The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment and Risk of Bias
3. Results
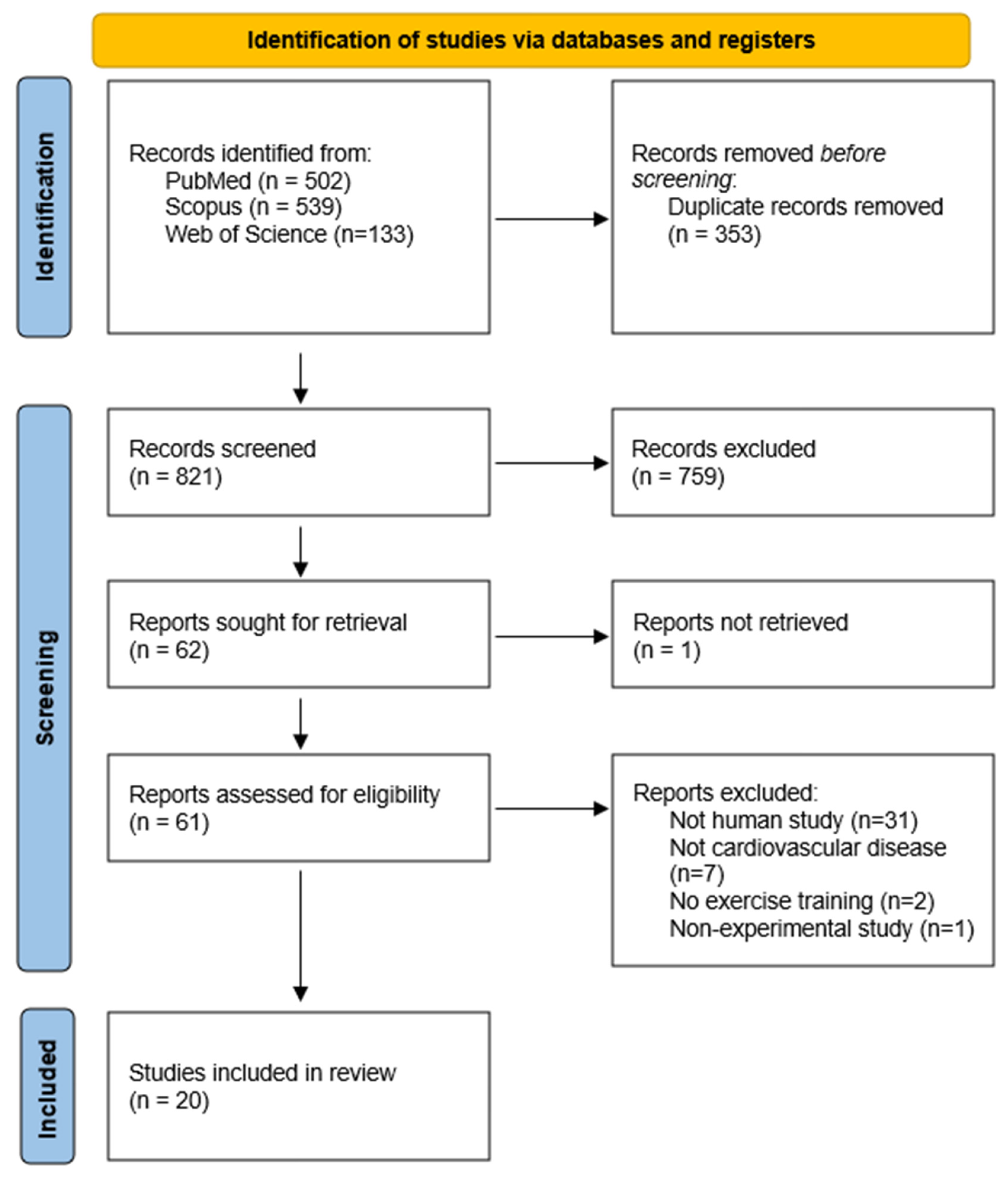
3.1. Study Selection
3.2. Study Characteristics
| Criteria | Eligibility Criteria Specified | Randomization Specified | Allocation Concealment | Groups Similar at Baseline | Blinding of all Assessors | Outcome Measures Assessed in 85% of Patients | Adverse Events Reported | Session Attendance Reported | Intent to Treat Analysis | Comparison Between Groups-Primary Outcome | Comparison between Groups-Secondary Outcome(s) | Point and Variability Measures | Activity Monitoring in Control Groups | Relative Exercise Intensity Remained Constant | Exercise Volume Characteristics and Energy Expenditure | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chou 2019 [22] | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 12/15 |
| Groennebaek 2019 [32] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 11/15 |
| Esposito 2018 [30] | No | No | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 8/15 |
| Southern 2015 [35] | Yes | No | No | Yes | No | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | 7/15 |
| Toth 2012 [37] | No | No | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | Yes | Yes | 7/15 |
| Esposito 2011 [31] | No | No | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | 7/15 |
| Williams 2007 [38] | Yes | Yes | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | 9/15 |
| Wisløff 2007 [42] | Yes | Yes | No | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 11/15 |
| Santoro 2002 [34] | Yes | No | No | No | No | Yes | No | No | No | No | No | Yes | No | Yes | Yes | 5/15 |
| Hambrecht 1997 [45] | No | Yes | No | Yes | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | 8/15 |
| Hambrecht 1995 [33] | No | Yes | No | Yes | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | 8/15 |
| Stratton 1994 [36] | No | No | No | No | No | Yes | Yes | Yes | No | No | No | Yes | No | No | No | 4/15 |
| Adamopoulos 1993 [29] | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | 10/15 |
| Lin 2021 [20] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 13/15 |
| Murrow 2019 [40] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 9/15 |
| van Schaardenburgh 2017 [41] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 13/15 |
| Hiatt 1996 [39] | No | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | 8/15 |
| Hsu 2019 [19] | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 11/15 |
| Zoll 2006 [43] | No | Yes | No | Yes | No | No | No | No | Yes | No | No | Yes | No | Yes | Yes | 6/15 |
| Fiorenza 2019 [44] | Yes | No | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 10/15 |
3.3. Physical Exercise Programs
3.4. Mitochondrial Outcomes
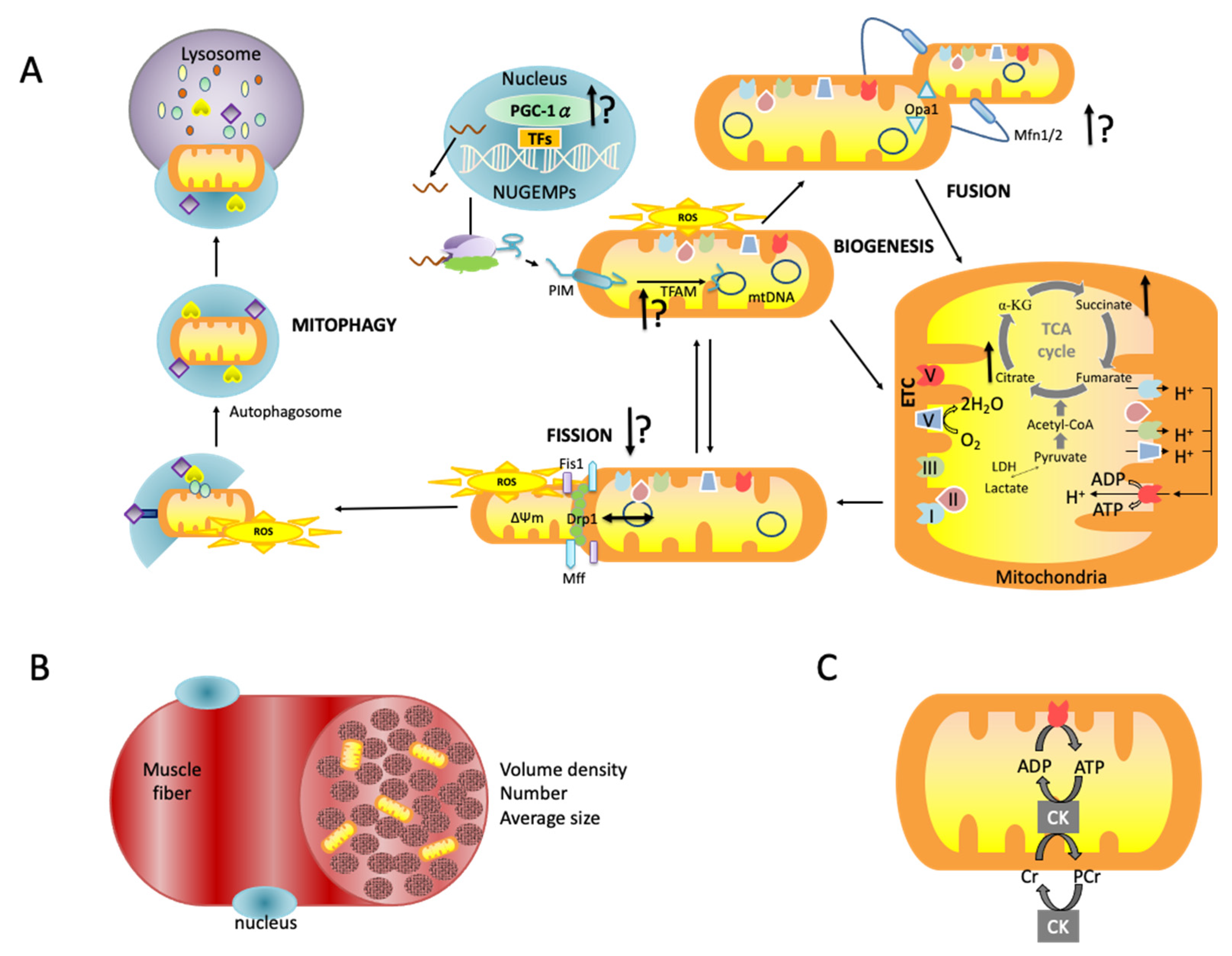
3.4.1. Mitochondrial Morphology
3.4.2. Mitochondrial Biogenesis and Dynamics
3.4.3. Mitochondrial Oxidative Capacity
3.4.4. Mitochondrial Antioxidant Capacity and Quality
4. Discussion
4.1. Study Quality
4.2. Exercise and Mitochondrial Oxidative Capacity
4.3. Exercise and Mitochondrial Biogenesis
4.4. Exercise and Mitochondrial Morphology, Antioxidant Capacity and Quality
4.5. Study Limitation
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Disesases; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tian, R.; Colucci, W.S.; Arany, Z.; Bachschmid, M.M.; Ballinger, S.W.; Boudina, S.; Bruce, J.E.; Busija, D.W.; Dikalov, S.; Dorn, G.W., II; et al. Unlocking the secrets of mitochondria in the cardiovascular system: Path to a cure in heart failure—A report from the 2018 national heart, lung, and blood institute workshop. Circulation 2019, 140, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Di Vaia, E.; Laccarino, G. Physical exercise: A novel tool to protect mitochondrial health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kraus, B.; Cain, H. Giant mitochondria in the human myocardium—Morphogenesis and fate. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1980, 33, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C.L.; Tandler, B.; Fujioka, H.; Riva, A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int. J. Biochem. Cell Biol. 2009, 41, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J.; Goldenthal, M.J.; Moe, G.W. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc. Res. 2001, 52, 103–110. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.F.; Orekhov, A.N. The role of mitochondria in cardiovascular diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in arherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef]
- Salnikova, D.; Orekhova, V.; Grechko, A.; Starodubova, A.; Bezsonov, E.; Popkova, T.; Orekhov, A. Mitochondrial dysfunction in vascular wall cells and its role in atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8990. [Google Scholar] [CrossRef]
- Petrucci, G.; Rizzi, A.; Hatem, D.; Tosti, G.; Rocca, B.; Pitocco, D. Role of oxidative stress in the pathogenesis of atherothrombotic diseases. Antioxidants 2022, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Forini, F.; Canale, P.; Nicolini, G.; Iervasi, G. Mitochondria-targeted drug delivery in cardiovascular disease: A long road to nano-cardio medicine. Pharmaceutics 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Rezende, L.F.M.; Joh, H.K.; Keum, N.; Ferrari, G.; Rey-Lopez, J.P.; Rimm, E.B.; Tabung, F.K.; Giovannucci, E.L. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: A prospective cohort of US adults. Circulation 2022, 146, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S. Strenuous, acute exercise suppresses polymorphonuclear leukocyte respiratory burst under adherence to surface-adherent platelets in men. Thromb. Haemost. 2004, 92, 1076–1085. [Google Scholar] [CrossRef]
- Tsai, H.H.; Chang, S.C.; Chou, C.H.; Weng, T.P.; Hsu, C.C.; Wang, J.S. Exercise training alleviates hypoxia-induced mitochondrial dysfunction in the lymphocytes of sedentary males. Sci. Rep. 2016, 6, 35170. [Google Scholar] [CrossRef]
- Wang, J.S.; Chen, Y.C.; Chen, W.L.; Lin, C.P. Effects of normoxic and hypoxic exercise regimens on lymphocyte apoptosis induced by oxidative stress in sedentary males. Eur. J. Appl. Physiol. 2017, 117, 2445–2455. [Google Scholar] [CrossRef]
- Wu, L.H.; Chang, S.C.; Fu, T.C.; Huang, C.H.; Wang, J.S. High-intensity interval training improves mitochondrial function and suppresses thrombin generation in platelets undergoing hypoxic stress. Sci. Rep. 2017, 7, 4191. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tsai, H.H.; Fu, T.C.; Wang, J.S. Exercise training enhances platelet mitochondrial bioenergetics in stroke patients: A randomized controlled trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef]
- Lin, M.L.; Fu, T.C.; Hsu, C.C.; Huang, S.C.; Lin, Y.T.; Wang, J.S. Cycling exercise training enhances platelet mitochondrial bioenergetics in patients with peripheral arterial disease: A randomized controlled trial. Thromb. Haemost. 2021, 121, 900–912. [Google Scholar] [CrossRef]
- Weng, T.P.; Fu, T.C.; Wang, C.H.; Hsu, C.C.; Wang, J.S. Activation of lymphocyte autophagy/apoptosis reflects haemodynamic inefficiency and functional aerobic impairment in patients with heart failure. Clin. Sci. 2014, 127, 589–602. [Google Scholar] [CrossRef]
- Chou, C.H.; Fu, T.C.; Tsai, H.H.; Hsu, C.C.; Wang, C.H.; Wang, J.S. High-intensity interval training enhances mitochondrial bioenergetics of platelets in patients with heart failure. Int. J. Cardiol. 2019, 274, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Rodriguez, D.A.; Hord, J.M. Mitochondria in the middle: Exercise preconditioning protection of striated muscle. J. Physiol. 2016, 594, 5161–5183. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Hung, C.H.; Shih, J.Y.; Chu, P.M.; Cheng, Y.H.; Lin, H.C.; Hsieh, P.L.; Tsai, K.L. Exercise intervention attenuates hyperhomocysteinemia-induced aortic endothelial oxidative injury by regulating SIRT1 through mitigating NADPH oxidase/LOX-1 signaling. Redox Biol. 2018, 14, 116–125. [Google Scholar] [CrossRef]
- Sties, S.W.; Andreato, L.V.; de Carvalho, T.; Gonzáles, A.I.; Angarten, V.G.; Ulbrich, A.Z.; de Mara, L.S.; Netto, A.S.; da Silva, E.L.; Andrade, A. Influence of exercise on oxidative stress in patients with heart failure. Heart Fail. Rev. 2018, 23, 225–235. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.A.U.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int. J. Evid. Based Healthc. 2015, 13, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Coats, A.J.; Brunotte, F.; Arnolda, L.; Meyer, T.; Thompson, C.H.; Dunn, J.F.; Stratton, J.; Kemp, G.J.; Radda, G.K. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J. Am. Coll. Cardiol. 1993, 21, 1101–1106. [Google Scholar] [CrossRef]
- Esposito, F.; Mathieu-Costello, O.; Wagner, P.D.; Richardson, R.S. Acute and chronic exercise in patients with heart failure with reduced ejection fraction: Evidence of structural and functional plasticity and intact angiogenic signalling in skeletal muscle. J. Physiol. 2018, 596, 5149–5161. [Google Scholar] [CrossRef]
- Esposito, F.; Reese, V.; Shabetai, R.; Wagner, P.D.; Richardson, R.S. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: The role of skeletal muscle convective and diffusive oxygen transport. J. Am. Coll. Cardiol. 2011, 58, 1353–1362. [Google Scholar] [CrossRef]
- Groennebaek, T.; Sieljacks, P.; Nielsen, R.; Pryds, K.; Jespersen, N.R.; Wang, J.; Carlsen, C.R.; Schmidt, M.R.; de Paoli, F.V.; Miller, B.F.; et al. Effect of blood flow restricted resistance exercise and remote ischemic conditioning on functional capacity and myocellular adaptations in patients with heart failure. Circ. Heart Fail. 2019, 12, e006427. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Niebauer, J.; Fiehn, E.; Kälberer, B.; Offner, B.; Hauer, K.; Riede, U.; Schlierf, G.; Kübler, W.; Schuler, G. Physical training in patients with stable chronic heart failure: Effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J. Am. Coll. Cardiol. 1995, 25, 1239–1249. [Google Scholar] [CrossRef]
- Santoro, C.; Cosmas, A.; Forman, D.; Morghan, A.; Bairos, L.; Levesque, S.; Roubenoff, R.; Hennessey, J.; Lamont, L.; Manfredi, T. Exercise training alters skeletal muscle mitochondrial morphometry in heart failure patients. J. Cardiovasc. Risk 2002, 9, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Southern, W.M.; Ryan, T.E.; Kepple, K.; Murrow, J.R.; Nilsson, K.R.; McCully, K.K. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol. Rep. 2015, 3, e12353. [Google Scholar] [CrossRef]
- Stratton, J.R.; Dunn, J.F.; Adamopoulos, S.; Kemp, G.J.; Coats, A.J.; Rajagopalan, B. Training partially reverses skeletal muscle metabolic abnormalities during exercise in heart failure. J. Appl. Physiol. 1994, 76, 1575–1582. [Google Scholar] [CrossRef]
- Toth, M.J.; Miller, M.S.; Ward, K.A.; Ades, P.A. Skeletal muscle mitochondrial density, gene expression, and enzyme activities in human heart failure: Minimal effects of the disease and resistance training. J. Appl. Physiol. 2012, 112, 1864–1874. [Google Scholar] [CrossRef]
- Williams, A.D.; Carey, M.F.; Selig, S.; Hayes, A.; Krum, H.; Patterson, J.; Toia, D.; Hare, D.L. Circuit resistance training in chronic heart failure improves skeletal muscle mitochondrial ATP production rate—A randomized controlled trial. J. Card. Fail. 2007, 13, 79–85. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Carry, M.R.; Brass, E.P. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J. Appl. Physiol. 1996, 81, 780–788. [Google Scholar] [CrossRef]
- Murrow, J.R.; Brizendine, J.T.; Djire, B.; Young, H.J.; Rathbun, S.; Nilsson, K.R., Jr.; McCully, K.K. Near infrared spectroscopy-guided exercise training for claudication in peripheral arterial disease. Eur. J. Prev. Cardiol. 2019, 26, 471–480. [Google Scholar] [CrossRef]
- Van Schaardenburgh, M.; Wohlwend, M.; Rognmo, Ø.; Mattsson, E. Calf raise exercise increases walking performance in patients with intermittent claudication. J. Vasc. Surg. 2017, 65, 1473–1482. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Steiner, R.; Meyer, K.; Vogt, M.; Hoppeler, H.; Flück, M. Gene expression in skeletal muscle of coronary artery disease patients after concentric and eccentric endurance training. Eur. J. Appl. Physiol. 2006, 96, 413–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiorenza, M.; Gunnarsson, T.P.; Ehlers, T.S.; Bangsbo, J. High-intensity exercise training ameliorates aberrant expression of markers of mitochondrial turnover but not oxidative damage in skeletal muscle of men with essential hypertension. Acta Physiol. 2019, 225, e13208. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Fiehn, E.; Yu, J.; Niebauer, J.; Weigl, C.; Hilbrich, L.; Adams, V.; Riede, U.; Schuler, G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1067–1073. [Google Scholar] [CrossRef]
- Carter, H.N.; Chen, C.C.; Hood, D.A. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Hardee, J.P.; VanderVeen, B.N.; Carson, J.A. Resistance exercise’s ability to reverse cancer-induced anabolic resistance. Exerc. Sport Sci. Rev. 2018, 46, 247–253. [Google Scholar] [CrossRef]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef]
- Oka, S.I.; Sabry, A.D.; Cawley, K.M.; Warren, J.S. Multiple levels of PGC-1α dysregulation in heart failure. Front. Cardiovasc. Med. 2020, 7, 2. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Hood, D.A. Exercise is mitochondrial medicine for muscle. Sports Med. Health Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef]
- Dorn, G.W.; Vega, R.B.; Kelly, D.P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015, 29, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Vitorino, R.; Padrão, A.I.; Espadas, G.; Mancuso, F.M.; Moreira-Gonçalves, D.; Castro-Sousa, G.; Henriques-Coelho, T.; Oliveira, P.A.; Barros, A.S.; et al. Lifelong exercise training modulates cardiac mitochondrial phosphoproteome in rats. J. Proteome Res. 2014, 13, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The role of exercise and TFAM in preventing skeletal muscle atrophy. J. Cell. Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Fu, T.C.; Lien, H.Y.; Wang, C.H.; Hsu, C.C.; Wu, W.C.; Chien, Y.W.; Cherng, W.J. Effect of aerobic interval training on erythrocyte rheological and hemodynamic functions in heart failure patients with anemia. Int. J. Cardiol. 2013, 168, 1243–1250. [Google Scholar] [CrossRef]
- Pang, B.P.S.; Chan, W.S.; Chan, C.B. Mitochondria homeostasis and oxidant/antioxidant balance in skeletal muscle-do myokines play a role? Antioxidants 2021, 10, 179. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, B.; Zhang, X.F.; Ma, Y.P.; Liu, J.D.; Wang, X.Z. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp. Gerontol. 2014, 56, 37–44. [Google Scholar] [CrossRef]

| Databases | Search String |
|---|---|
| PubMed | ((mitochondria*[Title/Abstract]) AND ((exercise[Title/Abstract]) OR (aerobic capacity[Title/Abstract])) AND (cardiovascular disease[MeSH Terms])) NOT (review[Publication Type]) |
| Scopus | (TITLE-ABS-KEY(mitochondria*) AND TITLE-ABS-KEY(exercise) AND TITLE-ABS-KEY ( "cardiovascular disease" OR "heart failure" OR "myocardiac infarct" OR stroke OR "peripheral artery disease")) AND (LIMIT-TO(DOCTYPE, "ar" )) AND (LIMIT-TO (LANGUAGE, "English" )) |
| Web of Science | mitochondria (All Fields) and exercise (All Fields) and cardiovascular disease (All Fields) |
| Authors | Disease | Study Design | Subject | Age | Exercise Program | Exercise Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Training | Protocol Duration | Frequency | Volume per Session | Intensity | Control/Healthy Group Activity | ||||||
| Chou et al., 2019 [22] | Heart failure | RCT | N: 34 TG: 17 CG: 17 | TG: 60.9 ± 0.5 CG: 59.7 ± 5.3 | Aerobic | HIIT cycling | 12 weeks | 5 days/week | 30 min | Five 3-min intervals at 40%, 80% VO2peak | CG: General health consultation |
| Groennebaek et al., 2019 [32] | Heart failure | RCT | N: 36 TG: 12 RICG: 12 CG: 12 | TG: 66 ± 7 RICG: 62 ± 9 CG: 63 ± 10 | Resistance | Blood flow restricted knee extension | 6 weeks | 3 days/week | 4 sets | 30% of 1RM | CG: no intervention RICG: once daily, 4 cycles of 5 min upper arm ischemia followed by 5 min of reperfusion |
| Esposito et al., 2018 [30] | Heart failure | Quasi-experiment | N: 12 TG: 6 HG: 6 | TG: 54 ± 14 HG: 51 ± 8 | Resistance | Knee extension | 8 weeks | 3 days/week | 100 min | 50% WRmax | HG: one-time assessment |
| Southern et al., 2015 [35] | Heart failure | Quasi-experiment | N: 12 TG: 7 HG: 5 | TG: 66 ± 4.1 HG: 61 ± 5.5 | Resistance | Non-dominant wrist flexor training with hand weight set | 4 weeks | 4 days/week | 30 min | 15% MVIC | HG: same training regimen |
| Toth et al., 2012 [37] | Heart failure | Quasi-experiment | N: 23 TG: 10 HG: 13 | TG: 71.8 ± 3.4 HG: 71.7 ± 1.7 | Resistance | Leg extension, leg press, leg curls, shoulder press, bench press, bicep curls, and lat pull-downs | 18 weeks | 3 days/week | Week 1: 50% of 1RM, 1 set 10 reps Week 2: 60% of 1RM, 2 sets 8 reps Week 3: 70% of 1RM, 3 sets 8 reps Week 4~18: 80% of 1RM, 3 sets 8 rep | HG: same training regimen | |
| Esposito et al., 2011 [31] | Heart failure | Quasi-experiment | N: 12 TG: 6 HG: 6 | TG: 54 ± 14 HG: 51 ± 8 | Resistance | Knee extension | 8 weeks | 3 days/week | 100 min | Not mentioned | HG: one-time assessment |
| Williams et al., 2007 [38] | Heart failure | RCT | N: 13 TG: 7 CG: 6 | TG: 67 ± 9 CG: 74 ± 4 | Resistance | Circuit training (Leg cycle, elbow extension/flexion, stair climbing, arm cycling, knee extension, and shoulder press) | 11 weeks | 3 days/week | 0.5~2 min for each | Moderate | Usual care |
| Wisløff et al., 2007 [42] | Heart failure | RCT | N: 27 MCTG: 9 AITG: 9 CG: 9 | MCTG: 74.4 ± 12 AITG: 76.5 ± 9 CG: 75.5 ± 13 | Aerobic | MCT or AIT Treadmill | 12 weeks | 3 days/week | MCTG: 47 min AITG: 38 min | MCTG: 70% HRpeak AITG: 95% HRpeak | CG: Standard advice regarding physical activity |
| Santoro et al., 2002 [34] | Heart failure | One group pre-posttest | N: 6 TG: 6 | TG: 73 (67~82) | Resistance | Leg press, knee curl, chest press, upper back machine, knee extension | 16 weeks | 3 days/week | 2 sets of 8 repetitions | 40~60% 1RM | No CG/HG |
| Hambrecht et al., 1997 [45] | Heart failure | RCT | N: 18 TG: 9 CG: 9 | TG: 50 ± 12 CG: 52 ± 8 | Aerobic | Cycling + group training (walking, calisthenics and ball games) | 24 weeks | Week 1–3: Cycling 6 times/day, 10 min each (inpatient) Week 4–24: Cycling 2 times/day, total 40 min (home); and group training 2 days/week | 70% HRpeak | Usual care | |
| Hambrecht et al., 1995 [33] | Heart failure | RCT | N: 22 TG: 12 CG: 10 | TG: 50 ± 12 CG: 52 ± 8 | Aerobic | Cycling + group training (walking, calisthenics and ball games) | 24 weeks | Week 1–3: Cycling 6 times/day, 10 min each (inpatient) Week 4–24: Cycling 2 times/day, total 40 min (home); and group training 2 days/week | 70% HRpeak | Usual care | |
| Stratton et al., 1994 [36] | Heart failure | One group pre-posttest | N: 10 TG: 10 | TG: 62 ± 11 | Resistance | Hand-held dynamometer | 4 weeks | 7 days/week | 2–3 sets of 10 reps of 10 s hold + 5 min of repeatedly squeezing | Not mentioned | No CG/HG |
| Adamopoulos et al., 1993 [29] | Heart failure | RCT crossover with HG comparison | N: 39 TG: 12 CG: 12 HG: 15 | TG: 62.4 ± 2.6 HG: 55.2 ± 2.8 | Aerobic | Cycling | 8 weeks | 5 days/week | 20 min | 70~80% HRmax | CG: avoidance of exercise HG: one-time assessment |
| Lin et al., 2021 [20] | Peripheral artery disease | RCT | N: 40 TG: 20 CG: 20 | TG: 71.1 ± 1.5 CG: 70.5 ± 1.9 | Aerobic | Cycling + general rehab | 12 weeks | 3 days/week | 30 min | At VT | CG: General rehab |
| Murrow et al., 2019 [40] | Peripheral artery disease | RCT | N: 18 NTG: 8 WTG: 10 | NTG: 72.0 ± 9.7 WTG: 71.6 ± 8.8 | Aerobic | Walking on treadmill | 12 weeks | 3 days/week | 30 min | NTG: Tissue saturation index reduced >15% WTG: claudication pain rating ≧6 | No CG/HG |
| van Schaardenburgh et al., 2017 [41] | Peripheral artery disease | RCT | N: 27 TTG: 13 CRTG: 14 | TTG: 70 ± 8.2 CRTG: 66 ± 9.3 | Aerobic vs. Resistance | Walking on treadmill vs. calf raises | 8 weeks | TTG: 3 days/week CRTG: 3 times/day | TTG: 30 min CRTG: not specified | TTG: near pain threshold CRTG: 5 more calf raise after pain felt | No CG/HG |
| Hiatt et al., 1996 [39] | Peripheral artery disease | RCT | N: 26 TTG: 10 STG: 8 CG: 8 | TTG: 67 ± 7 STG: 67 ± 6 CG: 67 ± 5 | Aerobic vs. Resistance | Walking on treadmill vs. lower limb exercise with cuff weight | 12 weeks | 3 days/week | TTG: 60 min STG: 3 sets of 6 reps | TTG: moderate claudication pain STG: not mentioned | Usual care |
| Hsu et al., 2019 [19] | Stroke | RCT | N: 30 TG: 15 CG: 15 | TG: 55.7 ± 3.0 CG: 57.8 ± 3.9 | Aerobic | Cycling + general rehab | 4 weeks | 5 days/week | 30 min | 60% VO2peak | CG: General rehab |
| Zoll et al., 2006 [43] | Coronary artery disease | RCT | N: 12 CETG: 6 EETG: 6 | Not mentioned | Aerobic | Cycling | 8 weeks | 3 days/week | 30 min | 60% VO2peak | No CG/HG |
| Fiorenza et al., 2019 [44] | Hypertension | Quasi-experiment | N: 37 TG: 24 HG: 13 | TG: 58.4 ± 2.5 HG: 60.8 ± 1.5 | Aerobic | HIIT Cycling | 6 weeks | 2~3 days/week | 2~3 sets of 5 min bout | 30~100% WRmax | HG: same training regimen |
| Author Year | Source of Mitochondria | Variables | Training | Healthy | Control |
|---|---|---|---|---|---|
| Chou et al., 2019 [22] | Blood sample (platelet) | OXPHOS (pmol/s/108 cells) | ↑ * | - | ↔ |
| ETS (pmol/s/108) | ↑ * | - | ↔ | ||
| Groennebaek et al., 2019 [32] | Muscle biopsy (vastus lateralis) | OXPHOS (pmol/s/mg) | ↑ ** | - | CG ↔ RIC ↔ |
| CS (μmol/min/g) | ↔ | - | CG ↔ RIC ↔ | ||
| Esposito et al., 2018 [30] | Muscle biopsy (vastus lateralis) | VVM (%) | ↑ * | - | - |
| Southern et al., 2015 [35] | Spectroscopy (wrist flexors) | Rate of recovery of mVO2 (min−1) | ↔ | ↑ * | - |
| Toth et al., 2012 [37] | Muscle biopsy (vastus lateralis) | Fractional density (%) | ↔ | ↔ | - |
| Number (μm2/area) | ↔ | ↔ | - | ||
| Average size (μm2) | ↔ | ↔ | - | ||
| PGC-1α (% of control) | ↔ | ↔ | - | ||
| PGC-1β (% of control) | ↔ | ↔ | - | ||
| NRF-1 (% of control) | ↔ | ↔ | - | ||
| TFAM (% of control) | ↑ ** | ↑ ** | - | ||
| COX-1 (% of control) | ↔ | ↓ ** | - | ||
| COX-5b (% of control) | ↔ | ↔ | - | ||
| COX (μmol/min/mg) | ↔ | ↔ | - | ||
| CS (μmol/min/mg) | ↔ | ↔ | - | ||
| Esposito et al., 2011 [31] | Muscle biopsy (vastus lateralis) | VVM (%) | ↑ * | - | - |
| Williams et al., 2007 [38] | Muscle biopsy (vastus lateralis) | MAPR (mmol ATP/min/kg) | ↑ * | - | ↓ * |
| CS (mmol/min/kg) | ↑ ** | - | ↔ | ||
| HAD (mmol/min/kg) | ↔ | - | ↔ | ||
| PFK (mmol/min/kg) | ↔ | - | ↔ | ||
| LDH (mmol/min/kg) | ↔ | - | ↔ | ||
| Wisløff et al., 2007 [42] | Muscle biopsy (vastus lateralis) | PGC-1α (arb. unit) | MCTG ↔ AITG ↑ ** | - | ↔ |
| Santoro et al., 2002 [34] | Muscle biopsy (vastus lateralis) | Area (μ2) | ↑ * | - | - |
| Elongation | ↔ | - | - | ||
| Hambrecht et al., 1997 [45] | Muscle biopsy (vastus lateralis) | SVMOcox+ (m2/cm3) | ↑ ** | - | ↔ |
| SVMOcox- (m2/cm3) | ↔ | - | ↔ | ||
| SVMIBM (m2/cm3) | ↑ ** | - | ↔ | ||
| SVMC (m2/cm3) | ↑ ** | - | ↔ | ||
| VVM (%) | ↑ ** | - | ↔ | ||
| Ncox+ (n) | ↔ | - | ↔ | ||
| Ncox- (n) | ↔ | - | ↔ | ||
| SVMOcox+/NSVMOcox+ (m2/cm3) | ↔ | - | ↔ | ||
| Hambrecht et al., 1995 [33] | Muscle biopsy (vastus lateralis) | VVMcox+ (%) | ↑ ** | - | ↔ |
| VVMcox- (%) | ↔ | - | ↔ | ||
| VVM (%) | ↑ * | - | ↔ | ||
| Stratton et al., 1994 [36] | Spectroscopy (forearm muscle) | PCr resynthesis rate (mM/min) | ↑ ** | - | - |
| MAPR (mM/min) | ↑ * | - | - | ||
| Adamopoulos et al., 1993 [29] | Spectroscopy (calf muscle) | PCr recovery time (min) | ↓ * | - | - |
| PCr resynthesis rate (mmol/liter/min) | ↑ * | - | - | ||
| MAPR (mmol/liter/min) | ↑ ** | - | - | ||
| Lin et al., 2021 [20] | Blood sample (platelet) | ETS (pmol/sec/108 cells) | ↑ * | - | ↔ |
| OXPHOS (pmol/sec/108 cells) | ↑ * | - | ↔ | ||
| Murrow et al., 2019 [40] | Spectroscopy (gastrocnemius) | Rate of recovery of mVO2 (rate constant) | NTG ↑ * WTG ↑ * | - | - |
| van Schaardenburgh et al., 2017 [41] | Muscle biopsy (gastrocnemius) | OXPHOS (pmol/mg/sec) | TTG ↔ CRTG ↔ | - | - |
| ETS (pmol/mg/sec) | TTG ↔ CRTG ↔ | - | - | ||
| CS (μmol/min/mg) | TTG ↔ CRTG ↑ * | - | - | ||
| Hiatt et al., 1996 [39] | Muscle biopsy (gastrocnemius) | LDH (mmol/min/g) | TTG ↔ STG ↔ | - | TTG ↔ STG ↔ |
| CS (μmol/min/g) | TTG ↔ STG ↓* | - | TTG ↔ STG ↔ | ||
| PFK (mmol/min/g) | TTG ↑ * STG ↔ | - | TTG ↔ STG ↔ | ||
| Carnitine (nmol/g) | TTG ↔ STG ↔ | - | TTG ↔ STG ↔ | ||
| Hsu et al., 2019 [19] | Blood sample (platelet) | OXPHOS (pmol/sec/108 cells) | ↑ * | - | ↔ |
| ETS (pmol/sec/108 cells) | ↑ * | - | ↔ | ||
| Zoll et al., 2006 [43] | Muscle biopsy (vastus lateralis) | VVM (%) | CETG ↔ EETG ↓ * | - | - |
| PGC-1α (mRNA level) | CETG ↔ EETG ↔ | - | - | ||
| TFAM (mRNA level) | CETG ↔ EETG ↔ | - | - | ||
| COX-1 (mRNA level) | CETG ↔ EETG ↔ | - | - | ||
| COX-4 (mRNA level) | CETG ↔ EETG ↓ * | - | - | ||
| Fiorenza et al., 2019 [44] | Muscle biopsy (vastus lateralis) | HAD (μmol/min/g) | ↑ ** | ↔ | - |
| CS activity (μmol/min/g) | ↑ *** | ↔ | - | ||
| CS content (arb. unit) | ↑ ** | ↔ | |||
| COX IV (arb. unit) | ↑ ** | ↔ | |||
| ERRα(arb. unit) | ↔ | ↔ | - | ||
| mitofusin 1 (arb. unit) | ↓ ** | ↔ | - | ||
| mitofusin 2 (arb. unit) | ↑ ** | ↔ | - | ||
| OPA1 (arb. unit) | ↓ ** | ↔ | |||
| Drp1 (arb. unit) | ↔ | ↔ | - | ||
| LC3-I (arb. unit) | ↔ | ↓ * | - | ||
| LC3-II (arb. unit) | ↔ | ↑ * | - | ||
| LC3-II/LC3-I ratio (arb. unit) | ↔ | ↑ ** | - | ||
| p62 (arb. unit) | ↑ *** | ↑ * | - | ||
| BAX (arb. unit) | ↑ *** | ↑ * | - | ||
| Bcl-2 (arb. unit) | ↓ ** | ↔ | - | ||
| BAX/Bcl-2 ratio (arb. unit) | ↑ *** | ↑ *** | - | ||
| SOD1 (arb. unit) | ↓ ** | ↔ | - | ||
| SOD2 (arb. unit) | ↑ * | ↑ * | - | ||
| GPX1 (arb. unit) | ↔ | ↑ * | - | ||
| Catalase (arb. unit) | ↑ * | ↔ | - | ||
| NOX (arb. unit) | ↑ * | ↑ * | - | ||
| Sirtuin 3 (arb. unit) | ↔ | ↔ | - | ||
| UCP3 (arb. unit) | ↔ | ↔ | |||
| HSP70 (arb. unit) | ↓ ** | ↔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.Y.; Chen, Y.-C.; Hsu, C.-C.; Fu, T.-C.; Wang, J.-S. The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12559. https://doi.org/10.3390/ijms232012559
Lim AY, Chen Y-C, Hsu C-C, Fu T-C, Wang J-S. The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(20):12559. https://doi.org/10.3390/ijms232012559
Chicago/Turabian StyleLim, Ai Yin, Yi-Ching Chen, Chih-Chin Hsu, Tieh-Cheng Fu, and Jong-Shyan Wang. 2022. "The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 23, no. 20: 12559. https://doi.org/10.3390/ijms232012559
APA StyleLim, A. Y., Chen, Y.-C., Hsu, C.-C., Fu, T.-C., & Wang, J.-S. (2022). The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 23(20), 12559. https://doi.org/10.3390/ijms232012559






