Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers
Abstract
:1. Introduction
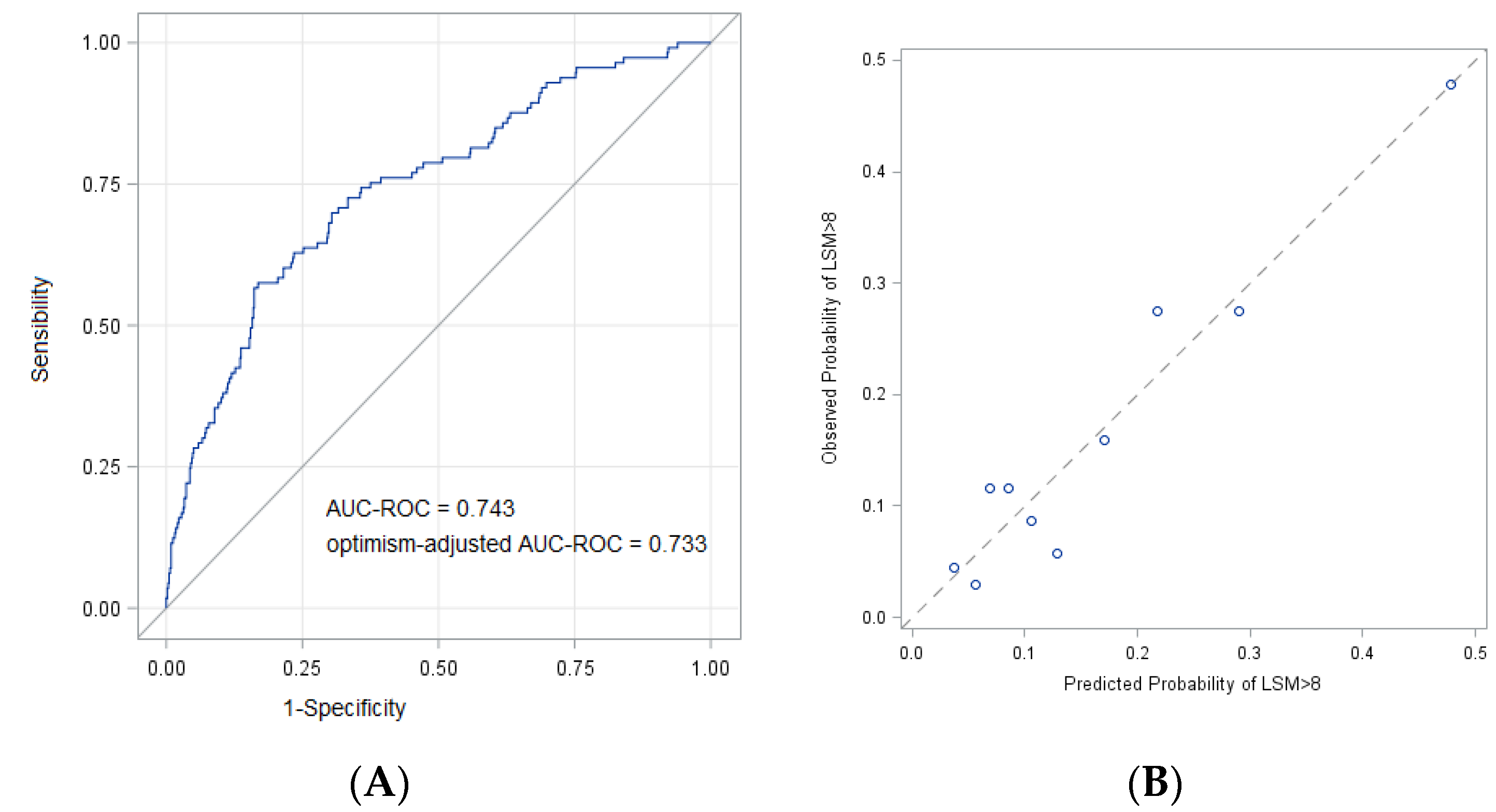
2. Results
FIB-4 and Transient Elastography
3. Discussion
4. Materials and Methods
Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Age ≥ 60 Years | DM | BMI ≥ 27 kg/m2 | GGT ≥ 25 UI/L | LSM ≥ 8 kPa n (%) | OR * (95% CI) (Ref = Remaining pts) |
|---|---|---|---|---|---|
| ☑ | ☑ | □ | □ | 30/82 (36.6) | 3.68 (2.13–6.34) |
| ☑ | □ | ☑ | □ | 36/116 (31.0) | 2.33 (1.40–3.91) |
| ☑ | □ | □ | ☑ | 37/116 (31.9) | 2.58 (1.58–4.21) |
| ☑ | ☑ | □ | ☑ | 23/52 (44.2) | 4.64 (2.53–8.53) |
| ☑ | □ | ☑ | ☑ | 29/73 (39.7) | 3.26 (1.88–5.64) |
| ☑ | ☑ | ☑ | □ | 23/58 (39.7) | 3.96 (2.16–7.23) |
| ☑ | ☑ | ☑ | ☑ | 21/39 (46.2) | 5.24 (2.68–10.25) |
| Predicted Probability | True Positive | True Negative | False Positive | False Negative | Sensibility | Specificity |
|---|---|---|---|---|---|---|
| 0.60 | 6 | 573 | 3 | 107 | 0.05 | 0.99 |
| 0.55 | 11 | 571 | 5 | 102 | 0.10 | 0.99 |
| 0.50 | 16 | 565 | 11 | 97 | 0.14 | 0.98 |
| 0.45 | 20 | 558 | 18 | 93 | 0.18 | 0.97 |
| 0.40 | 25 | 552 | 24 | 88 | 0.22 | 0.96 |
| 0.35 | 33 | 542 | 34 | 80 | 0.29 | 0.94 |
| 0.30 | 41 | 519 | 57 | 72 | 0.36 | 0.90 |
| 0.25 | 52 | 493 | 83 | 61 | 0.46 | 0.86 |
| 0.20 | 68 | 444 | 132 | 45 | 0.60 | 0.77 |
| 0.167 * | 77 | 404 | 172 | 36 | 0.68 | 0.70 |
| 0.15 | 80 | 387 | 189 | 33 | 0.71 | 0.67 |
| 0.10 | 90 | 271 | 305 | 23 | 0.80 | 0.47 |
| 0.05 | 110 | 69 | 507 | 3 | 0.97 | 0.12 |
Appendix B
- -
- p = probability of LSM ≥8 kPa;
- -
- Gender = 1 if the patient is a male, 0 otherwise:
- -
- Diabetes = 1 if the patient has diabetes, 0 otherwise;
- -
- The measurement units were: years for age, kg/m2 for body mass index and UI/L for serum-gamma-glutamyltransferase;
- -
- BMI: body mass index; GGT: serum-gamma-glutamyltransferase.
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gas-troenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Petta, S.; Sebastiani, G.; Viganò, M.; Ampuero, J.; Wai-Sun Wong, V.; Boursier, J.; Berzigotti, A.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced ahronic liver disease. Clin. Gastroenterol. Hepatol. 2021, 19, 806–815.e5. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; Sauver, J.S.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The Natural History of Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Björnsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [Green Version]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Grattagliano, I.; Ubaldi, E.; Napoli, L.; Marulli, C.F.; Nebiacolombo, C.; Cottone, C.; Portincasa, P. Utility of noninvasive methods for the characterization of nonalcoholic liver steatosis in the family practice. The “VARES” Italian multicenter study. Ann. Hepatol. 2013, 12, 70–77. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients With HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Clark, V.C.; Soldevilla-Pico, C.; Firpi-Morell, R.J.; Lai, J.; Shiffman, D.; Rowland, C.M.; Cusi, K. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 290–297. [Google Scholar] [CrossRef]
- Bertot, L.C.; Jeffrey, G.P.; de Boer, B.; MacQuillan, G.; Garas, G.; Chin, J.; Huang, Y.; Adams, L.A. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018, 38, 1793–1802. [Google Scholar] [CrossRef]
- Srivastava, A.; Jong, S.; Gola, A.; Srivastava, A.; Jong, S.; Gola, A.; Gailer, R.; Morgan, S.; Sennett, K.; Tanwar, S.; et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019, 19, 122. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Khac, E.; Thiele, M.; Voican, C.; Nahon, P.; Moreno, C.; Boursier, J.; Mueller, S.; de Ledinghen, V.; Stärkel, P.; Kim, S.G.; et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: An individual patient data meta-analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 614–625. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Boursier, J.; Vergniol, J.; Guillet, A.; Hiriart, J.-B.; Lannes, A.; Le Bail, B.; Michalak, S.; Chermak, F.; Bertrais, S.; Foucher, J.; et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 570–578. [Google Scholar] [CrossRef]
- Serra-Burriel, M.; Graupera, I.; Torán, P.; Thiele, M.; Roulot, D.; Wong, V.W.-S.; Guha, I.N.; Fabrellas, N.; Arslanow, A.; Expósito, C.; et al. Investigators of the LiverScreen Consortium. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J. Hepatol. 2019, 71, 1141–1151. [Google Scholar] [CrossRef]
- Graupera, I.; Thiele, M.; Serra-Burriel, M.; Caballeria, L.; Roulot, D.; Wong, G.L.H.; Fabrellas, N.; Guha, I.N.; Arslanow, A.; Expósito, C.; et al. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Lee, D.H. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2020, 35, 243–259. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Hastah, F.; Galan, M.V.; Gordon, S.C. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. Am. J. Clin. Pathol. 2005, 123, 382–387. [Google Scholar] [CrossRef]
- Foschi, F.G.; Domenicali, M.; Giacomoni, P.; Dall’Aglio, A.C.; Conti, F.; Borghi, A.; Bevilacqua, V.; Napoli, L.; Mirici, F.; Cucchetti, A.; et al. Is there an association between commonly employed biomarkers of liver fibrosis and liver stiffness in the general population? Ann. Hepatol. 2020, 19, 380–387. [Google Scholar] [CrossRef]
- Ooi, G.J.; Burton, P.R.; Doyle, L.; Wentworth, J.M.; Bhathal, P.S.; Sikaris, K.; Cowley, M.A.; Roberts, S.K.; Kemp, W.; O’Brien, P.E.; et al. Modified thresholds for fibrosis risk scores in non-alcoholic fatty liver disease arenecessary in the obese. Obes. Surg. 2017, 27, 115–125. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.-F.; Petta, S.; Romero-Gómez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampuero, J.; Pais, R.; Aller, R.; Gallego-Durán, R.; Crespo, J.; García-Monzón, C.; Boursier, J.; Vilar, E.; Petta, S.; Zheng, M.-H.; et al. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Ad-vanced Fibrosis. Clin. Gastroenterol. Hepatol. 2020, 18, 216–225.e215. [Google Scholar] [CrossRef] [PubMed]
- Harman, D.J.; Ryder, S.D.; James, M.W.; Jelpke, M.; Ottey, D.S.; Wilkes, E.A.; Card, T.R.; Aithal, G.P.; Guha, I.N. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: A cross-sectional diagnostic study utilising transient elastography. BMJ Open 2015, 5, e007516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S.; et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asphaug, L.; Thiele, M.; Krag, A.; Melberg, H.O. Cost-Effectiveness of Noninvasive Screening for Alcohol-Related Liver Fibrosis. Hepatology 2020, 71, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Albhaisi, S.A.M.; Sanyal, A.J. New drugs for NASH. Liver Int. 2021, 41 (Suppl. S1), 112–118. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E.; Borsboom, G.J.; Eijkemans, M.; Vergouwe, Y.; Habbema, J.F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Noma, H.; Shinozaki, T.; Iba, K.; Teramukai, S.; Furukawa, T.A. Confidence intervals of prediction accuracy measures for multi-variable prediction models based on the bootstrap-based optimism correction methods. Stat Med. 2021, 40, 5691–5701. [Google Scholar] [CrossRef]

| n = 1338 | |
|---|---|
| Age, mean (std) | 59 (13) |
| Male sex, n (%) | 769 (57) |
| Ethnicity, n (%) | |
| Caucasian | 1197 (89) |
| Hispanic | 67 (5) |
| Asian | 37 (3) |
| African | 37 (3) |
| BMI (kg/m2), median (IQR) | 28 (25.7–31.2) |
| Overweight, n (%) | 1065 (80) |
| DM, n (%) | 441 (32) |
| Hypertension, n (%) | 657 (49) |
| Dyslipidemia, n (%) | 602 (45) |
| Platelets (103/μL), median (IQR) | 227 (181–276) |
| Total bilirubin (mg/dL), median (IQR) | 0.7 (0.50–0.97) |
| AST (UI/L), mean (IQR) | 29 (22–42) |
| ALT (UI/L), median (IQR) | 36 (23–61) |
| ALP (UI/L), median (IQR) | 80 (70–129) |
| GGT (UI/L), median (IQR) | 49 (26–102) |
| INR, median (IQR) | 1 (1–1.1) |
| Albumin (g/dL), median (IQR) | 4.2 (4.0–4.4) |
| Total cholesterol (mg/dL), median (IQR) | 190 (162–220) |
| HDL cholesterol (mg/dL), median (IQR) | 49 (41–60) |
| Triglycerides (mg/dL), median (IQR) | 126 (92–175) |
| Glucose (mg/dL), median (IQR) | 102 (92–121) |
| n = 699 | |
|---|---|
| Age, mean (SD) | 52 (12.2) |
| Male, n (%) | 398 (56.9) |
| DM, n (%) | 172 (25) |
| Hypertension, n (%) | 259 (37) |
| Dyslipidemia, n (%) | 281 (40) |
| BMI (kg/m2), median (IQR) | 28 (25–31) |
| GGT (UI/L), median (IQR) | 40 (24–87) |
| Bilirubin (mg/dL), median (IQR) | 0.7 (0.5–0.9) |
| ALP (UI/L), median (IQR) | 85 (68–114) |
| Albumin (g/dL), median (IQR) | 4.3 (4.0–4.5) |
| LSM <8 kPa | 581 (83%) |
| LSM 8–12 kPa | 97 (14%) |
| LSM >12 kPa | 21 (3%) |
| LSM ≥ 8 kPa, n (%) (n = 118) | Unadjusted OR (95% CI) | Multiple-Adjusted OR * (95% CI) | |
|---|---|---|---|
| Age (five-years increase) | 1.13 (1.40–1.23) | ||
| Age, categories | |||
| <60 | 70/517 (13.5) | Ref | Ref |
| ≥60 | 48/182 (26.4) | 2.29 (1.51–3.46) | 1.96 (1.19–3.23) |
| Sex | |||
| Female | 44/301 (14.6) | Ref | Ref |
| Male | 74/398 (18.6) | 1.33 (0.89–2.01) | 1.33 (0.85–2.08) |
| DM | |||
| No | 64/527 (12.1) | Ref | Ref |
| Yes | 54/172 (31.4) | 3.31 (2.19–5.01) | 2.59 (1.63–4.13) |
| Hypertension | |||
| No | 57/440 (13.0) | Ref | Ref |
| Yes | 61/259 (23.6) | 2.07 (1.39–3.09) | 1.06 (0.66–1.70) |
| Dyslipidemia | |||
| No | 64/418 (15.3) | Ref | |
| Yes | 54/281 (19.2) | 1.32 (0.88–1.96) | |
| BMI (kg/m2), one-point increase | 1.12 (1.08–1.17) | ||
| BMI (kg/m2), categories | |||
| <27 | 27/281 (9.6) | Ref | Ref |
| ≥27 | 91/418 (21.7) | 2.62 (1.65–4.15) | 2.17 (1.33–3.56) |
| GGT, 10 UI/L increase | 1.05 (1.02–1.08) | ||
| GGT (UI/L), categories | |||
| <25 | 16/179 ^ (8.9) | Ref | Ref |
| ≥25 | 97/510 (19.0) | 2.39 (1.37–4.19) | 2.68 (1.49–4.84) |
| Bilirubin, 1 mg/dL increase | 0.73 (0.43–1.26) | ||
| ALP, 10 UI/L increase | 1.01 (0.97–1.04) | ||
| Albumin, 1 g/dL increase | 0.61 (0.34–1.10) | ||
| Total cholesterol, 10 mg/dL increase | 0.97 (0.92–1.02) | ||
| HDL cholesterol, 10 mg/dL increase | 0.90 (0.79–1.03) | ||
| Triglycerides, 10 mg/dL increase | 1.01 (0.98–1.03) |
| LSM ≥8 kPa n ^ (%) | Unadjusted OR (95% CI) | Sex-adjusted OR (95% CI) | |
|---|---|---|---|
| No. of risk factors * | |||
| 0 | 3/46 (6.5) | Ref | Ref |
| 1 | 12/212 (5.6) | ||
| 2 | 44/276 (15.9) | 3.07 (1.66–5.67) | 3.05 (1.65–5.63) |
| 3 | 36/116 (31.0) | 7.29 (3.79–14.01) | 7.29 (3.79–14.02) |
| 4 | 18/39 (46.2) | 13.89 (6.13–31.45) | 14.46 (6.36–32.91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viganò, M.; Pugliese, N.; Cerini, F.; Turati, F.; Cimino, V.; Ridolfo, S.; Rocchetto, S.; Foglio, F.; Terrin, M.; La Vecchia, C.; et al. Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers. Int. J. Mol. Sci. 2022, 23, 12489. https://doi.org/10.3390/ijms232012489
Viganò M, Pugliese N, Cerini F, Turati F, Cimino V, Ridolfo S, Rocchetto S, Foglio F, Terrin M, La Vecchia C, et al. Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers. International Journal of Molecular Sciences. 2022; 23(20):12489. https://doi.org/10.3390/ijms232012489
Chicago/Turabian StyleViganò, Mauro, Nicola Pugliese, Federica Cerini, Federica Turati, Vincenzo Cimino, Sofia Ridolfo, Simone Rocchetto, Francesca Foglio, Maria Terrin, Carlo La Vecchia, and et al. 2022. "Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers" International Journal of Molecular Sciences 23, no. 20: 12489. https://doi.org/10.3390/ijms232012489
APA StyleViganò, M., Pugliese, N., Cerini, F., Turati, F., Cimino, V., Ridolfo, S., Rocchetto, S., Foglio, F., Terrin, M., La Vecchia, C., Rumi, M. G., & Aghemo, A. (2022). Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers. International Journal of Molecular Sciences, 23(20), 12489. https://doi.org/10.3390/ijms232012489







