Anti-Inflammatory Effects of GLP-1R Activation in the Retina
Abstract
:1. Introduction
2. Inflammation in Ocular Disease
3. Anti-Inflammatory Effects of GLP-1 in the Retina
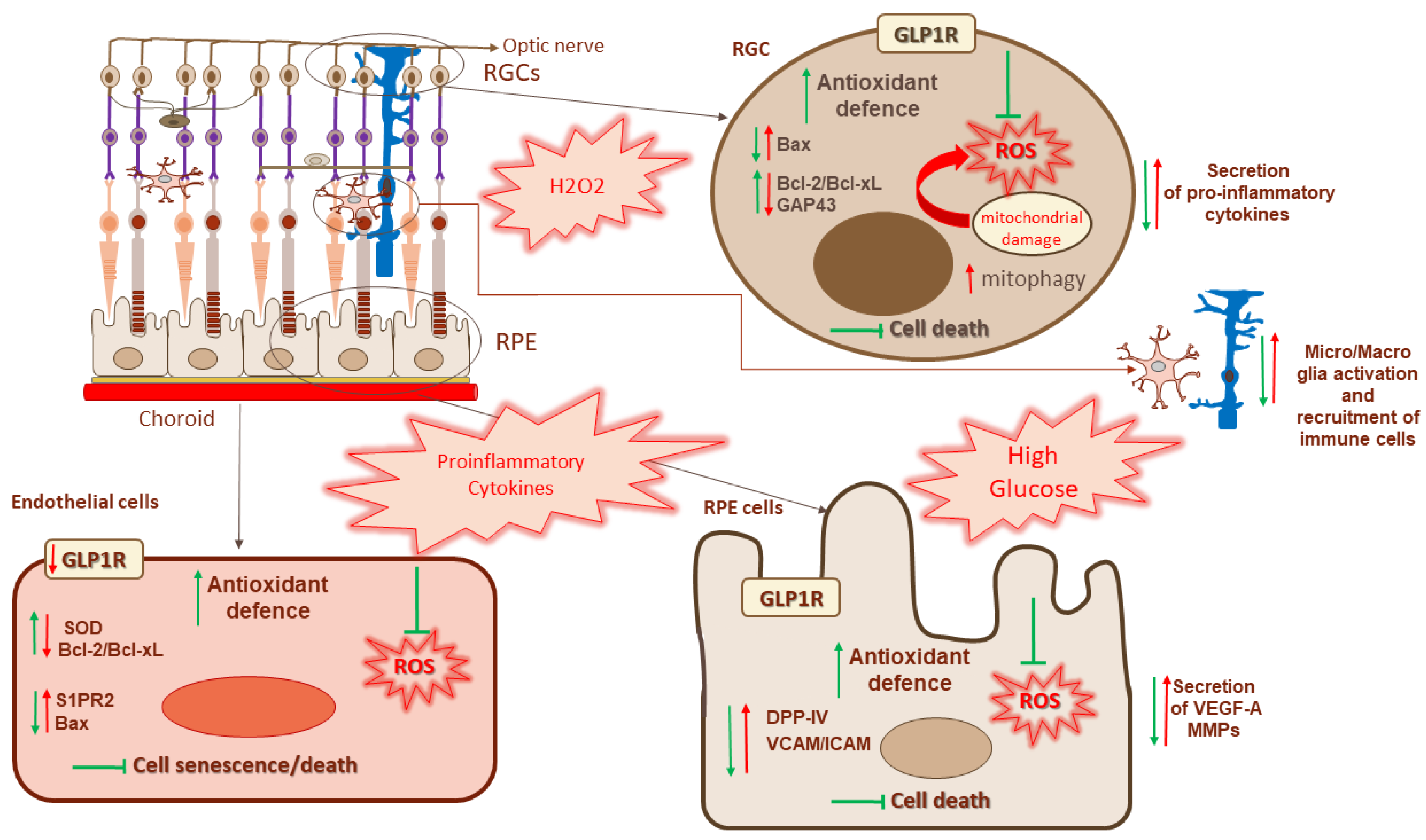
4. Retinal Ganglion Cells and Muller Cells
5. RPE Cells
6. Endothelial Cells and Pericytes
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, R.K. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin. Ther. 2011, 33, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [Green Version]
- Hogan, A.E.; Tobin, A.M.; Ahern, T.; Corrigan, M.A.; Gaoatswe, G.; Jackson, R.; O’Reilly, V.; Lynch, L.; Doherty, D.G.; Moynagh, P.N.; et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: Lessons from obesity, diabetes and psoriasis. Diabetologia 2011, 54, 2745–2754. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Anderson, R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puddu, A.; Maggi, D. Emerging Role of Caveolin-1 in GLP-1 Action. Front. Endocrinol. 2021, 12, 668012. [Google Scholar] [CrossRef]
- Roed, S.N.; Wismann, P.; Underwood, C.R.; Kulahin, N.; Iversen, H.; Cappelen, K.A.; Schaffer, L.; Lehtonen, J.; Hecksher-Soerensen, J.; Secher, A.; et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol. Cell. Endocrinol. 2014, 382, 938–949. [Google Scholar] [CrossRef]
- Fletcher, M.M.; Halls, M.L.; Zhao, P.; Clydesdale, L.; Christopoulos, A.; Sexton, P.M.; Wootten, D. Glucagon-like peptide-1 receptor internalisation controls spatiotemporal signalling mediated by biased agonists. Biochem. Pharmacol. 2018, 156, 406–419. [Google Scholar] [CrossRef]
- Gupta, V. Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 2013, 17, 413–421. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Mietlicki-Baase, E.G.; Kanoski, S.E.; de Jonghe, B.C. Incretins and amylin: Neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 2014, 34, 237–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes-state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef]
- Anagnostis, P.; Athyros, V.G.; Adamidou, F.; Panagiotou, A.; Kita, M.; Karagiannis, A.; Mikhailidis, D.P. Glucagon-like peptide-1-based therapies and cardiovascular disease: Looking beyond glycaemic control. Diabetes Obes. Metab. 2011, 13, 302–312. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [Green Version]
- Bakbak, E.; Terenzi, D.C.; Trac, J.Z.; Teoh, H.; Quan, A.; Glazer, S.A.; Rotstein, O.D.; Al-Omran, M.; Verma, S.; Hess, D.A. Lessons from bariatric surgery: Can increased GLP-1 enhance vascular repair during cardiometabolic-based chronic disease? Rev. Endocr. Metab. Disord. 2021, 22, 1171–1188. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, Z.; Zhang, H.; Zhou, X.; Guan, L.; Deng, W.; Xu, L. The Glucagon-Like Peptide-1 Analogue Liraglutide Inhibits Oxidative Stress and Inflammatory Response in the Liver of Rats with Diet-Induced Non-alcoholic Fatty Liver Disease. Biol. Pharm. Bull. 2015, 38, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Grieco, M.; Giorgi, A.; Gentile, M.C.; d’Erme, M.; Morano, S.; Maras, B.; Filardi, T. Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1112. [Google Scholar] [CrossRef]
- Insuela, D.B.R.; Carvalho, V.F. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur. J. Pharmacol. 2017, 812, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Bonora, B.M.; Cappellari, R.; Menegazzo, L.; Vedovato, M.; Iori, E.; Marescotti, M.C.; Albiero, M.; Avogaro, A. Acute Effects of Linagliptin on Progenitor Cells, Monocyte Phenotypes, and Soluble Mediators in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheen, A.J.; Esser, N.; Paquot, N. Antidiabetic agents: Potential anti-inflammatory activity beyond glucose control. Diabetes Metab. 2015, 41, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- El Mouhayyar, C.; Riachy, R.; Khalil, A.B.; Eid, A.; Azar, S. SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review. Int. J. Endocrinol. 2020, 2020, 1762164. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [Green Version]
- Trotta, M.C.; Gesualdo, C.; Petrillo, F.; Lepre, C.C.; Della Corte, A.; Cavasso, G.; Maggiore, G.; Hermenean, A.; Simonelli, F.; D’Amico, M.; et al. Resolution of Inflammation in Retinal Disorders: Briefly the State. Int. J. Mol. Sci. 2022, 23, 4501. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Nussenblatt, R.B.; Lightman, S.L.; Hollander, D.A. Inflammation in retinal disease. Int. J. Inflamm. 2013, 2013, 724648. [Google Scholar] [CrossRef]
- Goncalves, A.; Lin, C.M.; Muthusamy, A.; Fontes-Ribeiro, C.; Ambrosio, A.F.; Abcouwer, S.F.; Fernandes, R.; Antonetti, D.A. Protective Effect of a GLP-1 Analog on Ischemia-Reperfusion Induced Blood-Retinal Barrier Breakdown and Inflammation. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2584–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Vaz, J.; Faria de Abreu, J.R.; Campos, A.J. Early breakdown of the blood-retinal barrier in diabetes. Br. J. Ophthalmol. 1975, 59, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiec-Mroczek, J.; Oficjalska-Mlynczak, J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes--role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, M.L.; Iserovich, P.; Gotlinger, K.; Bellner, L.; Dunn, M.W.; Sartore, M.; Grazia Pertile, M.; Leonardi, A.; Sathe, S.; Beaton, A.; et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes 2010, 59, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [Green Version]
- Gesualdo, C.; Balta, C.; Platania, C.B.M.; Trotta, M.C.; Herman, H.; Gharbia, S.; Rosu, M.; Petrillo, F.; Giunta, S.; Della Corte, A.; et al. Fingolimod and Diabetic Retinopathy: A Drug Repurposing Study. Front. Pharmacol. 2021, 12, 718902. [Google Scholar] [CrossRef]
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Schwartz, T.D.; Dunaief, J.L.; Cui, Q.N. Myeloid cells in retinal and brain degeneration. FEBS J. 2022, 289, 2337–2361. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Q.N.; Stein, L.M.; Fortin, S.M.; Hayes, M.R. The role of glia in the physiology and pharmacology of glucagon-like peptide-1: Implications for obesity, diabetes, neurodegeneration and glaucoma. Br. J. Pharmacol. 2022, 179, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. J. Lab. Clin. Med. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Seve, P.; Cacoub, P.; Bodaghi, B.; Trad, S.; Sellam, J.; Bellocq, D.; Bielefeld, P.; Sene, D.; Kaplanski, G.; Monnet, D.; et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun. Rev. 2017, 16, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Liu, K.; Wang, Q.; Ruan, Y.; Ye, W.; Zhang, Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp. Eye Res. 2014, 127, 104–116. [Google Scholar] [CrossRef]
- Hernandez, C.; Bogdanov, P.; Corraliza, L.; Garcia-Ramirez, M.; Sola-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simo, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.M.; Jung, C.H. Effects of Incretin-Based Therapies on Diabetic Microvascular Complications. Endocrinol. Metab. 2017, 32, 316–325. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, H.; Kuang, H. The potential benefits of glucagon-like peptide-1 receptor agonists for diabetic retinopathy. Peptides 2018, 100, 123–126. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, K.; Wang, F.; Zhou, L.; Hu, Y.; Tang, M.; Zhang, S.; Jin, S.; Zhang, J.; Wang, J.; et al. The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp. Eye Res. 2016, 151, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynski, W.M.; Hankins, M.; Ong, S.H.; Richter, H.; Clemens, A.; Toussi, M. Microvascular Outcomes in Patients with Type 2 Diabetes Treated with Vildagliptin vs. Sulfonylurea: A Retrospective Study Using German Electronic Medical Records. Diabetes Ther. 2016, 7, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Nreu, B.; Scatena, A.; Andreozzi, F.; Sesti, G.; Mannucci, E.; Monami, M. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A meta-analysis of randomized controlled trials. Acta Diabetol. 2017, 54, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Sarangdhar, M.; Avogaro, A. Glucagon-like peptide-1 receptor agonists are not associated with retinal adverse events in the FDA Adverse Event Reporting System. BMJ Open Diabetes Res. Care 2018, 6, e000475. [Google Scholar] [CrossRef] [Green Version]
- Gaborit, B.; Julla, J.B.; Besbes, S.; Proust, M.; Vincentelli, C.; Alos, B.; Ancel, P.; Alzaid, F.; Garcia, R.; Mailly, P.; et al. Glucagon-like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J. Clin. Endocrinol. Metab. 2020, 105, e1549–e1560. [Google Scholar] [CrossRef]
- Saw, M.; Wong, V.W.; Ho, I.V.; Liew, G. New anti-hyperglycaemic agents for type 2 diabetes and their effects on diabetic retinopathy. Eye 2019, 33, 1842–1851. [Google Scholar] [CrossRef]
- Simo, R.; Hernandez, C. GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe? Diabetes 2017, 66, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.W.; Lee, J.H.; Lee, J.Y.; Ju, H.H.; Lee, Y.J.; Jee, D.H.; Ko, S.H.; Choi, J.A. The Anti-Inflammatory Effects of Glucagon-Like Peptide Receptor Agonist Lixisenatide on the Retinal Nuclear and Nerve Fiber Layers in an Animal Model of Early Type 2 Diabetes. Am. J. Pathol. 2020, 190, 1080–1094. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Oh, Y.; Gamuyao, R.; Lee, G.; Xie, Y.; Cho, H.; Lee, S.; Duh, E.J. Myeloid cell modulation by a GLP-1 receptor agonist regulates retinal angiogenesis in ischemic retinopathy. JCI Insight 2021, 6, e93382. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Li, M.; Huang, X.; Yang, Y.; Zeng, J.; Zhao, Y.; Wang, X.; Zhang, W.; Ying, Y. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3beta signaling. Neuropharmacology 2019, 153, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, N.; Kolibabka, M.; Busch, S.; Bugert, P.; Kaiser, U.; Lin, J.; Fleming, T.; Morcos, M.; Klein, T.; Schlotterer, A.; et al. The DPP4 Inhibitor Linagliptin Protects from Experimental Diabetic Retinopathy. PLoS ONE 2016, 11, e0167853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Q.; Zhang, J.; Lei, X.; Xu, G.T.; Ye, W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Wang, Q.; Lei, X.; Chu, Q.; Xu, G.T.; Ye, W. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Liu, K.; Wang, Q.; Ruan, Y.; Zhang, Y.; Ye, W. Exendin-4 protects retinal cells from early diabetes in Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax ratios and reducing reactive gliosis. Mol. Vis. 2014, 20, 1557–1568. [Google Scholar]
- Cai, X.; Li, J.; Wang, M.; She, M.; Tang, Y.; Li, J.; Li, H.; Hui, H. GLP-1 Treatment Improves Diabetic Retinopathy by Alleviating Autophagy through GLP-1R-ERK1/2-HDAC6 Signaling Pathway. Int. J. Med. Sci. 2017, 14, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, C.; Bogdanov, P.; Sola-Adell, C.; Sampedro, J.; Valeri, M.; Genis, X.; Simo-Servat, O.; Garcia-Ramirez, M.; Simo, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef] [Green Version]
- Seppa, K.; Toots, M.; Reimets, R.; Jagomae, T.; Koppel, T.; Pallase, M.; Hasselholt, S.; Krogsbaek Mikkelsen, M.; Randel Nyengaard, J.; Vasar, E.; et al. GLP-1 receptor agonist liraglutide has a neuroprotective effect on an aged rat model of Wolfram syndrome. Sci. Rep. 2019, 9, 15742. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Xu, H.; Hu, F.; Wu, J.; Kong, X.; Sun, X. Exendin-4, a GLP-1 receptor agonist regulates retinal capillary tone and restores microvascular patency after ischaemia-reperfusion injury. Br. J. Pharmacol. 2020, 177, 3389–3402. [Google Scholar] [CrossRef]
- Ramos, H.; Bogdanov, P.; Sampedro, J.; Huerta, J.; Simo, R.; Hernandez, C. Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants 2020, 9, 846. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1). Mediat. Inflamm. 2013, 2013, 975032. [Google Scholar] [CrossRef] [PubMed]
- Hebsgaard, J.B.; Pyke, C.; Yildirim, E.; Knudsen, L.B.; Heegaard, S.; Kvist, P.H. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes. Metab. 2018, 20, 2304–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Tribble, J.R.; Pepper, K.W.; Cross, S.D.; Morgan, B.P.; Morgan, J.E.; John, S.W.; Howell, G.R. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener. 2016, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Adamiec-Mroczek, J.; Zajac-Pytrus, H.; Misiuk-Hojlo, M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells During the Development of Diabetic Retinopathy. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2015, 24, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the pathogenesis of diabetic retinopathy: Molecular mechanisms and therapeutic implications. Curr. Med. Chem. 2013, 20, 3241–3250. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Rusenberg, B.; Pavlidis, M.; Stupp, T.; Thanos, S. Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Ross-Cisneros, F.N.; Sadun, A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004, 23, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Ma, X.F.; Lin, W.J.; Hao, M.; Yu, X.Y.; Li, H.X.; Xu, C.Y.; Kuang, H.Y. Neuroprotective Role of GLP-1 Analog for Retinal Ganglion Cells via PINK1/Parkin-Mediated Mitophagy in Diabetic Retinopathy. Front. Pharmacol. 2020, 11, 589114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, Y.X.; Gao, X.Y.; Kuang, H.Y.; Zhang, J.; Liu, R. Obestatin prevents H2O2-induced damage through activation of TrkB in RGC-5 cells. Biomed. Pharmacother. 2018, 97, 1061–1065. [Google Scholar] [CrossRef]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.; Li, T.; Zhang, P.; Wang, N.; Sun, X. Prolyl-4-Hydroxylases Inhibitor Stabilizes HIF-1alpha and Increases Mitophagy to Reduce Cell Death After Experimental Retinal Detachment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Miyai, T.; Vasanth, S.; Melangath, G.; Deshpande, N.; Kumar, V.; Benischke, A.S.; Chen, Y.; Price, M.O.; Price, F.W., Jr.; Jurkunas, U.V. Activation of PINK1-Parkin-Mediated Mitophagy Degrades Mitochondrial Quality Control Proteins in Fuchs Endothelial Corneal Dystrophy. Am. J. Pathol. 2019, 189, 2061–2076. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef]
- Fu, Z.; Kuang, H.Y.; Hao, M.; Gao, X.Y.; Liu, Y.; Shao, N. Protection of exenatide for retinal ganglion cells with different glucose concentrations. Peptides 2012, 37, 25–31. [Google Scholar] [CrossRef]
- Ma, X.; Lin, W.; Lin, Z.; Hao, M.; Gao, X.; Zhang, Y.; Kuang, H. Liraglutide alleviates H2O2-induced retinal ganglion cells injury by inhibiting autophagy through mitochondrial pathways. Peptides 2017, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dang, Y.; Dai, F.; Guo, Z.; Wu, J.; She, X.; Pei, Y.; Chen, Y.; Ling, W.; Wu, C.; et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J. Biol. Chem. 2003, 278, 29278–29287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Smith, M.D.; Sotirchos, E.S.; Jin, J.; Meyers, K.; Taylor, M.; Garton, T.; Bannon, R.; Lord, H.N.; Dawson, T.M.; et al. Therapeutic Potential of a Novel Glucagon-like Peptide-1 Receptor Agonist, NLY01, in Experimental Autoimmune Encephalomyelitis. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2021, 18, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.S.; Chintala, S.K. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS ONE 2011, 6, e18305. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Li, L.; Zhu, L.; Guo, Y.; Du, S.; Zhang, Y.; Wang, Z.; Zhang, Y.; Zhu, M. Geniposide Attenuates Hyperglycemia-Induced Oxidative Stress and Inflammation by Activating the Nrf2 Signaling Pathway in Experimental Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2021, 2021, 9247947. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Dorecka, M.; Siemianowicz, K.; Francuz, T.; Garczorz, W.; Chyra, A.; Klych, A.; Romaniuk, W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol. Rep. 2013, 65, 884–890. [Google Scholar] [CrossRef]
- Garczorz, W.; Gallego-Colon, E.; Kosowska, A.; Siemianowicz, K.; Klych-Ratuszny, A.; Wozniak, M.; Aghdam, M.R.F.; Francuz, T.; Dorecka, M. Exenatide modulates expression of metalloproteinases and their tissue inhibitors in TNF-alpha stimulated human retinal pigment epithelial cells. Pharmacol. Rep. 2019, 71, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Park, M.J.; Choi, J.H.; Lim, S.K.; Choi, H.J.; Park, S.H. Hyperglycemia-induced GLP-1R downregulation causes RPE cell apoptosis. Int. J. Biochem. Cell Biol. 2015, 59, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Al Sabaani, N. Exendin-4 inhibits high glucose-induced oxidative stress in retinal pigment epithelial cells by modulating the expression and activation of p(66)Shc. Cutan. Ocul. Toxicol. 2021, 40, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Tian, L.; Lu, D.; Li, H.; Cui, J. Exendin-4 Protects Human Retinal Pigment Epithelial Cells from H2O2-Induced Oxidative Damage via Activation of NRF2 Signaling. Ophthalmic Res. 2020, 63, 404–412. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Qiu, Z.; Li, L.; Qin, B.; Zhou, Y.; Liu, Y.; Liu, X.; Zhu, M.; Sang, A. Geniposide alleviates choroidal neovascularization by downregulating HB-EGF release from RPE cells by downregulating the miR-145-5p/NF-kappaB axis. Exp. Eye Res. 2021, 208, 108624. [Google Scholar] [CrossRef]
- Yu, D.Y.; Yu, P.K.; Cringle, S.J.; Kang, M.H.; Su, E.N. Functional and morphological characteristics of the retinal and choroidal vasculature. Prog. Retin. Eye Res. 2014, 40, 53–93. [Google Scholar] [CrossRef]
- Marklund, S.L. Expression of extracellular superoxide dismutase by human cell lines. Biochem. J. 1990, 266, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhao, Q. Exenatide regulates inflammation and the production of reactive oxygen species via inhibition of S1PR2 synthesis. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2021, 30, 555–561. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012, 34, 73–91. [Google Scholar] [CrossRef]
- Nian, S.; Mi, Y.; Ren, K.; Wang, S.; Li, M.; Yang, D. The inhibitory effects of Dulaglutide on cellular senescence against high glucose in human retinal endothelial cells. Hum. Cell 2022, 35, 995–1004. [Google Scholar] [CrossRef]
- Yasuda, H.; Ohashi, A.; Nishida, S.; Kamiya, T.; Suwa, T.; Hara, H.; Takeda, J.; Itoh, Y.; Adachi, T. Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells. J. Clin. Biochem. Nutr. 2016, 59, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Qin, W.; Kirchhof, B.; Mitsiades, N.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; Adamis, A.P. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am. J. Pathol. 2002, 160, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Egholm, C.; Khammy, M.M.; Dalsgaard, T.; Mazur, A.; Tritsaris, K.; Hansen, A.J.; Aalkjaer, C.; Dissing, S. GLP-1 inhibits VEGFA-mediated signaling in isolated human endothelial cells and VEGFA-induced dilation of rat mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1214–H1224. [Google Scholar] [CrossRef] [Green Version]
- Kolibabka, M.; Dietrich, N.; Klein, T.; Hammes, H.P. Anti-angiogenic effects of the DPP-4 inhibitor linagliptin via inhibition of VEGFR signalling in the mouse model of oxygen-induced retinopathy. Diabetologia 2018, 61, 2412–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, H.P.; Feng, Y.; Pfister, F.; Brownlee, M. Diabetic retinopathy: Targeting vasoregression. Diabetes 2011, 60, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.J.; Ma, X.F.; Hao, M.; Zhou, H.R.; Yu, X.Y.; Shao, N.; Gao, X.Y.; Kuang, H.Y. Liraglutide attenuates the migration of retinal pericytes induced by advanced glycation end products. Peptides 2018, 105, 7–13. [Google Scholar] [CrossRef]
- Yoshida, Y.; Joshi, P.; Barri, S.; Wang, J.; Corder, A.L.; O’Connell, S.S.; Fonseca, V.A. Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes—A systematic review and meta-analysis. J. Diabetes Complicat. 2022, 36, 108255. [Google Scholar] [CrossRef]
| Drug | Class | Description |
|---|---|---|
| exendin-4 | GLP-1RA | 39 amino acid peptide found in the saliva of the Gila monster (Heloderma suspectum), with 53% homology to human GLP-1 and resistance to DPP-4 inactivation. |
| exenatide | GLP-1RA | Synthetic analog of exendin-4 resistant to the proteolytic effect of DPP-4 |
| liraglutide | GLP-1RA | Modified human GLP-1(7–37) with a 97% homology and longer half-life (13 h). |
| semaglutide | GLP-1RA | Long-acting GLP-1 agonist with a 94% homology and a half-life of 1 week. |
| dulaglutide | GLP-1RA | Two GLP-1 molecules linked to an IgG4-Fc heavy chain and with a half-life of 4 days. |
| NLY01 | GLP-1RA | Pegylated exendin-4 analogue with an extended half-life. |
| geniposide | GLP-1RA | Iridoid glycoside, a natural compound found in several medicinal herbs. |
| linagliptin | DPP-IV inhibitor |
| Cell Type | RGCs | RPE Cells | Endothelial Cells | |
|---|---|---|---|---|
| Drugs | ||||
| exendin-4 | Mantains ratio between Bax and Bcl2/Bcl-xL | ↓ ROS production Increases the antioxidant response | ↑ eNOS phosphorylation | |
| exenatide | Improves mitochondrial function ↓ Bax ↑ Bcl-2 | Improves viability ↓ VCAM, ICAM-1 and MMPs ↑ TIMP-2 ↓ DPPIV activity | ↓ ROS production, caspase activation and apoptosis Induces expression of SOD ↓ Bax ↑ Bcl-2 | |
| liraglutide | Improves mitochondrial function ↓ mitophagy and autophagy Promotes mitochondrial generation Maintains expression of the axonal marker GAP43 | |||
| dulaglutide | ↓ ROS production Restores Gluthatione activity, levels of SIRT-1 and telomerase activity Prevents reduction in eNOS expression | |||
| NLY01 | ↓ release of proinflammatory cytokines by microglia ↓ expression of genes associated with neurotoxic astrocytes | |||
| geniposide | ↓ NF-kB activation | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puddu, A.; Maggi, D. Anti-Inflammatory Effects of GLP-1R Activation in the Retina. Int. J. Mol. Sci. 2022, 23, 12428. https://doi.org/10.3390/ijms232012428
Puddu A, Maggi D. Anti-Inflammatory Effects of GLP-1R Activation in the Retina. International Journal of Molecular Sciences. 2022; 23(20):12428. https://doi.org/10.3390/ijms232012428
Chicago/Turabian StylePuddu, Alessandra, and Davide Maggi. 2022. "Anti-Inflammatory Effects of GLP-1R Activation in the Retina" International Journal of Molecular Sciences 23, no. 20: 12428. https://doi.org/10.3390/ijms232012428
APA StylePuddu, A., & Maggi, D. (2022). Anti-Inflammatory Effects of GLP-1R Activation in the Retina. International Journal of Molecular Sciences, 23(20), 12428. https://doi.org/10.3390/ijms232012428






