ovarian somatic granulosa cells by activating the TP53INP1-p53-CDKN1A pathway. Development 2021, 148, dev199453. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, M.; Dal Pont, G.; Eom, J.; Schulte, P.M.; Wood, C.M. The effects of salinity and hypoxia exposure on oxygen consumption, ventilation, diffusive water exchange and ionoregulation in the Pacific hagfish (Eptatretus stoutii). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 232, 47–59. [Google Scholar] [CrossRef]
- Giacomin, M.; Bryant, H.J.; Val, A.L.; Schulte, P.M.; Wood, C.M. The osmorespiratory compromise: Physiological responses and tolerance to hypoxia are affected by salinity acclimation in the euryhaline Atlantic killifish (Fundulus heteroclitus). J. Exp. Biol. 2019, 222, jeb.206599. [Google Scholar] [CrossRef]
- Mu, Y.; Li, W.; Wei, Z.; He, L.; Zhang, W.; Chen, X. Transcriptome analysis reveals molecular strategies in gills and heart of large yellow croaker (Larimichthys crocea) under hypoxia stress. Fish Shellfish Immunol. 2020, 104, 304–313. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Oriol Sunyer, J. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011, 31, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gomez, F.; Greene, W.; Rego, K.; Hansen, J.D.; Costa, G.; Kataria, P.; Bromage, E.S. Discovery and Characterization of Secretory IgD in Rainbow Trout: Secretory IgD Is Produced through a Novel Splicing Mechanism. J. Immunol. 2012, 188, 1341. [Google Scholar] [CrossRef]
- Boardman, T.; Warner, C.; Ramirez-Gomez, F.; Matrisciano, J.; Bromage, E. Characterization of an anti-rainbow trout (Oncorhynchus mykiss) CD3ε monoclonal antibody. Vet. Immunol. Immunopathol. 2012, 145, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Gayle, M.A.; Slack, J.L.; Alderson, M.R.; Bird, T.A.; Giri, J.G.; Colotta, F.; Re, F.; Mantovani, A.; Shanebeck, K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 6155–6159. [Google Scholar] [CrossRef]
- Loitsch, S.M.; von Mallinckrodt, C.; Kippenberger, S.; Steinhilber, D.; Wagner, T.O.F.; Bargon, J. Reactive Oxygen Intermediates Are Involved in IL-8 Production Induced by Hyperosmotic Stress in Human Bronchial Epithelial Cells. Biochem. Biophys. Res. Commun. 2000, 276, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian-Ding, I.; Inamura, S.; Giese, T.; Moll, H.; Endres, S.; Sing, A.; Zähringer, U.; Hartmann, G. Staphylococcus aureus Protein A Triggers T Cell-Independent B Cell Proliferation by Sensitizing B Cells for TLR2 Ligands. J. Immunol. 2007, 178, 2803–2812. [Google Scholar] [CrossRef]
- Okamura, Y.; Miyanishi, H.; Kinoshita, M.; Kono, T.; Sakai, M.; Hikima, J.I. A defective interleukin-17 receptor A1 causes weight loss and intestinal metabolism-related gene downregulation in Japanese medaka, Oryzias latipes. Sci. Rep. 2021, 11, 12099. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zhao, J.; Liu, C.; Bulek, K.; Wu, L.; Chen, X.; Hao, Y.; Wang, Z.; Wang, X.; Ouyang, W.; et al. IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J. Immunol. Baltim. Md. 1950 2017, 199, 3849–3857. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of T(H)17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2022. [Google Scholar] [CrossRef]
- Chiu, R.; Boyle, W.J.; Meek, J.; Smeal, T.; Hunter, T.; Karin, M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 1988, 54, 541–552. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, X.; Chen, X.; Wu, H.; Zhang, H.; Xie, J.; Yang, X.; Gou, Z.; Ye, J. 17-β-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol. Vis. 2012, 18, 1115–1122. [Google Scholar]
- Li, D.-Q.; Luo, L.; Chen, Z.; Kim, H.-S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef]
- Choi, K.; Cope, W.G.; Harms, C.A.; Law, J.M. Rapid decreases in salinity, but not increases, lead to immune dysregulation in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2013, 36, 389–399. [Google Scholar] [CrossRef]
- Lin, G.; Li, S.; Huang, J.; Gao, D.; Lu, J. Hypoosmotic stress induced functional alternations of intestinal barrier integrity, inflammatory reactions, and neurotransmission along gut-brain axis in the yellowfin seabream (Acanthopagrus latus). Fish Physiol. Biochem. 2021, 47, 1725–1738. [Google Scholar] [CrossRef]
- Su, M.; Zhang, R.; Liu, N.; Zhang, J. Modulation of inflammatory response by cortisol in the kidney of spotted scat (Scatophagus argus) in vitro under different osmotic stresses. Fish Shellfish Immunol. 2020, 104, 46–54. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key Players in Innate and Adaptive Immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Akula, S.; Mohammadamin, S.; Hellman, L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS ONE 2014, 9, e96903. [Google Scholar] [CrossRef]
- Sutton, B.J.; Davies, A.M. Structure and dynamics of IgE-receptor interactions: FcεRI and CD23/FcεRII. Immunol. Rev. 2015, 268, 222–235. [Google Scholar] [CrossRef]
- Engeroff, P.; Vogel, M. The role of CD23 in the regulation of allergic responses. Allergy 2021, 76, 1981–1989. [Google Scholar] [CrossRef]
- Warr, G.W.; Magor, K.E.; Higgins, D.A. IgY: Clues to the origins of modern antibodies. Immunol. Today 1995, 16, 392–398. [Google Scholar] [CrossRef]
- Da’as, S.; Teh, E.M.; Dobson, J.T.; Nasrallah, G.K.; McBride, E.R.; Wang, H.; Neuberg, D.S.; Marshall, J.S.; Lin, T.J.; Berman, J.N. Zebrafish mast cells possess an FcɛRI-like receptor and participate in innate and adaptive immune responses. Dev. Comp. Immunol. 2011, 35, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mehlhop, P.D.; van de Rijn, M.; Goldberg, A.B.; Brewer, J.P.; Kurup, V.P.; Martin, T.R.; Oettgen, H.C. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. USA 1997, 94, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.R.; Raizman, M.B.; Gartner, C.S.; Scott, H.C.; Benson, A.C.; Austen, K.F. Secretory granule mediator release and generation of oxidative metabolites of arachidonic acid via Fc-IgG receptor bridging in mouse mast cells. J. Immunol. Baltim. Md. 1950 1992, 148, 868–871. [Google Scholar]
- Kong, X.; Wang, L.; Pei, C.; Zhang, J.; Zhao, X.; Li, L. Comparison of polymeric immunoglobulin receptor between fish and mammals. Vet. Immunol. Immunopathol. 2018, 202, 63–69. [Google Scholar] [CrossRef]
- Xu, G.; Zhan, W.; Ding, B.; Sheng, X. Molecular cloning and expression analysis of polymeric immunoglobulin receptor in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2013, 35, 653–660. [Google Scholar] [CrossRef]
- Banerjee, B.; Bhuyan, G.; Saha, N. Influence of environmental hypertonicity on the induction of ureogenesis and amino acid metabolism in air-breathing walking catfish (Clarias batrachus, Bloch). Indian J. Exp. Biol. 2014, 52, 728–738. [Google Scholar]
- Jiang, W.; Tian, X.; Fang, Z.; Li, L.; Dong, S.; Li, H.; Zhao, K. Metabolic responses in the gills of tongue sole (Cynoglossus semilaevis) exposed to salinity stress using NMR-based metabolomics. Sci. Total Environ. 2019, 653, 465–474. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, J.; Zhu, L.; Chen, A.; Cheng, Y. Label-free quantification proteomics analysis reveals acute hyper-osmotic responsive proteins in the gills of Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101009. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Liu, B.; Sun, C.; Song, C.; Liu, M.; Zhou, Q.; Zheng, X.; Liu, X. Response of microbiota and immune function to different hypotonic stress levels in giant freshwater prawn Macrobrachium rosenbergii post-larvae. Sci. Total Environ. 2022, 844, 157258. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Lao, H.; Yin, Z.; He, W.; Weng, S.; Yu, X.; Chan, S.; He, J. Molecular cloning and expression analysis of the ASC gene from mandarin fish and its regulation of NF-kappaB activation. Dev. Comp. Immunol. 2008, 32, 391–399. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Cheng, N.; Duan, J.; Wang, J.; Nagahama, Y.; Zhong, X.; Zhou, Q.; Wang, Y. Identification and expression profiles of prdm1 in medaka Oryzias latipes. Mol. Biol. Rep. 2014, 41, 617–626. [Google Scholar] [CrossRef]
- Nibona, E.; Xu, G.; Wu, K.; Shen, H.; Zhang, R.; Ke, X.; Al Hafiz, A.; Wang, Z.; Qi, C.; Zhao, H. Identification, characterization, expression profiles of OlHavcr2 in medaka (Oryzias latipes). Gen. Comp. Endocrinol. 2019, 277, 30–37. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Lau, M.C.; Kwong, E.M.; Lai, K.P.; Li, J.W.; Ho, J.C.; Chan, T.F.; Wong, C.K.; Jiang, Y.J.; Tse, W.K. Pathogenesis of POLR1C-dependent Type 3 Treacher Collins Syndrome revealed by a zebrafish model. Biochim. Et. Biophys. Acta 2016, 1862, 1147–1158. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]




| Disease or Functions Annotation | Activation Z-Score (SW/SFW) | Number of Genes (Interleukins Related) |
|---|---|---|
| Recruitment of phagocytes | −2.107 | 18 (IL1B) |
| Recruitment of leukocytes | −1.882 | 23 (IL1B; IL1R2) |
| Proliferation of immune cell | −0.606 | 39 (IL1B) |
| Cell death of immune cell | 1.183 | 36 (IL1B) |
| Transmigration of phagocytes | 0.834 | 9 (IL1B) |
| Systemic autoimmune syndrome | 0.640 | 61 (IL1B; IL1R2) |
| Activation of neutrophils | 0.579 | 10 (IL1B) |
| Gene | Primer—F (5′ --> 3′) | Primer—R (5′ --> 3′) |
|---|---|---|
| ass1 | GCAGAAATTTGGCATTCCGGT | GCCGGGTTTTTGGTCATCAAG |
| cad | ACGGGAACACCCAGAAATCC | CAGAGTAGTCGAACTCGCCC |
| ccnb1 | TGACTACGACAACCCCATGC | TGAGGATGGCTCGCATGTTT |
| ccne2 | CGCTTACTTGGCTCAGGACT | CAGGCTCCATCTGTGACGAA |
| cfos | GACAGCATCAAGTGCCTCCT | CACGTTTGGAAGAGCAAGCC |
| fcer2 | GAGGAAGAGATCCAATACTCCTCTG | ACAGCAGGTGATGAAACCATCT |
| il1-r2 | TGGATCCAGAGGTGGGGATT | GAGGAACCAGAGTTGGGTGG |
| il1β | GGGCATCAAGGACACCAAAC | GTGAGGGTGCTGAGGTTTCC |
| il8 | ACAATAACGGCCTTCGCGTT | GTTGGAAGTTGTGAGGGTGC |
| mmp9 | TTATCCTCCTGGTGAGGGCA | CGCCGAAAACAAAGGGGAAG |
| pigr | TGGTCACTCCACTACCCACA | CGCCAACAAGTGAGTGTGAC |
| plk1 | GGCTCGCTACTACATGACCC | GTGGCCAAACCAAAGTCACC |
| skp2 | CGGGTACAGAGAGAGCCTCA | GTGATAGCAGCGACTCAGGG |
| 18S | CCTGCGGCTTAATTTGACCC | GACAAATCGCTCCACCAACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Liu, J.; Leung, C.T.; Lin, X.; Chan, T.F.; Tse, W.K.F.; Lai, K.P. Transcriptomic Analysis in Marine Medaka Gill Reveals That the Hypo-Osmotic Stress Could Alter the Immune Response via the IL17 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12417. https://doi.org/10.3390/ijms232012417
Li R, Liu J, Leung CT, Lin X, Chan TF, Tse WKF, Lai KP. Transcriptomic Analysis in Marine Medaka Gill Reveals That the Hypo-Osmotic Stress Could Alter the Immune Response via the IL17 Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(20):12417. https://doi.org/10.3390/ijms232012417
Chicago/Turabian StyleLi, Rong, Jiaqi Liu, Chi Tim Leung, Xiao Lin, Ting Fung Chan, William Ka Fai Tse, and Keng Po Lai. 2022. "Transcriptomic Analysis in Marine Medaka Gill Reveals That the Hypo-Osmotic Stress Could Alter the Immune Response via the IL17 Signaling Pathway" International Journal of Molecular Sciences 23, no. 20: 12417. https://doi.org/10.3390/ijms232012417
APA StyleLi, R., Liu, J., Leung, C. T., Lin, X., Chan, T. F., Tse, W. K. F., & Lai, K. P. (2022). Transcriptomic Analysis in Marine Medaka Gill Reveals That the Hypo-Osmotic Stress Could Alter the Immune Response via the IL17 Signaling Pathway. International Journal of Molecular Sciences, 23(20), 12417. https://doi.org/10.3390/ijms232012417






