Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy
Abstract
:1. Introduction
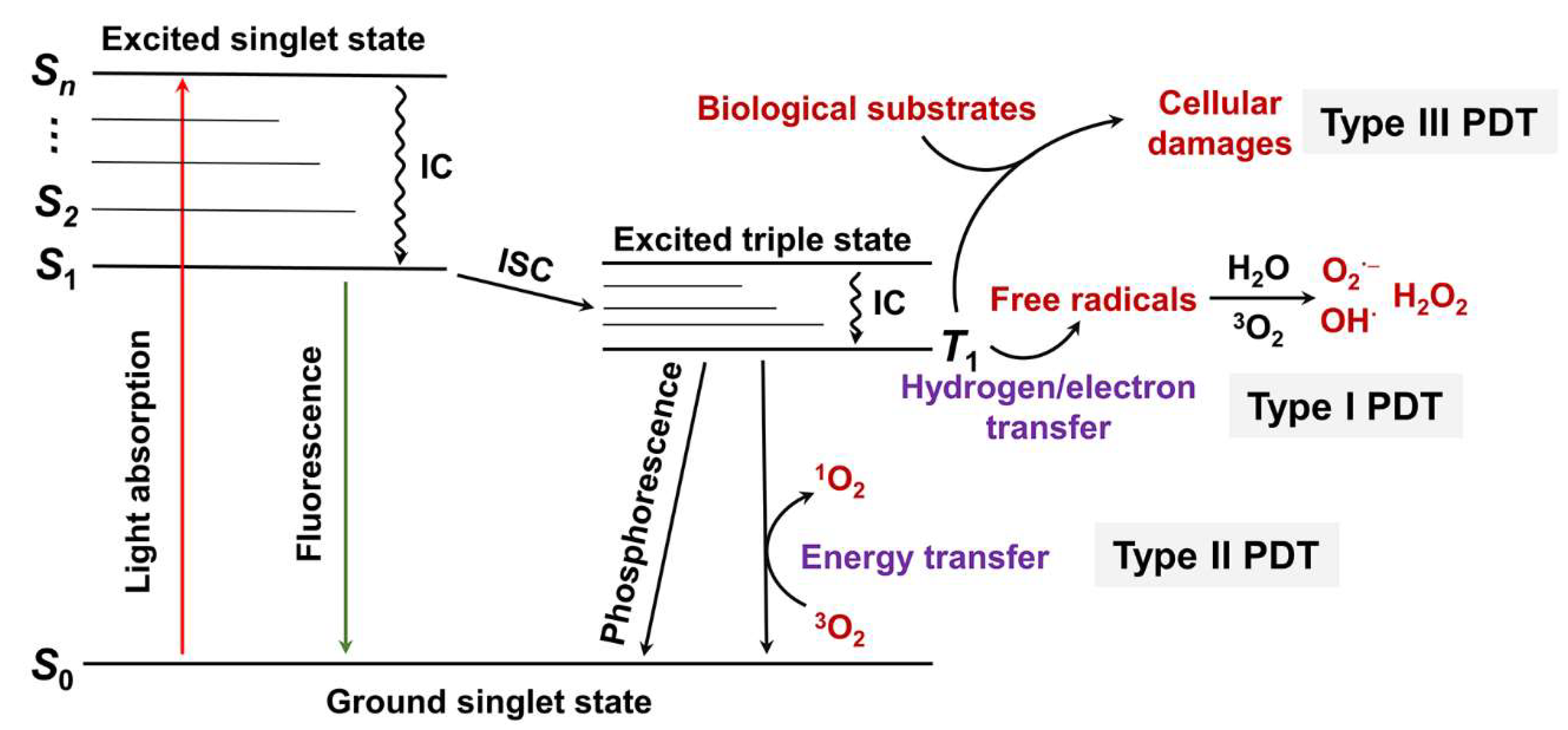
2. PDT Types
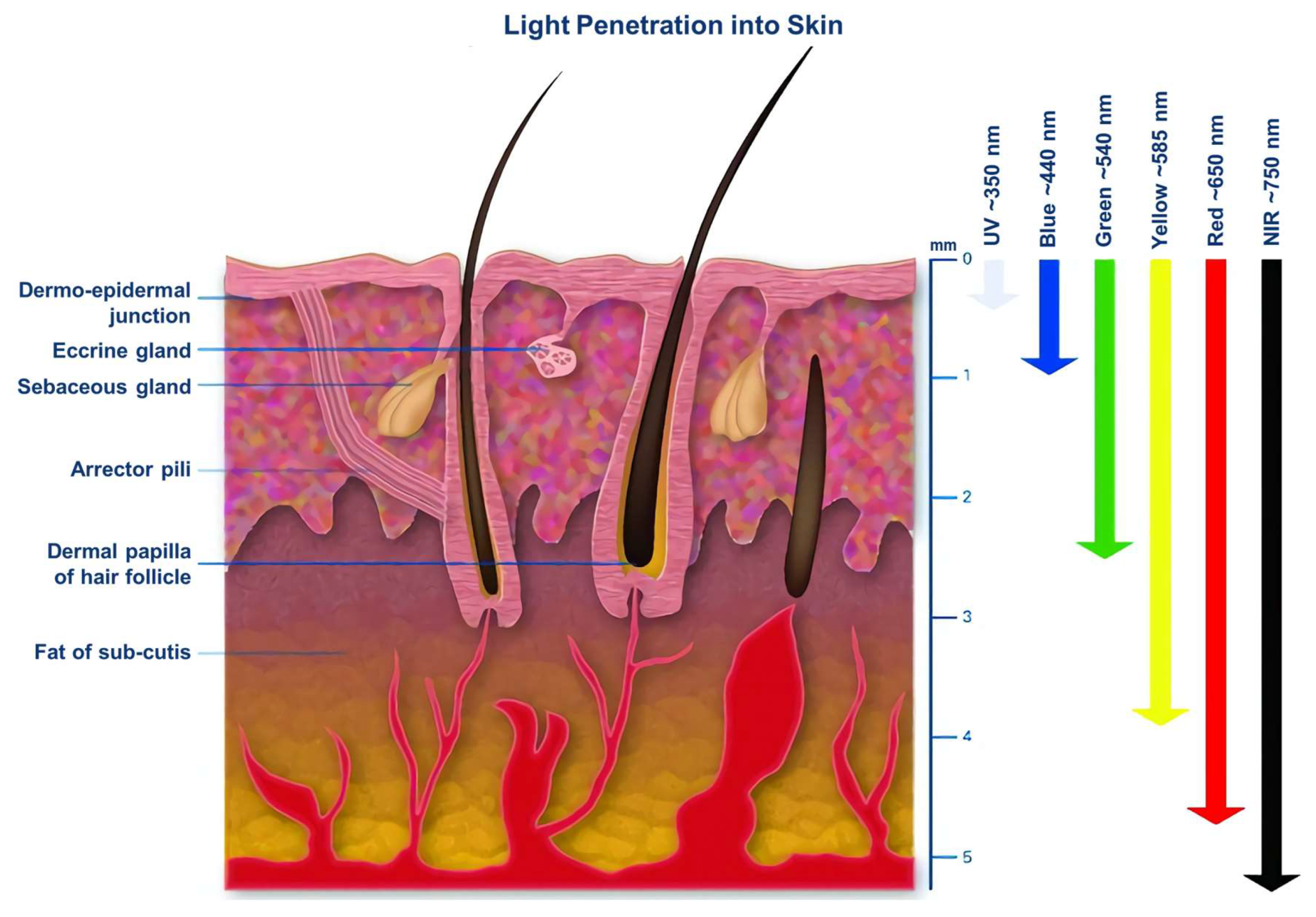
3. The Excitation Light in PDT
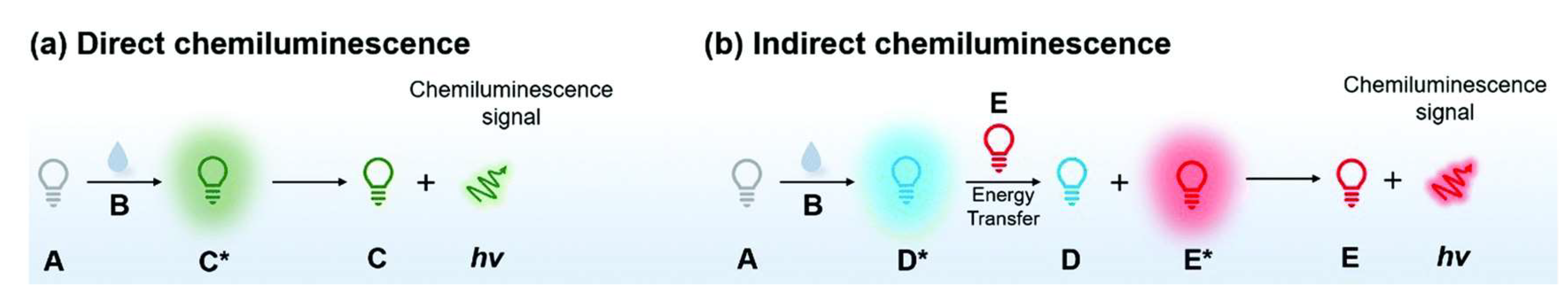
4. Chemiluminescence
5. CL-PDT System
5.1. Covalent CL-PDT System
5.2. Noncovalent CL-PDT System
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging Combination Strategies with Phototherapy in Cancer Nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-Infrared Photothermal Materials for Combinational Nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and Controlled Photosensitization with Bodipy Dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem. Int. Ed. 2019, 58, 2558–2569. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodriguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The Unique Features and Promises of Phthalocyanines as Advanced Photosensitisers for Photodynamic Therapy of Cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of Organic Semiconducting Materials for Deep-Tissue Optical Imaging, Phototherapy and Photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Ding, S.; Yang, M.; Lv, J.; Li, H.; Wei, G.; Gao, J.; Yuan, Z. Novel Lysosome-Targeting Fluorescence Off-on Photosensitizer for near-Infrared Hypoxia Imaging and Photodynamic Therapy in Vitro and in Vivo. Molecules 2022, 27, 3457. [Google Scholar] [CrossRef]
- Miao, Q.; Pu, K. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, e1801778. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ Heel of Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Y.; Chen, S.; Xu, M. Photodynamic Therapy of Cancers with Internal Light Sources: Chemiluminescence, Bioluminescence, and Cerenkov Radiation. Front. Chem. 2020, 8, 770. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for Bioimaging and Therapeutics: Recent Advances and Challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Huang, J.; Yu, B.; Pu, K.; Xu, F.J. Chemiluminescence: From Mechanism to Applications in Biological Imaging and Therapy. Aggregate 2021, 2, e140. [Google Scholar] [CrossRef]
- Magalhaes, C.M.; Esteves da Silva, J.C.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. Chemphyschem 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef]
- Bessière, A.; Durand, J.-O.; Noûs, C. Persistent Luminescence Materials for Deep Photodynamic Therapy. Nanophotonics 2021, 10, 2999–3029. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The Concept and Examples of Type-Iii Photosensitizers for Cancer Photodynamic Therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Clement, M.; Daniel, G.; Trelles, M. Optimising the Design of a Broad-Band Light Source for the Treatment of Skin. J. Cosmet. Laser Ther. 2005, 7, 177–189. [Google Scholar] [CrossRef]
- García-Campaña, A.M.; Baeyens, W.R.G. Principles and Recent Analytical Applications of Chemiluminescence. Analusis 2000, 28, 686–698. [Google Scholar] [CrossRef]
- Lou, J.; Tang, X.; Zhang, H.; Guan, W.; Lu, C. Chemiluminescence Resonance Energy Transfer Efficiency and Donor–Acceptor Distance: From Qualitative to Quantitative. Angew. Chem. Int. Ed. 2021, 60, 13029–13034. [Google Scholar] [CrossRef]
- White, E.H.; Roswell, D.F. Intramolecular Energy Transfer in Chemiluminescence. J. Am. Chem. Soc. 2002, 89, 3944–3945. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J. Nanomaterial-Based Chemiluminescence Resonance Energy Transfer: A Strategy to Develop New Analytical Methods. TrAC Trends Anal. Chem. 2012, 40, 77–89. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.-y.; Hai, X.; Song, W.; Ding, C.; Cao, J.; Bi, S. Chemiluminescence Resonance Energy Transfer: From Mechanisms to Analytical Applications. TrAC Trends Anal. Chem. 2020, 123, 115755. [Google Scholar] [CrossRef]
- Yesilgul, N.; Uyar, T.B.; Seven, O.; Akkaya, E.U. Singlet Oxygen Generation with Chemical Excitation of an Erythrosine-Luminol Conjugate. ACS Omega 2017, 2, 1367–1371. [Google Scholar] [CrossRef]
- Xu, X.; An, H.; Zhang, D.; Tao, H.; Dou, Y.; Li, X.; Huang, J.; Zhang, J. A Self-Illuminating Nanoparticle for Inflammation Imaging and Cancer Therapy. Sci. Adv. 2019, 5, eaat2953. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Guo, C.; Li, D.; Liu, R.; Xu, X.; Guo, J.; Ding, J.; Li, J.; Chen, W.; Zhang, J. Hydrogen Peroxide-Activatable Nanoparticles for Luminescence Imaging and in Situ Triggerable Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 17230–17243. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, A.; Sonkaya, O.; Soylukan, C.; Karaduman, T.; Algi, F. Bodipy and 2,3-Dihydrophthalazine-1,4-Dione Conjugates as Heavy Atom-Free Chemiluminogenic Photosensitizers. ACS Appl. Bio Mater. 2021, 4, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Digby, E.M.; Tung, M.T.; Kagalwala, H.N.; Ryan, L.S.; Lippert, A.R.; Beharry, A.A. Dark Dynamic Therapy: Photosensitization without Light Excitation Using Chemiluminescence Resonance Energy Transfer in a Dioxetane-Erythrosin B Conjugate. ACS Chem. Biol. 2022, 17, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Laptev, R.; Nisnevitch, M.; Siboni, G.; Malik, Z.; Firer, M.A. Intracellular Chemiluminescence Activates Targeted Photodynamic Destruction of Leukaemic Cells. Br. J. Cancer 2006, 95, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-C.; Huang, L.; Liu, C.-C.; Chao, P.-J.; Lin, F.-H. Luminol as the Light Source for in Situ Photodynamic Therapy. Process Biochem. 2012, 47, 1903–1908. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, L.; Ma, C.; Tu, Q.; Zhang, R.; Saeed, E.; Mahmoud, A.E.; Wang, J. Small Molecule-Initiated Light-Activated Semiconducting Polymer Dots: An Integrated Nanoplatform for Targeted Photodynamic Therapy and Imaging of Cancer Cells. Anal. Chem. 2014, 86, 3092–3099. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, H.; Liu, L.; Lv, F.; Ren, X.; Wang, S. Luminescent, Oxygen-Supplying, Hemoglobin-Linked Conjugated Polymer Nanoparticles for Photodynamic Therapy. Angew. Chem. Int. Ed. 2019, 58, 10660–10665. [Google Scholar] [CrossRef]
- Teng, Y.; Li, M.; Huang, X.; Ren, J. Singlet Oxygen Generation in Ferriporphyrin-Polymer Dots Catalyzed Chemiluminescence System for Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 5020–5029. [Google Scholar] [CrossRef]
- Jeon, J.; You, D.G.; Um, W.; Lee, J.; Kim, C.H.; Shin, S.; Kwon, S.; Park, J.H. Chemiluminescence Resonance Energy Transfer-Based Nanoparticles for Quantum Yield-Enhanced Cancer Phototheranostics. Sci. Adv. 2020, 6, eaaz8400. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Zhou, W.; Li, J.; Zhang, Y.; Liu, Y. Mitochondrion-Targeting Chemiluminescent Ternary Supramolecular Assembly for in Situ Photodynamic Therapy. Chem. Commun. 2020, 56, 8857–8860. [Google Scholar] [CrossRef]
- Wu, M.; Wu, L.; Li, J.; Zhang, D.; Lan, S.; Zhang, X.; Lin, X.; Liu, G.; Liu, X.; Liu, J. Self-Luminescing Theranostic Nanoreactors with Intraparticle Relayed Energy Transfer for Tumor Microenvironment Activated Imaging and Photodynamic Therapy. Theranostics 2019, 9, 20–33. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef] [Green Version]
- Berwin Singh, S.V.; Kim, J.; Park, H.; Khang, G.; Lee, D. Novel Chemi-Dynamic Nanoparticles as a Light-Free Photodynamic Therapeutic System for Cancer Treatment. Macromol. Res. 2017, 25, 749–755. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Gao, J.; Wu, W.; Hu, Y.; Jiang, X. Chemiluminescent Nanomicelles for Imaging Hydrogen Peroxide and Self-Therapy in Photodynamic Therapy. J. Biomed. Biotechnol. 2011, 2011, 679492. [Google Scholar] [CrossRef] [Green Version]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef] [Green Version]
- Beck, S.; Koster, H. Applications of Dioxetane Chemiluminescent Probes to Molecular Biology. Anal. Chem. 1990, 62, 2258–2270. [Google Scholar] [CrossRef]
- Schaap, A.P.; Handley, R.S.; Giri, B.P. Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 1: Aryl Esterase-Catalyzed Chemiluminescence from a Naphthyl Acetate-Substituted Dioxetane. Tetrahedron Lett. 1987, 28, 935–938. [Google Scholar] [CrossRef]
- Hananya, N.; Shabat, D. Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent. Sci. 2019, 5, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Haris, U.; Kagalwala, H.N.; Kim, Y.L.; Lippert, A.R. Seeking Illumination: The Path to Chemiluminescent 1,2-Dioxetanes for Quantitative Measurements and in Vivo Imaging. Acc. Chem. Res. 2021, 54, 2844–2857. [Google Scholar] [CrossRef] [PubMed]
- Hananya, N.; Shabat, D. A Glowing Trajectory between Bio- and Chemiluminescence: From Luciferin-Based Probes to Triggerable Dioxetanes. Angew. Chem. Int. Ed. 2017, 56, 16454–16463. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kim, Y.L.; Lippert, A.R. Chemiluminescence Measurement of Reactive Sulfur and Nitrogen Species. Antioxid. Redox Signal. 2022, 36, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.S.; Gerberich, J.; Haris, U.; Nguyen, D.; Mason, R.P.; Lippert, A.R. Ratiometric Ph Imaging Using a 1,2-Dioxetane Chemiluminescence Resonance Energy Transfer Sensor in Live Animals. ACS Sens. 2020, 5, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.S.; Gerberich, J.; Cao, J.; An, W.; Jenkins, B.A.; Mason, R.P.; Lippert, A.R. Kinetics-Based Measurement of Hypoxia in Living Cells and Animals Using an Acetoxymethyl Ester Chemiluminescent Probe. ACS Sens. 2019, 4, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.; Chen, X.; Su, D. Chemiluminescent Probes Based on 1,2-Dioxetane Structures for Bioimaging. Chem. Asian J. 2022, 17, e202200018. [Google Scholar] [CrossRef]
- Bastos, E.L.; Farahani, P.; Bechara, E.J.H.; Baader, W.J. Four-Membered Cyclic Peroxides: Carriers of Chemical Energy. J. Phys. Org. Chem. 2017, 30, e3725. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Yang, M.; Lv, J.; Li, H.; Gao, J.; Yuan, Z. A 1,2-Dioxetane-Based Chemiluminescent Probe for Highly Selective and Sensitive Detection of Superoxide Anions in Vitro and in Vivo. Chempluschem 2022, 87, e202200054. [Google Scholar] [CrossRef]
- Lu, X.; Song, X.; Wang, Q.; Hu, W.; Shi, W.; Tang, Y.; Wu, Z.; Fan, Q.; Huang, W. Chemiluminescent Organic Nanophotosensitizer for a Penetration Depth Independent Photodynamic Therapy. RSC Adv. 2020, 10, 11861–11864. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Chong, H.; Wang, B.; Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Chemical Molecule-Induced Light-Activated System for Anticancer and Antifungal Activities. J. Am. Chem. Soc. 2012, 134, 13184–13187. [Google Scholar] [CrossRef] [PubMed]
- Gnaim, S.; Gholap, S.P.; Ge, L.; Das, S.; Gutkin, S.; Green, O.; Shelef, O.; Hananya, N.; Baran, P.S.; Shabat, D. Modular Access to Diverse Chemiluminescent Dioxetane-Luminophores through Convergent Synthesis. Angew. Chem. Int. Ed. 2022, 61, e202202187. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.P.; Glesse, N.; Bonorino, C. Effect of Presumptive Tests Reagents on Human Blood Confirmatory Tests and DNA Analysis Using Real Time Polymerase Chain Reaction. Forensic Sci. Int. 2011, 206, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Yan, J.L.; Tu, Y.F. Study on a Luminol-Based Electrochemiluminescent Sensor for Label-Free DNA Sensing. Sensors 2010, 10, 9481–9492. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Song, Z. Study on the Proteins-Luminol Binding by Use of Luminol as a Fluorescence Probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 231–235. [Google Scholar] [CrossRef]
- Moyon, N.S.; Mitra, S. On the Interaction of Luminol with Human Serum Albumin: Nature and Thermodynamics of Ligand Binding. Chem. Phys. Lett. 2010, 498, 178–183. [Google Scholar] [CrossRef]
- Ikushima, T. Bimodal Induction of Sister-Chromatid Exchanges by Luminol, an Inhibitor of Poly(Adp-Ribose) Synthetase, During the S-Phase of the Cell Cycle. Chromosoma 1990, 99, 360–364. [Google Scholar] [CrossRef]


| Type | Chemiluminescent Substrate | PS | Cell | IC50 μM | Animal | Disease | Ref. |
|---|---|---|---|---|---|---|---|
| Covalent CL-PDT | luminol derivative | erythrosine | – | – | – | – | [30] |
| luminol | chlorin e6 | RAW264.7 B16F10 MOVAS MCF-7 A549 | 498.8 414.2 267.9 153.0 123.7 | male BALB/c mice C57BL/6 mice BALB/c nude mice | ulcerative colitis tumor | [31] | |
| luminol | chlorin e6 | 4T1 HCT116 A549 | 53.8 67.3 124.0 | Male BALB/c mice | tumor | [32] | |
| luminol derivative | BODIPY | Hep-2 | – | – | – | [33] | |
| Schaap’s adamantylidene-dioxetane | erythrosin B | MCF7 MDA-MB231 | 14.0 1.9 | – | – | [34] | |
| Noncovalent CL-PDT | luminol | hematoporphyrin | Friend’s Leukemia K-562 U-7.6 | 0.08 0.3 0.5 | – | – | [35] |
| luminol | 5-aminolevulinic acid | Caco-2 | – | – | – | [36] | |
| luminol | meta-tetra(hydroxyphenyl)- chlorin | MCF-7, C6, NIH 3T3 | – | – | – | [37] | |
| luminol | poly [2-methoxy-5-(2- ethylhexyloxy)-1,4-phenylenevinylene] | Hela | – | – | – | [38] | |
| luminol | Fe(III) Deuteroporphyrin IX | HeLa, MCF-7, H1299 cells, RAW264.7 | – | nude mice | tumor | [39] | |
| TCPO | chlorin e6 | HT29 cells | – | male BALB/c nude mice | tumor | [40] | |
| CPPO | DPAC-S | 293T, KYSE-150 | – | – | – | [41] | |
| CPPO | tetraphenylporphyrin | HeLa | – | BALB/c nude mice | tumor | [42] | |
| CPPO | TBD | 4T1 | – | BALB/c mice | tumor | [43] | |
| HPOX | protoporphyrin | SW620 | – | – | – | [44] | |
| Peroxalate ester oligomer | mesotetraphenylporphine | LoVo | – | – | – | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. https://doi.org/10.3390/ijms232012556
Gao J, Chen Z, Li X, Yang M, Lv J, Li H, Yuan Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. International Journal of Molecular Sciences. 2022; 23(20):12556. https://doi.org/10.3390/ijms232012556
Chicago/Turabian StyleGao, Jie, Zhengjun Chen, Xinmin Li, Mingyan Yang, Jiajia Lv, Hongyu Li, and Zeli Yuan. 2022. "Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy" International Journal of Molecular Sciences 23, no. 20: 12556. https://doi.org/10.3390/ijms232012556





