Zearalenone-Induced Mechanical Damage of Intestinal Barrier via the RhoA/ROCK Signaling Pathway in IPEC-J2 Cells
Abstract
:1. Introduction
2. Results
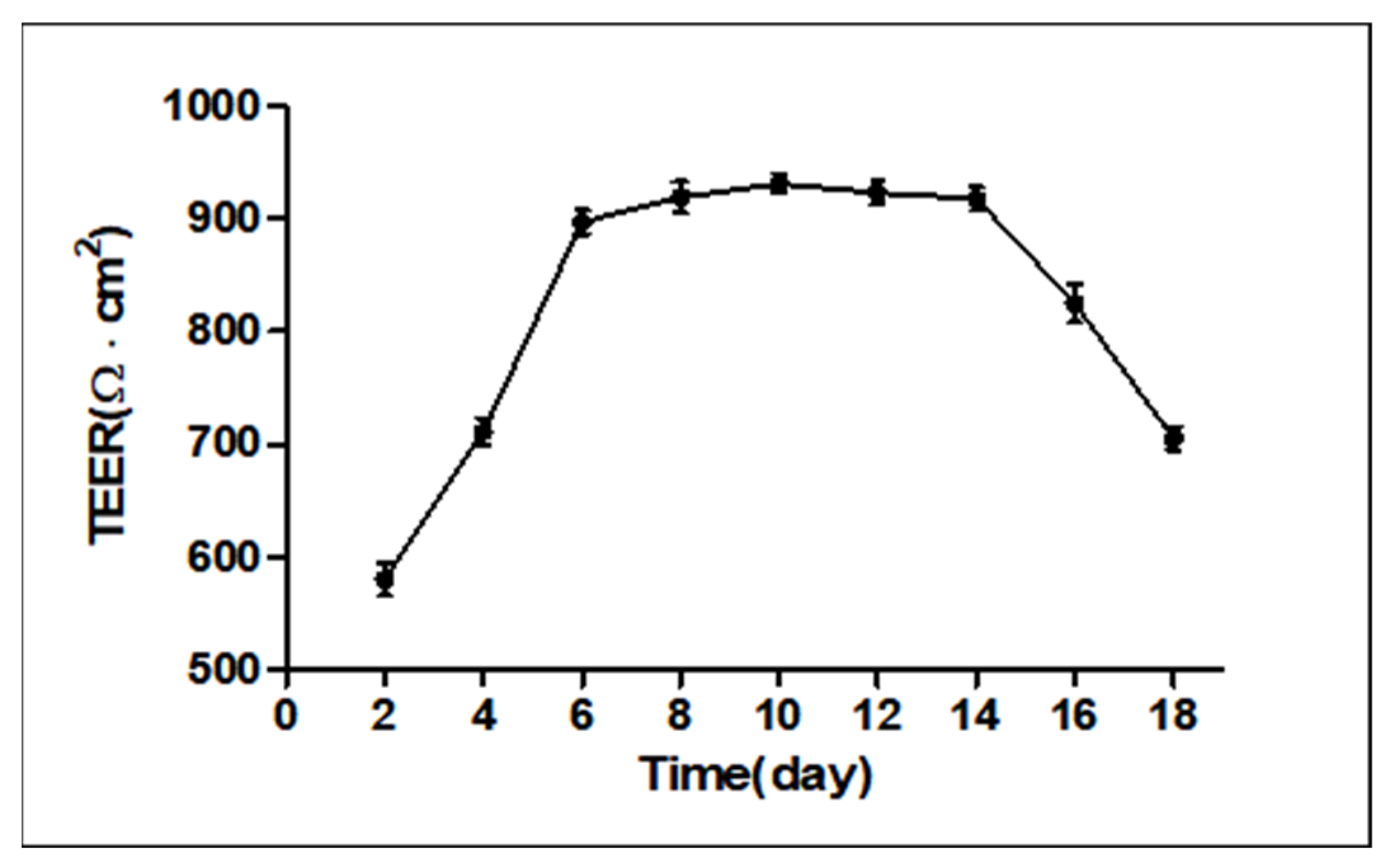
2.1. Measurement of Transepithelial Electrical Resistance (TEER)
2.2. Effects of ZEN on Permeability Barrier Function and Intestinal Integrity
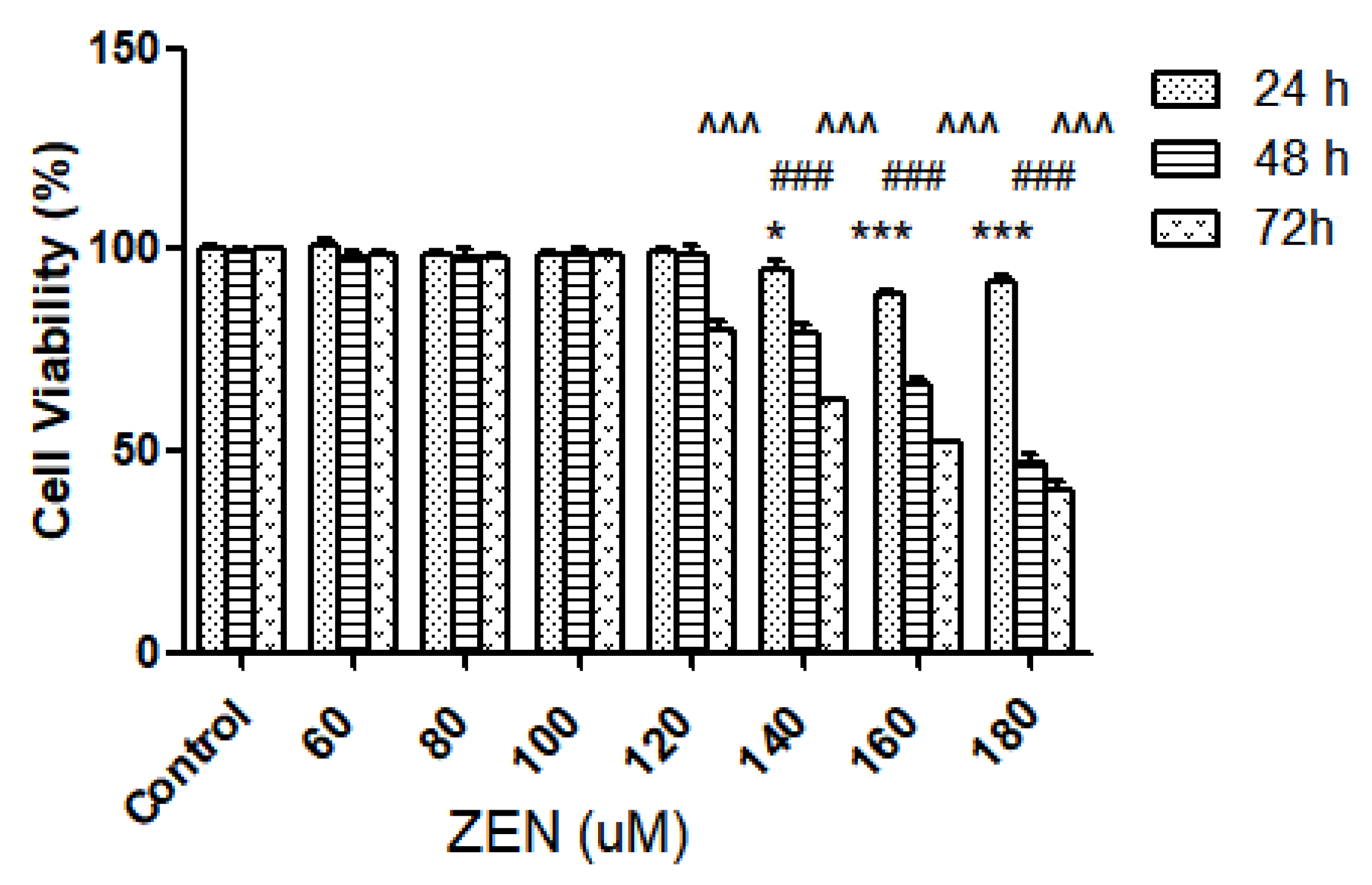
2.2.1. Effect of ZEN on Intestinal Epithelial Cell Viability
2.2.2. Alkaline Phosphatase Activity and The Permeation of FITC-Dextran
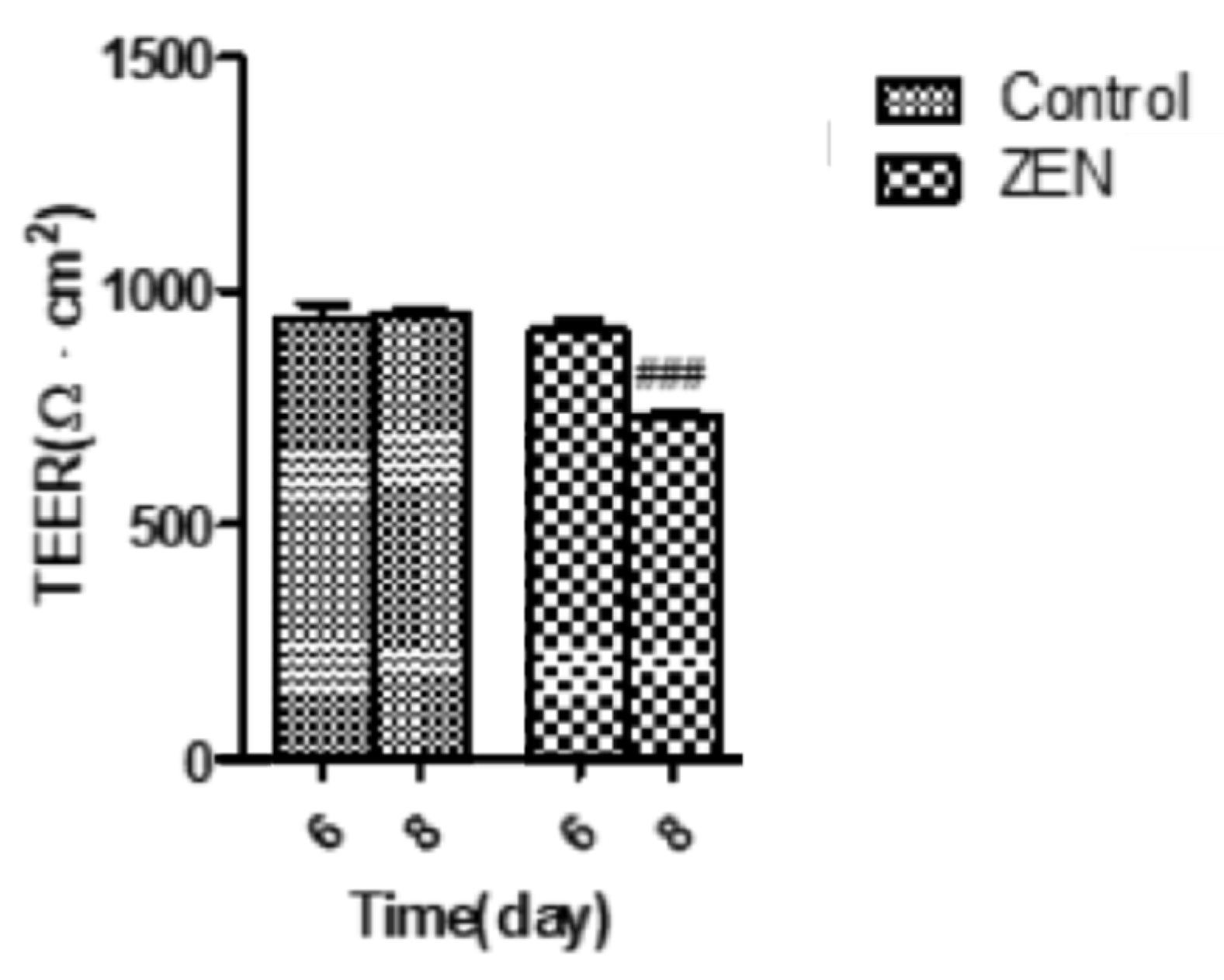
2.3. Analysis of Transepithelial Electrical Resistance (TEER)
2.4. Effect of ZEN on IPEC-J2 Intracellular ATP
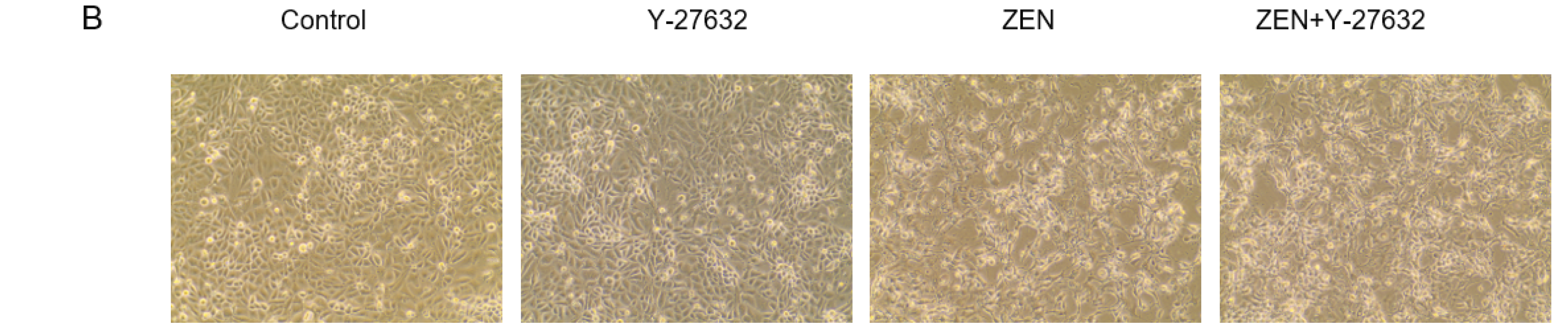
2.5. Effects of ZEN on IPEC-J2 Cytoskeletal Microfilament (F-Actin)
2.6. Effects of ZEN on the Distribution and Expression of TJ Proteins
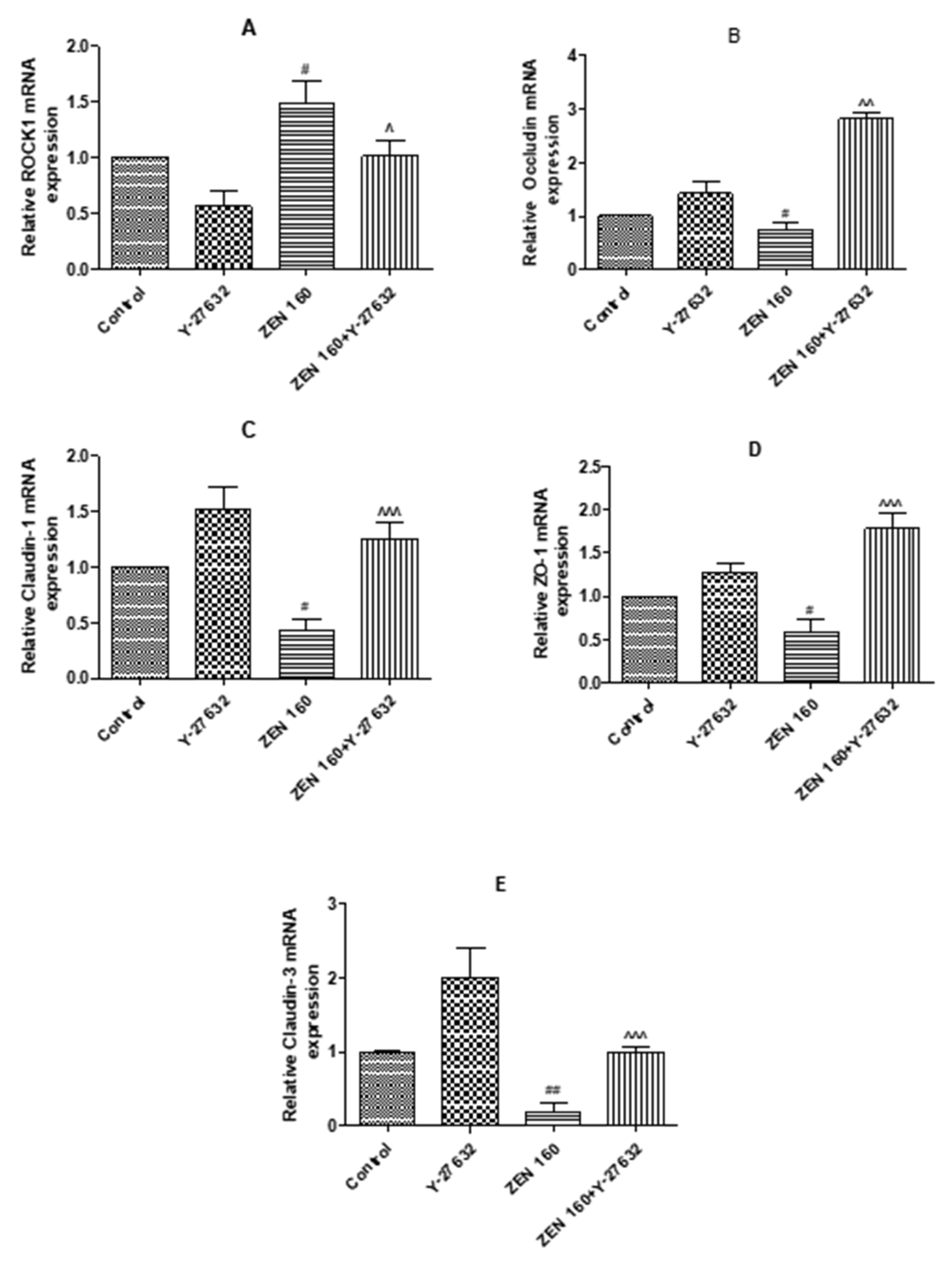
2.6.1. The Expression mRNA of ROCK1 and Tight Junction Proteins
2.6.2. Immunofluorescence
2.7. Western Blotting
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture Conditions
4.3. Transepithelial Electrical Resistance (TEER)
4.4. Cell Activity Assay
4.5. Alkaline Phosphatase Activity
4.6. Measurements of Paracellular Permeability
4.7. Design of RhoA/ROCK Signaling Pathway Validation Experiment
4.8. Quantitative Real-Time PCR
4.9. Immunofluorescence Staining
4.10. Intracellular ATP Determination
4.11. Filamentous Actin (F-actin) Staining Filaments of Actin of F-Actin Analysis
4.12. Western Blot Analysis
4.13. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification Strategies for Zearalenone Using Microorganisms: A Review. Microorganisms 2019, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo, A.; Ciasca, B.; Pascale, M.; Lattanzio, V.M.T. Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Tralamazza, S.M.; Piacentini, K.C.; Savi, G.D.; Carnielli-Queiroz, L.; Fontes, L.D.; Martins, C.S.; Correa, B.; Rocha, L.O. Wild rice (O. latifolia) from natural ecosystems in the Pantanal region of Brazil: Host to Fusarium incarnatum-equiseti species complex and highly contaminated by zearalenone. Int. J. Food Microbiol. 2021, 345, 8. [Google Scholar] [CrossRef] [PubMed]
- Lahouar, A.; Jedidi, I.; Sanchis, V.; Said, S. Aflatoxin B1, ochratoxin A and zearalenone in sorghum grains marketed in Tunisia. Food Addit. Contam. Part B-Surveill 2018, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.N.; Zheng, Y.D.; Tao, H.; Liu, J.; Zhao, P.; Yang, F.; Lv, Z.H.; Wang, J.Q. Effects of Bacillus subtilis ZJ-2019-1 on Zearalenone Toxicosis in Female Gilts. Toxins 2021, 13, 15. [Google Scholar] [CrossRef]
- Sabini, M.C.; Cariddi, L.N.; Escobar, F.M.; Manas, F.; Roma, D.; Candela, F.M.; Bagnis, G.; Soria, E.A.; Sabini, L.I.; Dalcero, A.M. Preventive effects of the antioxidant and antigenotoxic Achyrocline satureioides extract against zearalenone-induced mammal cytogenotoxicity and histological damage. World Mycotoxin J. 2021, 14, 401–409. [Google Scholar] [CrossRef]
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Wu, Y.; Xie, F.; Du, K.X.; Wang, Y.; Shi, L.X.; Ji, L.B.; Liu, T.Y.; Ma, X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017, 8, 44625–44638. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.H.; Zhang, M.L.; Yang, L.G.; Cheng, B.J.; Li, J.P.; Shan, A.S. Individual and combined effects of Fusarium toxins on apoptosis in PK15 cells and the protective role of N-acetylcysteine. Food Chem. Toxicol. 2018, 111, 27–43. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhang, W.; Gu, A.; Dong, J.; Li, J.; Shan, A. Protective Effect of N-Acetylcysteine against Oxidative Stress Induced by Zearalenone via Mitochondrial Apoptosis Pathway in SIEC02 Cells. Toxins 2018, 10, 407. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Zhao, L.; Krumm, C.S.; Qi, D.S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity (vol 39, pg 677, 2018). Trends Immunol. 2019, 40, 174. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Guo, W.; Wang, P.; Liu, Z.H.; Ye, P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int. J. Oral Sci. 2018, 10, e8. [Google Scholar] [CrossRef] [Green Version]
- Terry, S.; Nie, M.; Matter, K.; Balda, M.S. Rho signaling and tight junction functions. Physiology 2010, 25, 16–26. [Google Scholar] [CrossRef]
- Campos, S.B.; Ashworth, S.L.; Wean, S.; Hosford, M.; Sandoval, R.M.; Hallett, M.A.; Atkinson, S.J.; Molitoris, B.A. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am. J. Physiol. Renal Physiol. 2009, 296, F487–F495. [Google Scholar] [CrossRef] [Green Version]
- Cinel, I.; Ark, M.; Dellinger, P.; Karabacak, T.; Tamer, L.; Cinel, L.; Michael, P.; Hussein, S.; Parrillo, J.E.; Kumar, A.; et al. Involvement of Rho kinase (ROCK) in sepsis-induced acute lung injury. J. Thorac. Dis. 2012, 4, 30–39. [Google Scholar]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Bandarage, U.K.; Cao, J.; Come, J.H.; Court, J.J.; Gao, H.; Jacobs, M.D.; Marhefka, C.; Nanthakumar, S.; Green, J. ROCK inhibitors 3: Design, synthesis and structure-activity relationships of 7-azaindole-based Rho kinase (ROCK) inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 2622–2626. [Google Scholar] [CrossRef]
- Tong, J.; Wang, Y.; Chang, B.; Zhang, D.; Wang, B. Y-27632 inhibits ethanol-induced increase in intestinal epithelial barrier permeability. Mol. Med. Rep. 2014, 9, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.; Binion, D.G.; Nelson, V.M.; Kanaa, Y.; Javadi, P.; Lazarova, Z.; Andrekopoulos, C.; Kalyanaraman, B.; Otterson, M.F.; Rafiee, P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G1323–G1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci. USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, E.; Dubey, B.N.; Zhang, S.C.; Gremer, L.; Dvorsky, R.; Moll, J.M.; Taha, M.S.; Nagel-Steger, L.; Piekorz, R.P.; Somlyo, A.V.; et al. Rho-kinase: Regulation, (dys) function, and inhibition. Biol. Chem. 2013, 394, 1399–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Bhetwal, B.P.; Gunst, S.J. Rho kinase collaborates with p21-activated kinase to regulate actin polymerization and contraction in airway smooth muscle. J. Physiol. 2018, 596, 3617–3635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, D.; Han, R.; Zhang, X.; Zhang, S.; Qin, G. Soybean allergen glycinin induced the destruction of the mechanical barrier function in IPEC-J2. Food Agric. Immunol. 2015, 26, 601–609. [Google Scholar] [CrossRef]
- Pastorelli, L.; De Salvo, C.; Mercado, J.R.; Vecchi, M.; Pizarro, T.T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front. Immunol. 2013, 4, 280. [Google Scholar] [CrossRef] [Green Version]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. JALA 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Gil-Cardoso, K.; Gines, I.; Pinent, M.; Ardevol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Geens, M.M.; Niewold, T.A. Preliminary Characterization of the Transcriptional Response of the Porcine Intestinal Cell Line IPEC-J2 to Enterotoxigenic Escherichia coli, Escherichia coli, and E. coli Lipopolysaccharide. Comp. Funct. Genom. 2010, 2010, 469583. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Pasmans, F.; De Backer, P.; Croubels, S. An in vitro model using the IPEC-J2 cell line for efficacy and drug interaction testing of mycotoxin detoxifying agents. Toxicol. In Vitro 2013, 27, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Song, S.K.; Lee, I.K.; Ko, S.; Han, S.E.; Bae, S.; Ji, S.Y.; Park, B.C.; Song, K.D.; Lee, H.K.; et al. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Vet. Res. 2016, 47, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Zhang, T.; Gu, A.; Li, J.; Shan, A. The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells. Toxins 2021, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shi, J.; Zou, X.; Wang, C.; Yang, Y.; Zhang, H. Silver nanoparticles interact with the cell membrane and increase endothelial permeability by promoting VE-cadherin internalization. J. Hazard. Mater. 2016, 317, 570–578. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Xue, Y.X.; Liu, L.B.; Liu, Y.H. Endothelial-monocyte-activating polypeptide II increases blood-tumor barrier permeability by down-regulating the expression levels of tight junction associated proteins. Brain Res. 2010, 1319, 13–20. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.; Chen, Z.; Chen, Z.; Zhang, W.; Ma, X.; Wang, L.; Yang, X.; Jiang, Z. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef] [Green Version]
- Schnittler, H.; Taha, M.; Schnittler, M.O.; Taha, A.A.; Lindemann, N.; Seebach, J. Actin filament dynamics and endothelial cell junctions: The Ying and Yang between stabilization and motion. Cell Tissue Res. 2014, 355, 529–543. [Google Scholar] [CrossRef]
- Zhu, H.; Yao, X.M.; Qian, J.P.; Yang, J.; Pan, X.D.; Chen, X.D. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5239–5246. [Google Scholar]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; Pavlyuk, R.; Hildebrand, J.; et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusrat, A.; von Eichel-Streiber, C.; Turner, J.R.; Verkade, P.; Madara, J.L.; Parkos, C.A. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 2001, 69, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Betanzos, A.; Javier-Reyna, R.; Garcia-Rivera, G.; Banuelos, C.; Gonzalez-Mariscal, L.; Schnoor, M.; Orozco, E. The EhCPADH112 Complex of Entamoeba histolytica Interacts with Tight Junction Proteins Occludin and Claudin-1 to Produce Epithelial Damage. PLoS ONE 2013, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Wojciak-Stothard, B.; Ridley, A.J. Rho GTPases and the regulation of endothelial permeability. Vasc. Pharmacol. 2002, 39, 187–199. [Google Scholar] [CrossRef]
- Arnold, T.R.; Stephenson, R.E.; Miller, A.L. Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function. Exp. Cell Res. 2017, 358, 20–30. [Google Scholar] [CrossRef]
- Utech, M.; Ivanov, A.I.; Samarin, S.N.; Bruewer, M.; Turner, J.R.; Mrsny, R.J.; Parkos, C.A.; Nusrat, A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: Myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell 2005, 16, 5040–5052. [Google Scholar] [CrossRef] [Green Version]
- Narumiya, S.; Ishizaki, T.; Uehata, M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000, 325, 273–284. [Google Scholar]
- Alevizopoulos, K.; Dimas, K.; Papadopoulou, N.; Schmidt, E.M.; Tsapara, A.; Alkahtani, S.; Honisch, S.; Prousis, K.C.; Alarifi, S.; Calogeropoulou, T.; et al. Functional characterization and anti-cancer action of the clinical phase II cardiac Na+/K+ ATPase inhibitor istaroxime: In vitro and in vivo properties and cross talk with the membrane androgen receptor. Oncotarget 2016, 7, 24415–24428. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wen, J.; Luo, W. Rhoassociated kinase inhibitor, Y27632, inhibits the invasion and proliferation of T24 and 5367 bladder cancer cells. Mol. Med. Rep. 2015, 12, 7526–7530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca(2+)-mediated MLCK activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Koh, H.; Yamada, W.; Shimizu, M.; Ogawa, Y.; Hasegawa, N.; Yamaguchi, K.; Ishii, Y.; Richer, S.E.; Doerschuk, C.M.; et al. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am. J. Respir. Cell Mol. Biol. 2005, 32, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic acid enhances tight junctions and ameliorates inflammation in Salmonella typhimurium-induced diarrhea in mice via the TLR4/NF-kappaB and MAPK pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef]






| Genes | Orientation | Sequences (5′-3′) | Fragments Size (bp) | Accession Number |
|---|---|---|---|---|
| GAPDH | Forward | GATGGTGAAGGTCGGAGTGAAC | 153 | NM_001206359.1 |
| Reversed | TGGGTGGAATCATACTGGAACA | |||
| Occludin | Forward | ACGAGCAGCAAAGGGATTCTTC | 152 | NM_001163647.2 |
| Reversed | TCACACCCAGGATAGCACTCATT | |||
| Claudin-1 | Forward | TGCCTCAGTGGAAGATTTACTCC | 147 | NM_001244539.1 |
| Reversed | TGGTGTTCAGATTCAGCAAGGA | |||
| ZO-1 | Forward | AGTTTGATAGTGGCGTTGACAC | 106 | XM_005659811.1 |
| Reversed | GCTGAAGGACTCACAGGAACA | |||
| Claudin-3 | Forward | GTCCATGGGCCTGGAGAT | 130 | NM_001160075.1 |
| Reversed | GATCTGCGCTGTGATAATGC | |||
| ROCK1 | Forward | TTGTGCCTTCCTTACTGACAGG | 125 | XM_005653400.3 |
| Reversed | CTGGTGCCACAGTGTCTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Wang, J.; Gu, A.; Wang, T.; Li, J.; Shan, A. Zearalenone-Induced Mechanical Damage of Intestinal Barrier via the RhoA/ROCK Signaling Pathway in IPEC-J2 Cells. Int. J. Mol. Sci. 2022, 23, 12550. https://doi.org/10.3390/ijms232012550
Huang B, Wang J, Gu A, Wang T, Li J, Shan A. Zearalenone-Induced Mechanical Damage of Intestinal Barrier via the RhoA/ROCK Signaling Pathway in IPEC-J2 Cells. International Journal of Molecular Sciences. 2022; 23(20):12550. https://doi.org/10.3390/ijms232012550
Chicago/Turabian StyleHuang, Biying, Jingjing Wang, Aixin Gu, Tianhu Wang, Jianping Li, and Anshan Shan. 2022. "Zearalenone-Induced Mechanical Damage of Intestinal Barrier via the RhoA/ROCK Signaling Pathway in IPEC-J2 Cells" International Journal of Molecular Sciences 23, no. 20: 12550. https://doi.org/10.3390/ijms232012550






