Polydopamine Copolymers for Stable Drug Nanoprecipitation
Abstract
:1. Introduction
2. Results and Discussion
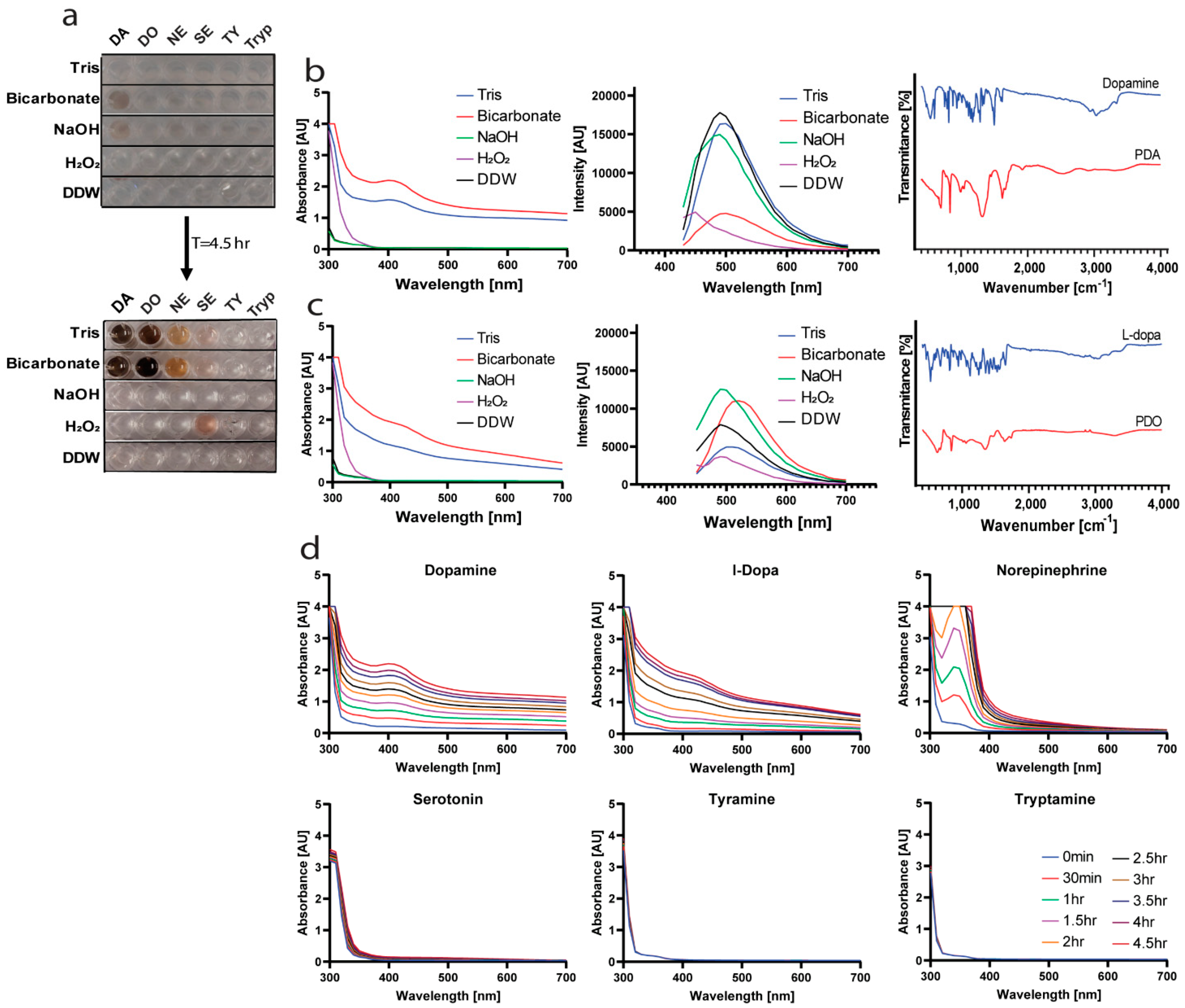
2.1. Finding the Optimal Conditions for Polymerization
2.2. Optimal Copolymer for Hydrophobic Drugs Stabilization
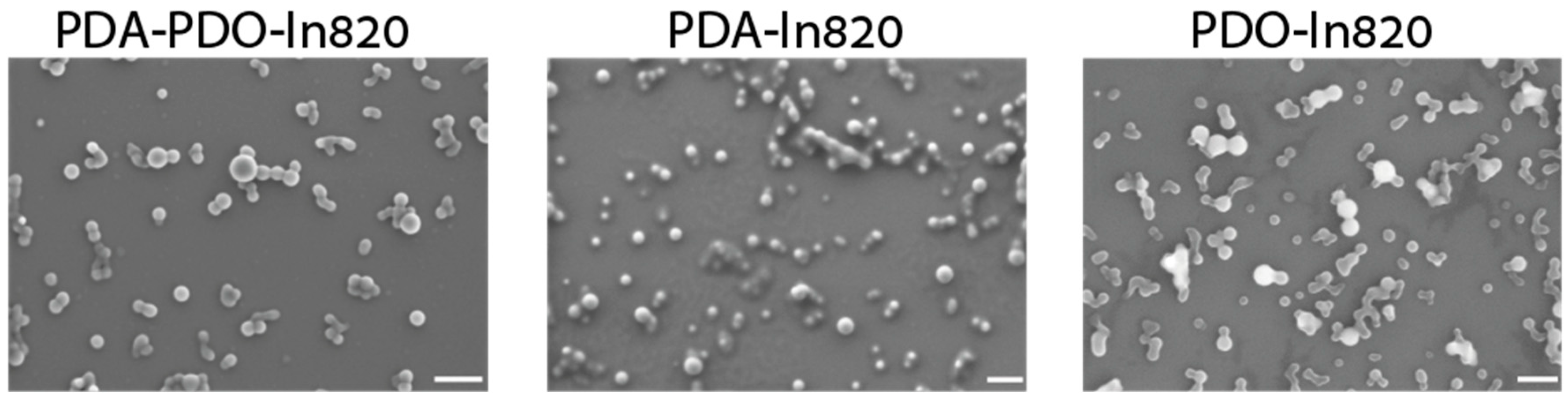
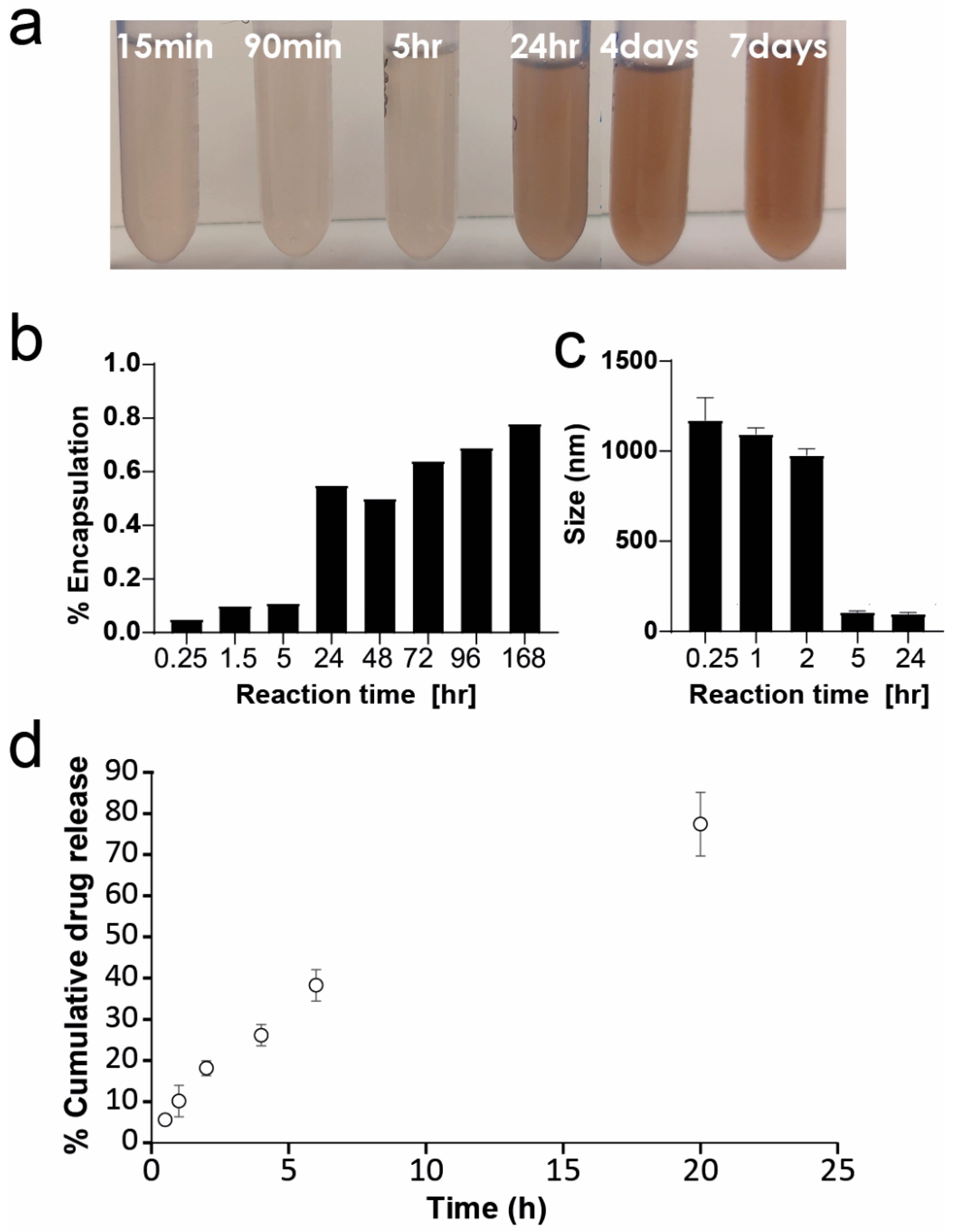
2.3. Optimization of PDA-PDO-In820 Synthesis
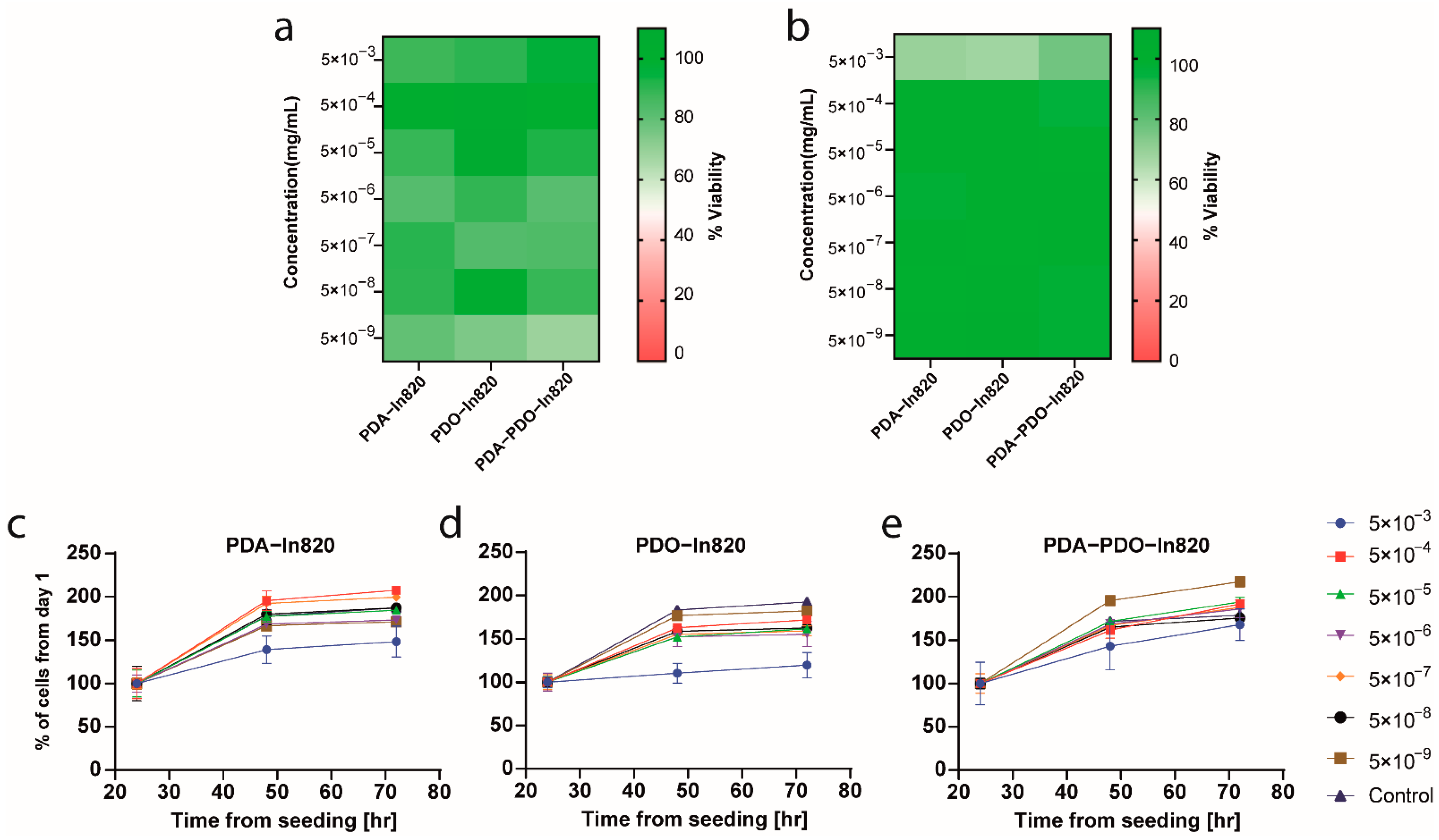
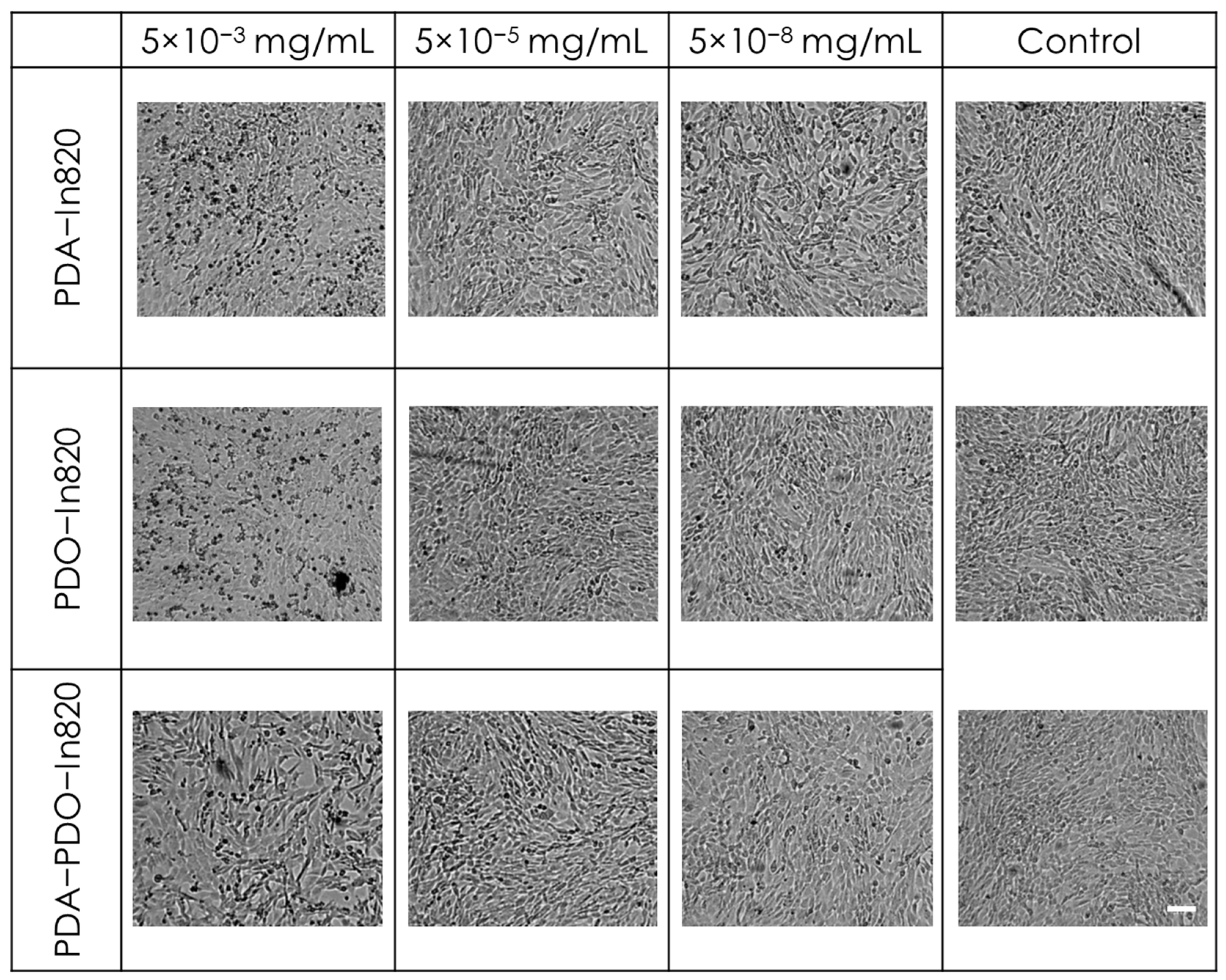
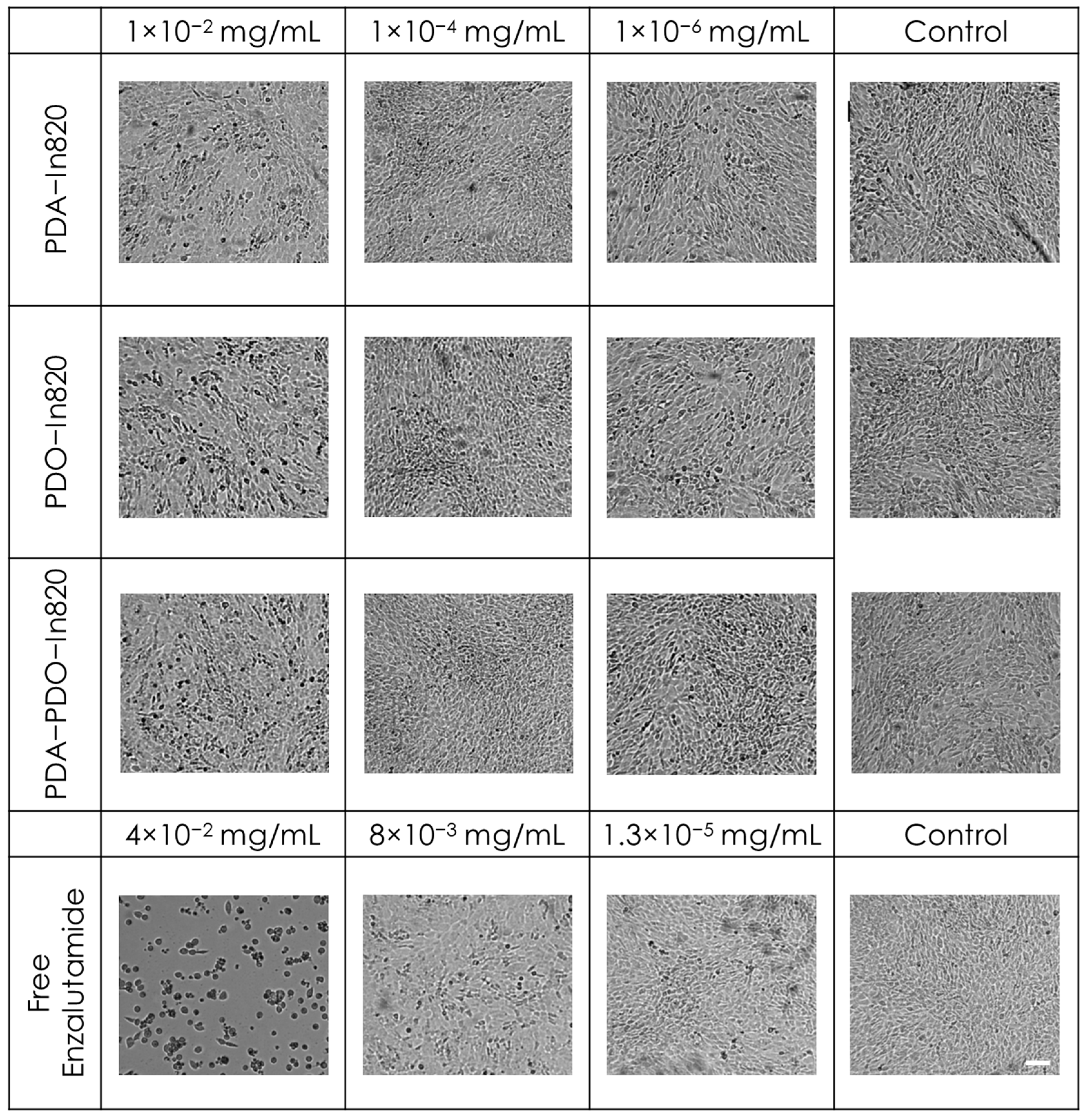
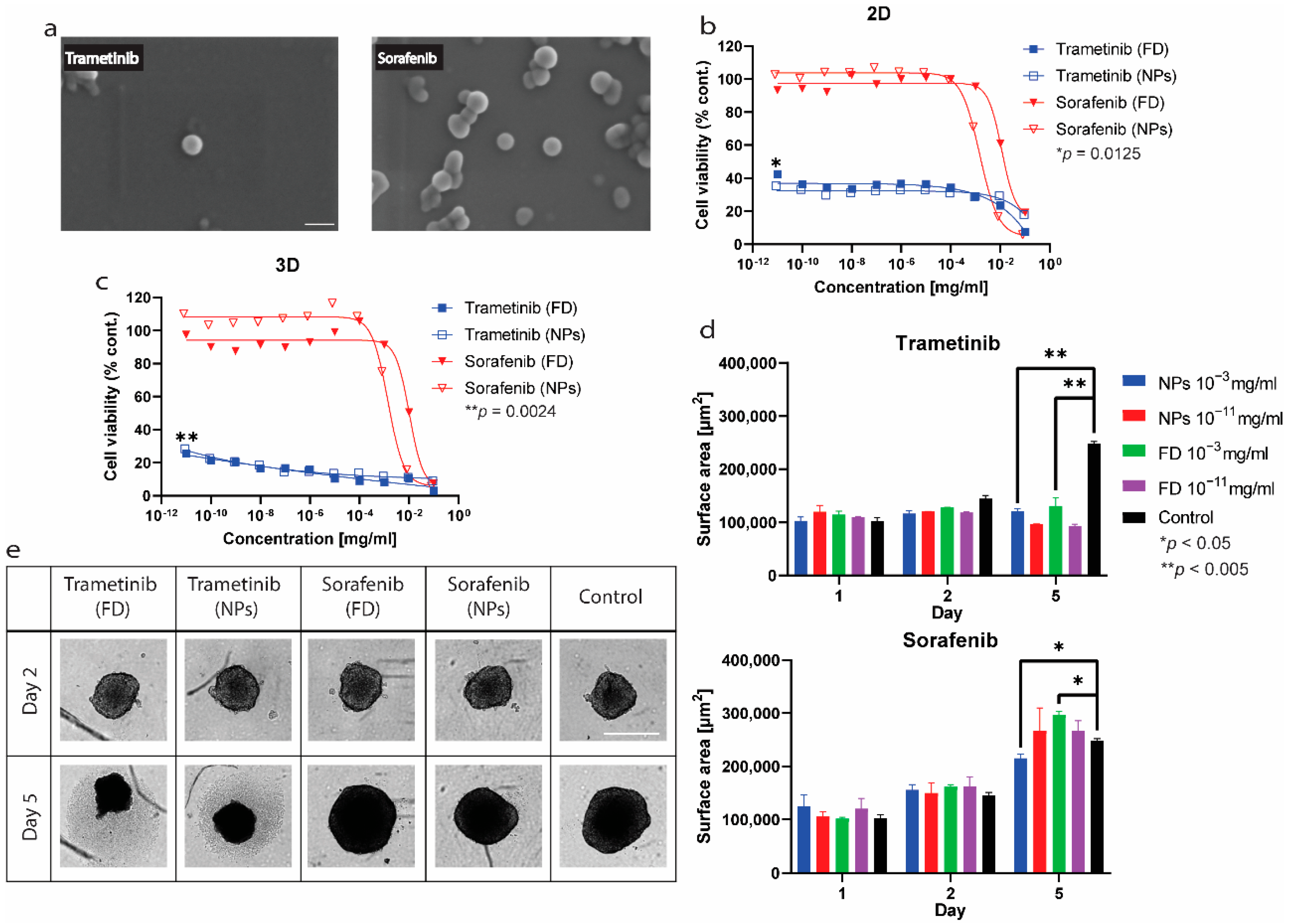
2.4. In Vitro Toxicity and Antitumor Efficacy Experiments
3. Methods
3.1. Materials and Reagents
3.2. Polymerization Process
3.3. Absorbance Measurements
3.4. ATR-FTIR
3.5. Imaging Fluorescent Drug Aggregates with Automated Microscopy
3.6. Preparation of Nanoparticles
3.7. Characterization of Nanoparticles
3.8. HR-SEM
3.9. Cell Cultures
3.10. Polymers Toxicity Assay in 2D
3.11. Cell Viability Assay in 2D
3.12. Cell Viability Assay in 3D
3.13. Drug Release Profile
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Unravelling the polydopamine mystery: Is the end in sight? Polym. Chem. 2019, 10, 5771–5777. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batul, R.; Yu, A.; Bhave, M.; Khaliq, A. Synthesis of Polydopamine Nanoparticles for Drug Delivery Applications. Microsc. Microanal. 2018, 24, 1758–1759. [Google Scholar] [CrossRef] [Green Version]
- Van Der Westen, R.; Hosta-Rigau, L.; Sutherland, D.S.; Goldie, K.N.; Albericio, F.; Postma, A.; Städler, B. Myoblast Cell Interaction with Polydopamine Coated Liposomes. Biointerphases 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Yang, H.-C.; Xu, X.-L.; Wu, J.; Xu, Z.-K. Polydopamine as a Catalyst for Thiol Coupling. ChemCatChem 2015, 7, 3822–3825. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.-P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Chen, H.; Sui, L.; Feng, L.; Liu, Z.; Liu, Y.; Wei, G.; Lu, W. Enhanced mucosal penetration and efficient inhibition efficacy against cervical cancer of PEGylated docetaxel nanocrystals by TAT modification. J. Control. Release 2021, 336, 572–582. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhao, F. Recent Advances in Intrinsically Fluorescent Polydopamine Materials. Appl. Sci. 2022, 12, 4560. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur. J. Pharm. Sci. 2005, 24, 67–75. [Google Scholar] [CrossRef]
- Shamay, Y.; Shah, J.; Işık, M.; Mizrachi, A.; Leibold, J.; Tschaharganeh, D.F.; Roxbury, D.; Budhathoki-Uprety, J.; Nawaly, K.; Sugarman, J.L.; et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 2018, 17, 361–368. [Google Scholar] [CrossRef]
- Harris, Y.; Sason, H.; Niezni, D.; Shamay, Y. Automated discovery of nanomaterials via drug aggregation induced emission. Biomaterials 2022, 289, 121800. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Liu, X.; Hu, R.; Qin, A.; Tang, B.Z. Aggregation-Induced Emission Materials that Aid in Pharmaceutical Research. Adv. Healthc. Mater. 2021, 10, 2101067. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Yan, Y. Self-Assembly of Aggregation-Induced-Emission Molecules. Chem. Asian J. 2018, 14, 730–750. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Hu, H.; Dyke, J.C.; Bowman, B.A.; Ko, C.-C.; You, W. Investigation of Dopamine Analogues: Synthesis, Mechanistic Understanding, and Structure–Property Relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef]
- Tan, X.; Gao, P.; Li, Y.; Qi, P.; Liu, J.; Shen, R.; Wang, L.; Huang, N.; Xiong, K.; Tian, W.; et al. Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: Comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices. Bioact. Mater. 2020, 6, 285–296. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, H.; Zhang, Y.; Hu, Z.; Niu, N.; Yu, C. Controlled synthesis of polydopamine: A new strategy for highly sensitive fluorescence turn-on detection of acetylcholinesterase activity. Mikrochim. Acta 2018, 185, 132. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. PH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Xia, S.; Tao, L.; Wei, Y. A Facile Preparation of Mussel-Inspired Poly(dopamine phosphonate-co-PEGMA)s via a One-Pot Multicomponent Polymerization System. Macromol. Rapid Commun. 2019, 41, e1900533. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, M.-Q.; Chen, T.-T.; Zhang, H.; Hu, D.-F.; Wu, B.-H.; Ji, J.; Xu, Z.-K. Dopamine-Triggered One-Step Polymerization and Codeposition of Acrylate Monomers for Functional Coatings. ACS Appl. Mater. Interfaces 2017, 9, 34356–34366. [Google Scholar] [CrossRef]
- Liang, S.; Yu, S.; Gao, C. Preparation of complementary glycosylated hyperbranched polymer/poly(ethylene glycol) brushes and their selective interactions with hepatocytes. Colloids Surf. B Biointerfaces 2016, 145, 309–318. [Google Scholar] [CrossRef]
- Prostota, Y.; Coelho, P.J. Cationic 3H-indolium dyes by ring-opening of benzo[1,3]oxazine. Dye. Pigment. 2013, 98, 93–99. [Google Scholar] [CrossRef]
- Huefner, A.; Kuan, W.L.; Müller, K.H.; Skepper, J.N.; Barker, R.A.; Mahajan, S. Characterization and visualization of vesicles in the endo-lysosomal pathway with surface-enhanced raman spectroscopy and chemometrics. ACS Nano 2016, 10, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Enzalutamide: A Review in Castration-Resistant Prostate Cancer. Drugs 2018, 78, 1913–1924. [Google Scholar] [CrossRef]
- Eder, T.; Weber, A.; Neuwirt, H.; Grünbacher, G.; Ploner, C.; Klocker, H.; Sampson, N.; Eder, I.E. Cancer-Associated Fibroblasts Modify the Response of Prostate Cancer Cells to Androgen and Anti-Androgens in Three-Dimensional Spheroid Culture. Int. J. Mol. Sci. 2016, 17, 1458. [Google Scholar] [CrossRef]
- Zhao, C.; Xiao, H.; Wu, X.; Li, C.; Liang, G.; Yang, S.; Lin, J. Rational combination of MEK inhibitor and the STAT3 pathway modulator for the therapy in K-Ras mutated pancreatic and colon cancer cells. Oncotarget 2015, 6, 14472–14487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, T.; Mitchell, C.; Park, M.A.; Yacoub, A.; Graf, M.; Rahmani, M.; Houghton, P.J.; Voelkel-Johnson, C.; Grant, S.; Dent, P. Sorafenib and Vorinostat Kill Colon Cancer Cells by CD95-Dependent and -Independent Mechanisms. Mol. Pharmacol. 2009, 76, 342–355. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niezni, D.; Harris, Y.; Sason, H.; Avrashami, M.; Shamay, Y. Polydopamine Copolymers for Stable Drug Nanoprecipitation. Int. J. Mol. Sci. 2022, 23, 12420. https://doi.org/10.3390/ijms232012420
Niezni D, Harris Y, Sason H, Avrashami M, Shamay Y. Polydopamine Copolymers for Stable Drug Nanoprecipitation. International Journal of Molecular Sciences. 2022; 23(20):12420. https://doi.org/10.3390/ijms232012420
Chicago/Turabian StyleNiezni, Danna, Yuval Harris, Hagit Sason, Maytal Avrashami, and Yosi Shamay. 2022. "Polydopamine Copolymers for Stable Drug Nanoprecipitation" International Journal of Molecular Sciences 23, no. 20: 12420. https://doi.org/10.3390/ijms232012420





